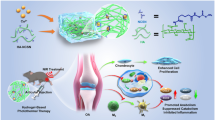
Abstract
BACKGROUND:
The extracellular matrix (ECM) has many functions, such as segregating tissues, providing support, and regulating intercellular communication. Cartilage-derived ECM (CECM) can be prepared via consecutive processes of chemical decellularization and enzyme treatment. The purpose of this study was to improve and treat osteoarthritis (OA) using porcine knee articular CECM.
METHODS:
We assessed the rheological characteristics and pH of CECM solutions. Furthermore, we determined the effects of CECM on cell proliferation and cytotoxicity in the chondrocytes of New Zealand rabbits. The inhibitory effect of CECM on tumor necrosis factor (TNF)-α-induced cellular apoptosis was assessed using New Zealand rabbit chondrocytes and human synoviocytes. Finally, we examined the in vivo effects of CECM on inflammation control and cartilage degradation in an experimental OA-induced rat model. The rat model of OA was established by injecting monosodium iodoacetate into the intra-articular knee joint. The rats were then injected with CECM solution. Inflammation control and cartilage degradation were assessed by measuring the serum levels of proinflammatory cytokines and C-telopeptide of type II collagen and performing a histomorphological analysis.
RESULTS:
CECM was found to be biocompatible and non-immunogenic, and could improve cell proliferation without inducing a toxic reaction. CECM significantly reduced cellular apoptosis due to TNF-α, significantly improved the survival of cells in inflammatory environments, and exerted anti-inflammatory effects.
CONCLUSION:
Our findings suggest that CECM is an appropriate injectable material that mediates OA-induced inflammation.






Similar content being viewed by others
References
Hunter DJ, Guermazi A, Roemer F, Zhang Y, Neogi T. Structural correlates of pain in joints with osteoarthritis. Osteoarthritis Cartilage. 2013;21:1170–8.
Chapple CM, Nicholson H, Baxter GD, Abbott JH. Patient characteristics that predict progression of knee osteoarthritis: a systematic review of prognostic studies. Arthritis Care Res (Hoboken). 2011;63:1115–25.
Laasila K, Laasonen L, Leirisalo-Repo M. Antibiotic treatment and long term prognosis of reactive arthritis. Ann Rheum Dis. 2003;62:655–8.
Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47.
Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73.
Bhatia D, Bejarano T, Novo M. Current interventions in the management of knee osteoarthritis. J Pharm Bioallied Sci. 2013;5:30–8.
Billesberger LM, Fisher KM, Qadri YJ, Boortz-Marx RL. Procedural treatments for knee osteoarthritis: a review of current injectable therapies. Pain Res Manag. 2020;2020:3873098.
Jacobs HN, Rathod S, Wolf MT, Elisseeff JH. Intra-articular injection of urinary bladder matrix reduces osteoarthritis development. AAPS J. 2017;19:141–9.
Li Y, Cao J, Han S, Liang Y, Zhang T, Zhao H, et al. ECM based injectable thermo-sensitive hydrogel on the recovery of injured cartilage induced by osteoarthritis. Artif Cells Nanomed Biotechnol. 2018;46:152–60.
Proffen BL, Sieker JT, Murray MM, Akelman MR, Chin KE, Perrone GS, et al. Extracellular matrix-blood composite injection reduces post-traumatic osteoarthritis after anterior cruciate ligament injury in the rat. J Orthop Res. 2016;34:995–1003.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Kumar V, Abbas AK, Fausto N, Aster JC. Robbins and Cotran pathologic basis of disease. 9th edition. Professional edition e-book. Amsterdam: Elsevier health sciences; 2014.
Lo CM, Wang HB, Dembo M, Wang Y. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52.
Hadjipanayi E, Mudera V, Brown RA. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J Tissue Eng Regen Med. 2009;3:77–84.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50.
Beachley VZ, Wolf MT, Sadtler K, Manda SS, Jacobs H, et al. Tissue matrix arrays for high-throughput screening and systems analysis of cell function. Nat Methods. 2015;12:1197–204.
Kim HJ, Lee S, Yun HW, Yin XY, Kim SH, Choi BH, et al. In vivo degradation profile of porcine cartilage-derived extracellular matrix powder scaffolds using a non-invasive fluorescence imaging method. J Biomater Sci Polym Ed. 2016;27:177–90.
Jhun J, Lee SH, Na HS, Seo HB, Kim EK, Moon SJ, et al. The chicken combs extract alleviates pain and cartilage degradation in rat model osteoarthritis. Tissue Eng Regen Med. 2015;12:352–61.
Imamura M, Ezquerro F, Marcon Alfieri F, Vilas Boas L, Tozetto-Mendoza TR, Chen J, et al. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int J Inflamm. 2015;2015:1–8.
Li TZ, Jin CZ, Choi BH, Kim MS, Kim YJ, Park SR, et al. Using cartilage extracellular matrix (CECM) membrane to enhance the reparability of the bone marrow stimulation technique for articular cartilage defect in canine model. Adv Func Mater. 2012;22:4292–300.
Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration. Arthritis Rheum. 2002;46:1986–96.
Grogan SP, Chen X, Sovani S, Taniguchi N, Colwell CW Jr, Lotz MK, et al. Influence of cartilage extracellular matrix molecules on cell phenotype and neocartilage formation. Tissue Eng Part A. 2014;20:264–74.
Tian G, Jiang S, Li J, Wei F, Li X, Ding Y, et al. Cell-free decellularized cartilage extracellular matrix scaffolds combined with interleukin 4 promote osteochondral repair through immunomodulatory macrophages: in vitro and in vivo preclinical study. Acta Biomater. 2021;127:131–45.
Choi BH, Choi KH, Lee HS, Song BR, Park SR, Yang JW, et al. Inhibition of blood vessel formation by a chondrocyte-derived extracellular matrix. Biomaterials. 2014;35:5711–20.
Kwon JS, Yoon SM, Shim SW, Park JH, Min KJ, Oh HJ, et al. Injectable extracellular matrix hydrogel developed using porcine articular cartilage. Int J Pharm. 2013;454:183–91.
De Lange-Brokaar BJ, Ioan-Facsinay A, Van Osch GJ, Zuurmond A-M, Schoones J, Toes RE, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–99.
Revell PA, Mayston V, Lalor P, Mapp P. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–7.
Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7.
Bhuanantanondh P, Grecov D, Kwok E. Rheological study of viscosupplements and synovial fluid in patients with osteoarthritis. CMBES Proc. 2010;33:522.
Park DY, Yun HW, Lim S, Truong MD, Yin XY, Park J, et al. Cross-linked cartilage acellular matrix film decreases postsurgical peritendinous adhesions. Artif Organs. 2020;44:E136–49.
Nicholls M, Manjoo A, Shaw P, Niazi F, Rosen J. Rheological properties of commercially available hyaluronic acid products in the United States for the treatment of osteoarthritis knee pain. Clin Med Insights Arthritis Musculoskelet Disord. 2018;11:1179544117751622.
Lynn AK, Yannas IV, Bonfield W. Antigenicity and immunogenicity of collagen. J Biomed Mater Res Part B: Appl Biomater Off J Soc Biomater Japan Soc Biomater Aust Soc Biomater Korean Soc Biomater. 2004;71:343–54.
Kim HY, Kim WU, Cho ML, Lee SK, Youn J, Kim SI, et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2085–93.
Ria F, Penitente R, De Santis M, Nicolò C, Di Sante G, Orsini M, et al. Collagen-specific T-cell repertoire in blood and synovial fluid varies with disease activity in early rheumatoid arthritis. Arthritis Res Ther. 2008;10:R135.
Myers LK, Tang B, Rosioniec EF, Stuart JM, Kang AH. PART III Autoimmunity an altered peptide ligand of type ii collagen suppresses autoimmune arthritis. Crit RevTM Immunol. 2007;27:345–56.
Cremer MA, Xiu JY, Myers LK, Brand DD, Rosloniec EF, Kang AH. T cell immunity to type II collagen in the biobreeding rat: The identification and characterization of RT1u-restricted T cell epitopes on α1 (II). J Immunol. 2004;173:1795–801.
Im G-I. Perspective on intra-articular injection cell therapy for osteoarthritis treatment. Tissue Eng Regen Med. 2019;16:357–63.
Lee SW, Lee HJ, Chung WT, Choi SM, Rhyu SH, Kim DK, et al. TRAIL induces apoptosis of chondrocytes and influences the pathogenesis of experimentally induced rat osteoarthritis. Arthritis Rheum. 2004;50:534–42.
Lee SW, Song YS, Lee SY, Yoon YG, Lee SH, Park BS, et al. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163.
Coustry F, Posey KL, Liu P, Alcorn JL, Hecht JT. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am J Pathol. 2012;180:738–48.
Akkiraju H, Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J dev biol. 2015;3:177–92.
Toshihiko I, Mitsumori S, Hiroko K, Haruko Y, Kayoko NI. Morphology and functional roles of synoviocytes in the joint. Arch Histol Cytol. 2000;63:17–31.
Lee SG, Lee EJ, Park WD, Kim JB, Kim EO, Choi SW. Anti-inflammatory and anti-osteoarthritis effects of fermented Achyranthes japonica Nakai. J Ethnopharmacol. 2012;142:634–41.
Mobasheri A. Intersection of inflammation and herbal medicine in the treatment of osteoarthritis. Curr Rheumatol Rep. 2012;14:604–16.
Ferrero-Miliani L, Nielsen OH, Andersen PS, Girardin S. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1β generation. Clin Exp Immunol. 2007;147:227–35.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42.
Jeong JH, Moon SJ, Jhun JY, Yang EJ, Cho ML, Min JK. Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS One. 2015;10:e0130882.
Khodir SA, Al-Gholam MA, Salem HR. L-Carnitine potentiates the anti-inflammatory and antinociceptive effects of diclofenac sodium in an experimentally-induced knee osteoarthritis rat model. Iran J Basic Med Sci. 2020;23:1035-44.
Mobasheri A. Osteoarthritis year 2012 in review: biomarkers. Osteoarthritis Cartilage. 2012;20:1451–64.
Felson DT. Developments in the clinical understanding of osteoarthritis. Arthritis Res Ther. 2009;11:203.
Acknowledgements
Everyone who contributed significantly to the work has been listed. This research was supported by the National Research Foundation Grant (NRF- 2019M3E5D1A02070861.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical statement
All animal studies were approved by the Institutional Review Board at the Pukyong National University (PKNUIACUC-2020–03).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, SH., Jo, SH., Kim, SH. et al. Anti-Osteoarthritic Effects of Cartilage-Derived Extracellular Matrix in a Rat Osteoarthritis Model. Tissue Eng Regen Med 20, 83–92 (2023). https://doi.org/10.1007/s13770-022-00508-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-022-00508-7