Abstract
Background:
Human umbilical cord blood-derived MSCs (hUCB-MSCs) have been studied in osteoarthritis (OA) and cartilage regeneration. Our previous study demonstrated that hUCB-MSCs combined with cartilage acellular matrix injection (CAM Inj.) represent potential therapeutic agents for structural improvement and anti-inflammatory effects in a rabbit model of OA.
Methods:
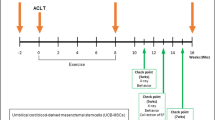
Based on a previous study, this study has evaluated the safety and efficacy of hUCB-MSCs combined with CAM Inj. in an anterior cruciate ligament transection (ACLT) with medial meniscectomy (MMx) in a goat model. In this study, 27 goats were divided into 5 groups: normal (n = 3), OA (n = 6), OA + CAM Inj. (n = 6), OA + hUCB-MSCs (n = 6), and OA + hUCB-MSCs + CAM Inj. (n = 6). Lameness and radiographic parameters were assessed 6 months after administration, and macroscopic and histological evaluations of the goat articular cartilage were performed 6 months after intervention.
Results:
The results showed significant improvement in lameness score only in the OA + hUCB-MSCs group at 5 months after treatment (*p < 0.05), whereas the K&L score showed significant improvement only in the OA + hUCB-MSCs + CAM Inj. group 6 months after intervention (*p < 0.05). In addition, the gross findings showed significance in OA + CAM Inj. and OA + hUCB-MSCs + CAM Inj. groups 6 months after treatment (*p < 0.05 and **p < 0.01).
Conclusion:
In conclusion, treatment with a combination of hUCB-MSCs and CAM Inj. reduced OA symptoms and induced effective cartilage tissue repair in a goat model. We suggest the combination of hUCB-MSCs and CAM Inj. as an alternative therapy for OA.





Similar content being viewed by others
References
Shane Anderson A, Loeser RF. Why is osteoarthritis an age-related disease? Best Pract Res Clin Rheumatol. 2010;24:15–26.
Valdes AM, Stocks J. Osteoarthritis and ageing. EMJ. 2018;3:116–23.
Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707.
Pelletier JP, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45:S22-7.
Cho SK, Kim H, Park HR, Choi W, Choi S, Jung SY, et al. Nonsteroidal anti-inflammatory drugs-sparing effect of symptomatic slow-acting drugs for osteoarthritis in knee osteoarthritis patients. Int J Rheum Dis. 2019;26:179–85.
Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328:869.
Shimizu M, Higuchi H, Takagishi K, Shinozaki T, Kobayashi T. Clinical and biochemical characteristics after intra-articular injection for the treatment of osteoarthritis of the knee: prospective randomized study of sodium hyaluronate and corticosteroid. J Orthop Sci. 2010;15:51–6.
Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014;5:351–61.
Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321.
Zhang W, Ouyang H, Dass CR, Xu J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040.
Grässel S, Muschter D. Recent advances in the treatment of osteoarthritis. F1000Res. 2020;9:F1000 Faculty Rev-325.
Le H, Xu W, Zhuang X, Chang F, Wang Y, Ding J. Mesenchymal stem cells for cartilage regeneration. J Tissue Eng. 2020;11:2041731420943839.
Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cell. 2019;8:886.
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–26.
Chanda D, Kumar S, Ponnazhagan S. Therapeutic potential of adult bone marrow-derived mesenchymal stem cells in diseases of the skeleton. J Cell Biochem. 2010;111:249–57.
Zhang R, Ma J, Han J, Zhang W, Ma J. Mesenchymal stem cell related therapies for cartilage lesions and osteoarthritis. Am J Transl Res. 2019;11:6275–89.
Huang YZ, Xie HQ, Silini A, Parolini O, Zhang Y, Deng L, et al. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: current status and future perspectives. Stem Cell Rev. 2017;13:575–86.
Park YB, Ha CW, Lee CH, Yoon YC, Park YG. Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl Med. 2017;6:613–21.
Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regen Ther. 2020;14:32–9.
Cui B, Li E, Yang B, Wang B. Human umbilical cord bloodderived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7:1233–6.
Pham PV, Vu NB, Pham VM, Truong NH, Pham TL, Dang LT, et al. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med. 2014;12:56.
Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells. 2010;2:34–8.
Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–32.
Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66–72.
Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Yin H, Wang Y, Sun Z, Sun X, Xu Y, Li P, et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109.
Jeon HJ, Yoon KA, An ES, Kang TW, Sim YB, Ahn J, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells combined with cartilage acellular matrix mediated via bone morphogenic protein 6 in a rabbit model of articular cruciate ligament transection. Stem Cell Rev Rep. 2020;16:596–611.
Cope P, Ourradi K, Li Y, Sharif M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis Cartilage. 2019;27:230–9.
Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–76.
Moran CJ, Ramesh A, Brama PA, O’Byrne JM, O’Brien FJ, Levingstone TJ. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. 2016;3:1.
Vandeweerd JM, Kirschvink N, Muylkens B, Depiereux E, Clegg P, Herteman N, et al. A study of the anatomy and injection techniques of the ovine stifle by positive contrast arthrography, computed tomography arthrography and gross anatomical dissection. Vet J. 2012;193:426–32.
Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19:493–9.
Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464–74.
Kazemi D, Shams Asenjan K, Dehdilani N, Parsa H. Canine articular cartilage regeneration using mesenchymal stem cells seeded on platelet rich fibrin: macroscopic and histological assessments. Bone Joint Res. 2017;6:98–107.
Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87:2671–86.
McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, et al. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552–61.
Levingstone TJ, Ramesh A, Brady RT, Brama PA, Kearney C, Gleeson JP, et al. Cell-free multi-layered collagen-based scaffolds demonstrate layer specific regeneration of functional osteochondral tissue in caprine joints. Biomaterials. 2016;87:69–81.
Xu X, Liang Y, Li X, Ouyang K, Wang M, Cao T, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2021;269:120539.
Xia H, Liang C, Luo P, Huang J, He J, Wang Z, et al. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res Ther. 2018;9:174.
Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–16.
Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, et al. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25:570–80.
Park YB, Song M, Lee CH, Kim JA, Ha CW. Cartilage repair by human umbilical cord blood-derived mesenchymal stem cells with different hydrogels in a rat model. J Orthop Res. 2015;33:1580–6.
Kilmer CE, Battistoni CM, Cox A, Breur GJ, Panitch A, Liu JC. Collagen type I and II blend hydrogel with autologous mesenchymal stem cells as a scaffold for articular cartilage defect repair. ACS Biomater Sci Eng. 2020;6:3464–76.
Burnsed OA, Schwartz Z, Marchand KO, Hyzy SL, Olivares-Navarrete R, Boyan BD. Hydrogels derived from cartilage matrices promote induction of human mesenchymal stem cell chondrogenic differentiation. Acta Biomater. 2016;43:139–49.
Rothrauff BB, Yang G, Tuan RS. Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:133.
Yun HW, Song BR, Shin DI, Yin XY, Truong MD, Noh S, et al. Fabrication of decellularized meniscus extracellular matrix according to inner cartilaginous, middle transitional, and outer fibrous zones result in zone-specific protein expression useful for precise replication of meniscus zones. Mater Sci Eng C Mater Biol Appl. 2021;128:112312.
Keane TJ, Swinehart IT, Badylak SF. Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods. 2015;84:25–34.
Zhong Y, Jiang A, Sun F, Xiao Y, Gu Y, Wu L, et al. A comparative study of the effects of different decellularization methods and genipin-cross-linking on the properties of tracheal matrices. Tissue Eng Regen Med. 2019;16:39–50.
Singh S, Afara IO, Tehrani AH, Oloyede A. Effect of decellularization on the load-bearing characteristics of articular cartilage matrix. Tissue Eng Regen Med. 2015;12:294–305.
Khajavi M, Hajimoradloo A, Zandi M, Pezeshki-Modaress M, Bonakdar S, Zamani A. Fish cartilage: a promising source of biomaterial for biological scaffold fabrication in cartilage tissue engineering. J Biomed Mater Res A. 2021;109:1737–50.
Acknowledgements
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: H I20C0233).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Authors declare there is no conflict of interest with the manuscript.
Ethics statement
Animal experiments were approved by the Experimental Animal Ethics Committee of KPC Co., Ltd. (approval no.: P18042).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13770_2021_407_MOESM2_ESM.tif
Supplementary Figure S2. Clinical analysis of OA symptoms in a goat model (Test 2). A: Main lameness score over the study period. B: Representative radiological images of knee joints of experimental goats at 12 months. C: Radiological score according to the K&L scale at initial, 3, 6, 9 and 12 months. D: Representative gross images of the femoral condyle. The black circle indicates the defects or repaired lesion. E: Comparison of the gross finding scores of the lesion in the affected articular cartilage. F: Microscopic lesions of the articular cartilage in the affected knees of all experimental groups (Scale bars = 1 mm). G: Comparison of the modified OARSI histopathologic scores in the affected articular cartilage. Results represent means ± SEM, *p < 0.05. OA: osteoarthritis, K&L: Kellgren & Lawrence, COL2: Type 2 collagen. (TIF 19941 kb)
13770_2021_407_MOESM3_ESM.tif
Supplementary Figure S3. Histological evaluation of the synthesis of cartilaginous ECM on hUCB-MSCs pellet. A: Protein expression levels of COL2 in CAM Inj.. B: COL2 staining of hUCB-MSCs pellets (Scale bars = 200 μm). ECM; extracellular matrix, hUCB-MSCs: Human Umbilical cord blood-mesenchymal stem cell, COL2: Type 2 collagen. (TIF 27699 kb)
Rights and permissions
About this article
Cite this article
Kim, M., Ahn, J., Lee, J. et al. Combined Mesenchymal Stem Cells and Cartilage Acellular Matrix Injection Therapy for Osteoarthritis in Goats. Tissue Eng Regen Med 19, 177–187 (2022). https://doi.org/10.1007/s13770-021-00407-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-021-00407-3