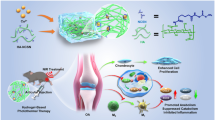
Abstract
Background:
Mesenchymal stem cells (MSCs) are used for tissue regeneration due to their wide differentiation capacity and anti-inflammatory effects. Extracellular vesicles (EVs) derived from MSCs are also known for their regenerative effects as they contain nucleic acids, proteins, lipids, and cytokines similar to those of parental cells. There are several studies on the use of MSCs or EVs for tissue regeneration. However, the combinatorial effect of human MSCs (hMSCs) and EVs is not clear. In this study, we investigated the combinatorial effect of hMSCs and EVs on cartilage regeneration via co-encapsulation in a hyaluronic-acid (HA)-based hydrogel.
Methods:
A methacrylic-acid-based HA hydrogel was prepared to encapsulate hMSCs and EVs in hydrogels. Through in vitro and in vivo analyses, we investigated the chondrogenic potential of the HA hydrogel-encapsulated with hMSCs and EVs.
Results:
Co-encapsulation of hMSCs with EVs in the HA hydrogel increased the chondrogenic differentiation of hMSCs and regeneration of damaged cartilage tissue compared with that of the HA hydrogel loaded with hMSCs only.
Conclusion:
Co-encapsulation of hMSCs and EVs in the HA hydrogel effectively enhances cartilage tissue regeneration due to the combinatorial therapeutic effect of hMSCs and EVs. Thus, in addition to cartilage tissue regeneration for the treatment of osteoarthritis, this approach would be a useful strategy to improve other types of tissue regeneration.




Similar content being viewed by others
References
Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002;14:573–7.
Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427:S6-15.
Sacitharan PK. Ageing and osteoarthritis. Subcell Biochem. 2019;91:123–59.
Simon LS. Osteoarthritis: a review. Clin Cornerstone. 1999;2:26–37.
Stewart HL, Kawcak CE. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front Vet Sci. 2018;5:178.
Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2021;80:413–22.
Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75.
Yu D, Xu J, Liu F, Wang X, Mao Y, Zhu Z. Subchondral bone changes and the impacts on joint pain and articular cartilage degeneration in osteoarthritis. Clin Exp Rheumatol. 2016;34:929–34.
Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1825–34.
De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74–80.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707.
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580–92.
Grässel S, Aszodi A. Osteoarthritis and cartilage regeneration: focus on pathophysiology and molecular mechanisms. Int J Mol Sci. 2019;20:6156.
Li M, Yin H, Yan Z, Li H, Wu J, Wang Y, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022;140:23–42.
Armiento AR, Stoddart MJ, Alini M, Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20.
Hafezi M, Nouri Khorasani S, Zare M, Esmaeely Neisiany R, Davoodi P. Advanced hydrogels for cartilage tissue engineering: recent progress and future directions. Polymers (Basel). 2021;13:4199.
Nabizadeh Z, Nasrollahzadeh M, Daemi H, BaghabanEslaminejad M, Shabani AA, Dadashpour M, et al. Micro- and nanotechnology in biomedical engineering for cartilage tissue regeneration in osteoarthritis. Beilstein J Nanotechnol. 2022;13:363–89.
Kumar R, Griffin M, Butler PE. A review of current regenerative medicine strategies that utilize nanotechnology to treat cartilage damage. Open Orthop J. 2016;10:862–76.
Duarte Campos DF, Drescher W, Rath B, Tingart M, Fischer H. Supporting biomaterials for articular cartilage repair. Cartilage. 2012;3:205–21.
Hwang NS, Zhang C, Hwang YS, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1:97–106.
Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19–31.
Barry F. MSC therapy for osteoarthritis: an unfinished story. J Orthop Res. 2019;37:1229–35.
Jevotovsky DS, Alfonso AR, Einhorn TA, Chiu ES. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilge. 2018;26:711–29.
Ahn J, Arai Y, Kim BJ, Seo YK, Moon JJ, Shin DA, et al. Combinatorial physicochemical stimuli in the three-dimensional environment of a hyaluronic acid hydrogel amplify chondrogenesis by stimulating phosphorylation of the Smad and MAPK signaling pathways. NPG Asia Mater. 2022;14:1–15.
Mancuso P, Raman S, Glynn A, Barry F, Murphy JM. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Front Bioeng Biotechnol. 2019;7:9.
Pers YM, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase i dose-escalation trial. Stem Cells Transl Med. 2016;5:847–56.
de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, et al. Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells. 2018;36:602–15.
Najar M, Martel-Pelletier J, Pelletier JP, Fahmi H. Mesenchymal stromal cell immunology for efficient and safe treatment of osteoarthritis. Front Cell Dev Biol. 2020;8:567813.
Yao Y, Huang J, Geng Y, Qian H, Wang F, Liu X, et al. Paracrine action of mesenchymal stem cells revealed by single cell gene profiling in infarcted murine hearts. PLoS One. 2015;10:e0129164.
Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Babu GS, Badrish Y, Oswal VM, Jeyaraman N, Prajwal GS, Jeyaraman M, et al. Immunomodulatory actions of mesenchymal stromal cells (MSCs) in osteoarthritis of the knee. Osteology. 2021;1:209–24.
Zhao X, Zhao Y, Sun X, Xing Y, Wang X, Yang Q. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8:575057.
Kou M, Huang L, Yang J, Chiang Z, Chen S, Liu J, et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: a next generation therapeutic tool? Cell Death Dis. 2022;13:580.
Jeyaraman M, Muthu S, Shehabaz S, Jeyaraman N, Rajendran RL, Hong CM, et al. Current understanding of MSC-derived exosomes in the management of knee osteoarthritis. Exp Cell Res. 2022;418:113274.
He C, Zheng S, Luo Y, Wang B. Exosome theranostics: biology and translational medicine. Theranostics. 2018;8:237–55.
Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19:47.
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18:1852.
Patel JM, Saleh KS, Burdick JA, Mauck RL. Bioactive factors for cartilage repair and regeneration: improving delivery, retention, and activity. Acta Biomater. 2019;93:222–38.
Yaghoubi Y, Movassaghpour A, Zamani M, Talebi M, Mehdizadeh A, Yousefi M. Human umbilical cord mesenchymal stem cells derived-exosomes in diseases treatment. Life Sci. 2019;233:116733.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Bao C, He C. The role and therapeutic potential of MSC-derived exosomes in osteoarthritis. Arch Biochem Biophys. 2021;710:109002.
Lankford KL, Arroyo EJ, Nazimek K, Bryniarski K, Askenase PW, Kocsis JD. Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS One. 2018;13:e0190358.
Sun G, Li G, Li D, Huang W, Zhang R, Zhang H, et al. hucMSC derived exosomes promote functional recovery in spinal cord injury mice via attenuating inflammation. Mater Sci Eng C Mater Biol Appl. 2018;89:194–204.
Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal stromal cell exosomes ameliorate experimental bronchopulmonary dysplasia and restore lung function through macrophage immunomodulation. Am J Respir Crit Care Med. 2018;197:104–16.
Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017;35:1208–21.
Fan B, Li C, Szalad A, Wang L, Pan W, Zhang R, et al. Mesenchymal stromal cell-derived exosomes ameliorate peripheral neuropathy in a mouse model of diabetes. Diabetologia. 2020;63:431–43.
Yang Y, Hong Y, Cho E, Kim GB, Kim IS. Extracellular vesicles as a platform for membrane-associated therapeutic protein delivery. J Extracell Vesicles. 2018;7:1440131.
Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol. 2013;165:77–84.
Yamashita T, Takahashi Y, Nishikawa M, Takakura Y. Effect of exosome isolation methods on physicochemical properties of exosomes and clearance of exosomes from the blood circulation. Eur J Pharm Biopharm. 2016;98:1–8.
Matsumoto A, Takahashi Y, Chang HY, Wu YW, Yamamoto A, Ishihama Y, et al. Blood concentrations of small extracellular vesicles are determined by a balance between abundant secretion and rapid clearance. J Extracell Vesicles. 2020;9:1696517.
Hemmati-Sadeghi S, Ringe J, Dehne T, Haag R, Sittinger M. Hyaluronic acid influence on normal and osteoarthritic tissue-engineered cartilage. Int J Mol Sci. 2018;19:1519.
Wu SC, Chen CH, Chang JK, Fu YC, Wang CK, Eswaramoorthy R, et al. Hyaluronan initiates chondrogenesis mainly via CD44 in human adipose-derived stem cells. J Appl Physiol (1985). 2013;114:1610–8.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Lesley J, Hascall VC, Tammi M, Hyman R. Hyaluronan binding by cell surface CD44. J Biol Chem. 2000;275:26967–75.
Tzavlaki K, Moustakas A. TGF-β signaling. Biomolecules. 2020;10:487.
Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, et al. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J Biol Chem. 2003;278:41227–36.
Bai J, Zhang Y, Zheng X, Huang M, Cheng W, Shan H, et al. LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 2020;11:763.
Duan L, Liang Y, Xu X, Xiao Y, Wang D. Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment. Arthritis Res Ther. 2020;22:194.
Zhang Y, Li S, Jin P, Shang T, Sun R, Lu L, et al. Dual functions of microRNA-17 in maintaining cartilage homeostasis and protection against osteoarthritis. Nat Commun. 2022;13:2447.
Shang X, Fang Y, Xin W, You H. The application of extracellular vesicles mediated miRNAs in osteoarthritis: current knowledge and perspective. J Inflamm Res. 2022;15:2583–99.
Loussouarn C, Pers YM, Bony C, Jorgensen C, Noël D. Mesenchymal stromal cell-derived extracellular vesicles regulate the mitochondrial metabolism via transfer of miRNAs. Front Immunol. 2021;12:623973.
Park KS, Bandeira E, Shelke GV, Lässer C, Lötvall J. Enhancement of therapeutic potential of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:288.
Kawata K, Koga H, Tsuji K, Miyatake K, Nakagawa Y, Yokota T, et al. Extracellular vesicles derived from mesenchymal stromal cells mediate endogenous cell growth and migration via the CXCL5 and CXCL6/CXCR2 axes and repair menisci. Stem Cell Res Ther. 2021;12:414.
Dabrowska S, Andrzejewska A, Janowski M, Lukomska B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. 2020;11:591065.
Xie M, Xiong W, She Z, Wen Z, Abdirahman AS, Wan W, et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front Immunol. 2020;11:13.
Acknowledgements
This work was funded by the Korean government (MSIT, MOE, and MOHW) (NRF-2022R1A2C3004850, NRF-2020R1I1A1A01074331, NRF-2019M3A9H1032376, and 21C0703L1).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
The study protocol was approved by the institutional review board of Dongguk University (IRB No. DUIH2020-03-012-022). Informed consent was confirmed by the IRB. The animal studies were performed after receiving approval of the Institutional Animal Care and Use Committee (IACUC) in Dongguk University (IACUC approval no. IACUC-2021-018-2).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, W.J., Ahn, J., Lee, M. et al. Combinatorial Effect of Mesenchymal Stem Cells and Extracellular Vesicles in a Hydrogel on Cartilage Regeneration. Tissue Eng Regen Med 20, 143–154 (2023). https://doi.org/10.1007/s13770-022-00509-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00509-6