Abstract
Background:
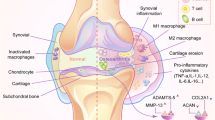
Extracellular vesicles (EVs) exhibit potential as functional biomolecules for tissue regeneration and immunomodulation as they play important roles in the physiological communication between cells. EV internal cargo contains miRNAs, proteins, lipids, and so on. Osteoarthritis (OA) is a common joint disease causing disability owing to impaired joint function and pain. EVs originating from animal cells and tissue matrices are also being considered for OA, in addition to research involving non-steroidal therapeutic agents. However, there are no studies on EVs from marine organisms. Hence, we focused on sea cucumber-derived EVs and conducted experiments to set up an extraction protocol and to demonstrate their efficacy to modulate the inflammatory environment.
Methods:
Sea cucumber extracellular matrices (SECMs) were prepared by a decellularization process. Lyophilized SECMs were treated with collagenase and filtered to isolate sea cucumber extracellular vesicles (SEVs). After isolation, we conducted physical characterization and cell activation studies including cytotoxicity, proliferation, and anti-inflammation effect assays.
Results:
The physical characterization results showed circular SEVs in the size range of 66–480 nm. These SEVs contained large amounts of protein cargo, infiltrated the synoviocyte membrane without damage, and had a suppressive effect on inflammatory cytokines.
Conclusion:
This study established an extraction process for EVs from sea cucumber and reported the anti-inflammatory ability of SEVs. Isolated SEVs can be further utilized for tissue regeneration studies and can be compared to various marine or animal-derived EVs.





Similar content being viewed by others
References
Ahmed AU. An overview of inflammation: mechanism and consequences. Front Biol. 2011;6:274.
Chen Y, Jiang W, Yong H, He M, Yang Y, Deng Z, et al. Macrophages in osteoarthritis: pathophysiology and therapeutics. Am J Transl Res. 2020;12:261–8.
Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42.
Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70:185–93.
Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214.
Toh WS, Lai RC, Hui JHP, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Zhang S, Chuah SJ, Lai RC, Hui JHP, Lim SK, Toh WS. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27.
Wang Y, Yu D, Liu Z, Zhou F, Dai J, Wu B, et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189.
Liu Y, Zou R, Wang Z, Wen C, Zhang F, Lin F. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475:3629–38.
Edgar JR. Q&A: What are exosomes, exactly? BMC Biol. 2016;14:46.
De Broe ME, Wieme RJ, Logghe GN, Roels F. Spontaneous shedding of plasma membrane fragments by human cells in vivo and in vitro. Clin Chim Acta. 1977;81:237–45.
Buzas EI, György B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–64.
Takahashi A, Okada R, Nagao K, Kawamata Y, Hanyu A, Yoshimoto S, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287.
Cho BS, Kim JO, Ha DH, Yi YW. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res Ther. 2018;9:187.
Lim W, Kim HS. Exosomes as therapeutic vehicles for cancer. Tissue Eng Regen Med. 2019;16:213–23.
Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–44.
Chen J, Chopp M. Exosome therapy for stroke. Stroke. 2018;49:1083–90.
Zhang ZG, Chopp M. Exosomes in stroke pathogenesis and therapy. J Clin Invest. 2016;126:1190–7.
Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells. 2017;35:851–8.
Hussey GS, Keane TJ, Badylak SF. The extracellular matrix of the gastrointestinal tract: a regenerative medicine platform. Nat Rev Gastroenterol Hepatol. 2017;14:540–52.
Kim S, Kim BS. Control of adult stem cell behavior with biomaterials. Tissue Eng Regen Med. 2014;11:423–30.
Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83.
Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12:367–77.
Park DY, Yun HW, Lim S, Truong MD, Yin XY, Park J, et al. Cross-linked cartilage acellular matrix film decreases postsurgical peritendinous adhesions. Artif Organs. 2020;44:E136–49.
Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, et al. Matrix-bound nanovesicles recapitulate extracellular matrix effects on macrophage phenotype. Tissue Eng Part A. 2017;23:1283–94.
Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, et al. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2:e1600502.
van der Merwe Y, Faust AE, Sakalli ET, Westrick CC, Hussey G, Chan KC, et al. Matrix-bound nanovesicles prevent ischemia-induced retinal ganglion cell axon degeneration and death and preserve visual function. Sci Rep. 2019;9:1–15.
An M, Kwon K, Park J, Ryu DR, Shin JA, Lee Kang J, et al. Extracellular matrix-derived extracellular vesicles promote cardiomyocyte growth and electrical activity in engineered cardiac atria. Biomaterials. 2017;146:49–59.
Park SY, Lim HK, Lee S, Hwang HC, Cho SK, Cho M. Pepsin-solubilised collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCaT cell migration. Food Chem. 2012;132:487–92.
Silva TH, Moreira-Silva J, Marques AL, Domingues A, Bayon Y, Reis RL. Marine origin collagens and its potential applications. Mar Drugs. 2014;12:5881–901.
Binnewerg B, Schubert M, Voronkina A, Muzychka L, Wysokowski M, Petrenko I, et al. Marine biomaterials: biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater Sci Eng C Mater Biol Appl. 2020;109:110566.
Kim SK, Ngo DH, Vo TS, Ryu B. Industry perspectives of marine-derived proteins as biomaterials. In: Kim SK, editor. Marine biomaterials: characterization, isolation and applications. Boca Raton, FL, USA: CRC Press; 2013.
Ohta N, Sato M, Ushida K, Kokubo M, Baba T, Taniguchi K, et al. Jellyfish mucin may have potential disease-modifying effects on osteoarthritis. BMC Biotechnol. 2009;9:98.
Gomes AR, Freitas AC, Duarte AC, Rocha-Santos TA. Echinoderms: a review of bioactive compounds with potential health effects. Amsterdam: Elsevier; 2016. p. 1–54.
Carnevali MC. Regeneration in echinoderms: repair, regrowth, cloning. Invertebrate Surviv J. 2006;3:64–76.
San Miguel-Ruiz JE, García-Arrarás JE. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev Biol. 2007;7:115.
Sun L, Chen M, Yang H, Wang T, Liu B, Shu C, et al. Large scale gene expression profiling during intestine and body wall regeneration in the sea cucumber Apostichopus japonicus. Comp Biochem Physiol Part D Genomics Proteomics. 2011;6:195–205.
García-Arrarás JE, Dolmatov IY. Echinoderms: potential model systems for studies on muscle regeneration. Curr Pharm Des. 2010;16:942–55.
Smith GN Jr. Regeneration in the sea cucumber Leptosynapta. II. The regenerative capacity. J Exp Zool. 1971;177:331–42.
Siahaan EA, Pangestuti R, Munandar H, Kim SK. Cosmeceuticals properties of sea cucumbers: prospects and trends. Cosmetics. 2017;4:26.
Kijjoa A, Sawangwong P. Drugs and cosmetics from the sea. Mar Drugs. 2004;2:73–82.
Li X, Roginsky AB, Ding XZ, Woodward C, Collin P, Newman RA, et al. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, frondoside a. Ann N Y Acad Sci. 2008;1138:181–98.
Oh GW, Ko SC, Lee DH, Heo SJ, Jung WK. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. J Fish Aquat Sci. 2017;20:28.
Janakiram NB, Mohammed A, Rao CV. Sea cucumbers metabolites as potent anti-cancer agents. Mar Drugs. 2015;13:2909–23.
Kim JL, Park SH, Jeong S, Kim BR, Na YJ, Jo MJ, et al. Sea cucumber (Stichopus japonicas) F2 enhanced TRAIL-induced apoptosis via XIAP ubiquitination and ER stress in colorectal cancer cells. Nutrients. 2019;11:1061.
Himaya SW, Ryu B, Qian ZJ, Kim SK. Sea cucumber, Stichopus japonicus ethyl acetate fraction modulates the lipopolysaccharide induced iNOS and COX-2 via MAPK signaling pathway in murine macrophages. Environ Toxicol Pharmacol. 2010;30:68–75.
Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science. 2014;343:183–6.
Soler N, Krupovic M, Marguet E, Forterre P. Membrane vesicles in natural environments: a major challenge in viral ecology. ISME J. 2015;9:793–6.
Toyofuku M, Morinaga K, Hashimoto Y, Uhl J, Shimamura H, Inaba H, et al. Membrane vesicle-mediated bacterial communication. ISME J. 2017;11:1504–9.
Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. N Engl J Med. 2018;379:958–66.
Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39:240–55.
Zhang P, Li C, Zhang R, Zhang W, Jin C, Wang L, et al. The roles of two miRNAs in regulating the immune response of sea cucumber. Genetics. 2015;201:1397–410.
Zhan Y, Liu L, Zhao T, Sun J, Cui D, Li Y, et al. MicroRNAs involved in innate immunity regulation in the sea cucumber: a review. Fish Shellfish Immunol. 2019;95:297–304.
Acknowledgement
This research was supported by the National Research Foundation Grant (NRF- 2019M3E5D1A02070861.)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, SH., Kim, C. & Park, SH. Novel Marine Organism-Derived Extracellular Vesicles for Control of Anti-Inflammation. Tissue Eng Regen Med 18, 71–79 (2021). https://doi.org/10.1007/s13770-020-00319-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00319-8