Abstract
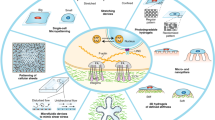
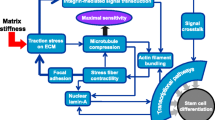
Tissue-resident stem cells are surrounded by a microenvironment known as ‘stem cell niche’ which is specific for each stem cell type. This niche comprises of cell-intrinsic and -extrinsic factors like biochemical and biophysical signals, which regulate stem cell characteristics and differentiation. Biochemical signals have been thoroughly studied however, the effect of biophysical signals on stem cell regulation is yet to be completely understood. Biomaterials have aided in addressing this issue since they can provide a defined and tuneable microenvironment resembling in vivo conditions. We review various biomaterials used in many studies which have shown a connection between biomaterial-generated mechanical signals and alteration in stem cell behaviour. Researchers probed to understand the mechanism of mechanotransduction and reported that the signals from the extracellular matrix regulate a transcription factor yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ), which is a downstream-regulator of the Hippo pathway and it transduces the mechanical signals inside the nucleus. We highlight the role of the YAP/TAZ as mechanotransducers in stem cell self-renewal and differentiation in response to substrate stiffness, also the possibility of mechanobiology as the emerging field of regenerative medicines and three-dimensional tissue printing.



Similar content being viewed by others
References
Ranga A, Gjorevski N, Lutolf M. Drug discovery through stem cell-based organoid models. Adv Drug Deliv Rev. 2014;69:19–28.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–97.
Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753–9.
Steinbeck JA, Studer L. Moving stem cells to the clinic: potential and limitations for brain repair. Neuron. 2015;86:187–206.
Caglayan S, Ahrens TD, Cieślar-Pobuda A, Staerk J. Modern ways of obtaining stem cells. In: Łos MJ, Hudecki A, Wiecheć E, editors. Stem cells and biomaterials for regenerative medicine. Cambridge: Academic Press; 2019. p. 17–36.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Yamanaka S. Patient-specific pluripotent stem cells become even more accessible. Cell Stem Cell. 2010;7:1–2.
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55.
Zhang J, Li L. BMP signaling and stem cell regulation. Dev Biol. 2005;284:1–11.
Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–509.
Kaverina I, Krylyshkina O, Beningo K, Anderson K, Wang YL, Small JV. Tensile stress stimulates microtubule outgrowth in living cells. J Cell Sci. 2002;115:2283–91.
Vining KH, Mooney DJ. Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol. 2017;18:728–42.
Eyckmans J, Boudou T, Yu X, Chen CS. A hitchhiker’s guide to mechanobiology. Dev Cell. 2011;21:35–47.
Pelham RJ, Wang YL. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–5.
Huch M, Knoblich JA, Lutolf M, Martinez-Arias A. The hope and the hype of organoid research. Development. 2017;144:938–41.
Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6.
Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71.
Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9.
Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–21.
Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–15.
Huch M, Dorrell C, Boj SF, Van Es JH, Li VS, Van De Wetering M, et al. In vitro expansion of single Lgr5 + liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–50.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Múnera JO, Wells JM. Generation of gastrointestinal organoids from human pluripotent stem cells. Organ Regen. 2017;1597:167–77.
Wimmer RA, Leopoldi A, Aichinger M, Kerjaschki D, Penninger JM. Generation of blood vessel organoids from human pluripotent stem cells. Nat Prot. 2019;14:3082–100.
Yue B. Biology of the extracellular matrix: an overview. J Glaucoma. 2014;23 Suppl 1:S20–3.
Cancedda R. Cartilage and bone extracellular matrix. Curr Pharma Design. 2009;15:1334–48.
Ruoslahti E. Brain extracellular matrix. Glycobiology. 1996;6:489–92.
Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–400.
Budday S, Nay R, de Rooij R, Steinmann P, Wyrobek T, Ovaert TC, et al. Mechanical properties of gray and white matter brain tissue by indentation. J Mech Behav Biomed. 2015;46:318–30.
Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophy J. 2000;79:144–52.
Toh WS, Lim TC, Kurisawa M, Spector M. Modulation of mesenchymal stem cell chondrogenesis in a tuneable hyaluronic acid hydrogel microenvironment. Biomaterials. 2012;33:3835–45.
Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89.
García A, Reyes CD. Bio-adhesive surfaces to promote osteoblast differentiation and bone formation. J Dent Res. 2005;84:407–13.
Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411-5.
Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res. 2001;49:647–58.
Engler AJ, Richert L, Wong JY, Picart C, Discher DE. Surface probe measurements of the elasticity of sectioned tissue, thin gels and polyelectrolyte multilayer films: correlations between substrate stiffness and cell adhesion. Surface Sci. 2004;570:142–54.
Gilbert M, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81.
Kolahi KS, Donjacour A, Liu X, Lin W, Simbulan RK, Bloise E, et al. Effect of substrate stiffness on early mouse embryo development. PLoS One. 2012;7:e41717.
Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput. 2000;38:211–8.
Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of α-smooth muscle actin to stress fibres. J Cell Biol. 2006;172:259–68.
Muduli S, Chen LH, Li M, Heish ZW, Liu CH, Kumar S, et al. Stem cell culture on polyvinyl alcohol hydrogels having different elasticity and immobilized with ECM-derived oligopeptides. J Polym Eng. 2017;37:647–60.
Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26.
Théry M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci. 2010;123:4201–13.
Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600.
Maldonado M, Wong LY, Echeverria C, Ico G, Low K, Fujimoto T, et al. The effects of electrospun substrate-mediated cell colony morphology on the self-renewal of human induced pluripotent stem cells. Biomaterials. 2015;50:10–9.
Abbas Y, Carnicer-Lombarte A, Gardner L, Thomas J, Brosens JJ, Moffett A, et al. Tissue stiffness at the human maternal–fetal interface. Hum Reprod. 2019;34:1999–2008.
Braam SR, Zeinstra L, Litjens S, Ward-van Oostwaard D, van den Brink S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via αVβ5 integrin. Stem Cells. 2008;26:2257–65.
Musah S, Morin SA, Wrighton J, Zwick DB, Jin S, Kiessling LL. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano. 2012;6:10168–77.
Musah S, Wrighton J, Zaltsman Y, Zhong X, Zorn S, Parlato MB, et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc Natl Acad Sci U S A. 2014;111:13805–10.
Chen YF, Li Y, Chou CH, Chiew MY, Huang HD, Ho JHC, et al. Control of matrix stiffness promotes endodermal lineage specification by regulating SMAD2/3 via lncRNA LINC00458. Sci Adv. 2020;6:eaay0264.
Chuah YJ, Koh YT, Lim K, Menon NV, Wu Y, Kang Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci Rep. 2015;5:18162.
Evans ND, Minelli C, Gentleman E, LaPointe V, Patankar SN, Kallivretaki M, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cells Mater. 2009;18:1–13.
Palchesko RN, Zhang L, Sun Y, Feinberg AW. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One. 2012;7:e51499.
Yeh YC, Corbin EA, Caliari SR, Ouyang L, Vega SL, Truitt R, et al. Mechanically dynamic PDMS substrates to investigate changing cell environments. Biomaterials. 2017;145:23–32.
Maldonado M, Ico G, Low K, Luu RJ, Nam J. Enhanced lineage-specific differentiation efficiency of human induced pluripotent stem cells by engineering colony dimensionality using electrospun scaffolds. Adv Healthc Mater. 2016;5:1408–12.
Maldonado M, Luu RJ, Ico G, Ospina A, Myung D, Shih H, et al. Lineage-and developmental stage-specific mechanomodulation of induced pluripotent stem cell differentiation. Stem Cell Res Ther. 2017;8:216.
Wu Y, Xiang Y, Fang J, Li X, Lin Z, Dai G, et al. The influence of the stiffness of GelMA substrate on the outgrowth of PC12 cells. Biosci Rep. 2019;39:BSR20181748.
Celikkin N, Mastrogiacomo S, Jaroszewicz J, Walboomers XF, Swieszkowski W. Gelatin methacrylate scaffold for bone tissue engineering: the influence of polymer concentration. J Biomed Mater Res A. 2018;106:201–9.
Peter M, Singh A, Mohankumar K, Jeenger R, Joge A, Gatne MM, et al. Gelatin-based matrices as a tunable platform to study in vitro and in vivo 3D cell invasion. ACS Appl Bio Mater. 2019;2:916–29.
Singh A, Tayalia P. Three-dimensional cryogel matrix for spheroid formation and anti-cancer drug screening. J Biomed Mater Res A. 2020;108:365–76.
Kang H, Shih YRV, Hwang Y, Wen C, Rao V, Seo T, et al. Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta Biomater. 2014;10:4961–70.
Llames S, García-Pérez E, Meana Á, Larcher F, del Río M. Feeder layer cell actions and applications. Tissue Eng Part B Rev. 2015;21:345–53.
Puck TT, Marcus I. A rapid method for viable cell titration and clone production with HeLa cells in tissue culture: the use of X-irradiated cells to supply conditioning factors. Proc Natl Acad Sci U S A. 1955;41:432–7.
Rodin S, Antonsson L, Hovatta O, Tryggvason K. Monolayer culturing and cloning of human pluripotent stem cells on laminin-521–based matrices under xeno-free and chemically defined conditions. Nat Protoc. 2014;9:2354–68.
Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One. 2015;10:e0145068.
Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer-and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–45.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, et al. Chemically defined conditions for human iPSC derivation and culture. Nat Methods. 2011;8:424–9.
Philp D, Chen SS, Fitzgerald W, Orenstein J, Margolis L, Kleinman HK. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288–96.
Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86.
Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–90.
Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen–hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6:3957–68.
Pawelec KM, Best SM, Cameron RE. Collagen: a network for regenerative medicine. J Mater Chem B. 2016;4:6484–96.
Chen C, Zhao ML, Zhang RK, Lu G, Zhao CY, Fu F, et al. Collagen/heparin sulfate scaffolds fabricated by a 3D bioprinter improved mechanical properties and neurological function after spinal cord injury in rats. J Biomed Mater Res A. 2017;105:1324–32.
Khatami N, Khoshfetrat AB, Khaksar M, Zamani ARN, Rahbarghazi R. Collagen-alginate-nano-silica microspheres improved the osteogenic potential of human osteoblast-like MG-63 cells. J Cell Biochem. 2019;120:15069–82.
Hughes CS, Radan L, Betts D, Postovit LM, Lajoie GA. Proteomic analysis of extracellular matrices used in stem cell culture. Proteomics. 2011;11:3983–91.
Baxter MA, Camarasa MV, Bates N. Analysis of the distinct functions of growth factors and tissue culture substrates necessary for the long-term self-renewal of human embryonic stem cell lines. Stem Cell Res. 2009;3:28–38.
Jacobsen MM, Li D, Rim NG, Backman D, Smith ML, Wong JY. Silk-fibronectin protein alloy fibres support cell adhesion and viability as a high strength, matrix fibre analogue. Sci Rep. 2017;7:45653.
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4.
Kohen NT, Little LE, Healy KE. Characterization of Matrigel interfaces during defined human embryonic stem cell culture. Biointerphases. 2009;4:69–79.
Semler EJ, Ranucci CS, Moghe V. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol Bioeng. 2000;69:359–69.
Soofi SS, Last JA, Liliensiek SJ, Nealey F, Murphy CJ. The elastic modulus of Matrigel™ as determined by atomic force microscopy. J Struct Biol. 2009;167:216–9.
Takahashi Y, Sato S, Kurashima Y, Yamamoto T, Kurokawa S, Yuki Y, et al. A refined culture system for human induced pluripotent stem cell-derived intestinal epithelial organoids. Stem Cell Rep. 2018;10:314–28.
Khaing ZZ, Seidlits SK. Hyaluronic acid and neural stem cells: implications for biomaterial design. J Mater Chem B. 2015;3:7850–66.
Bierman R. Hyaluronic acid-based hydrogels as an in vitro approach to neural stem/progenitor cell differentiation [Doctoral dissertation]. Los Angeles: University of California, Los Angeles; 2018.
Wang HB, Dembo M, Wang YL. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol. 2000;279:C1345–50.
Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir. 2003;19:1908–13.
Sachot N, Engel E, Castaño O. Hybrid organic-inorganic scaffolding biomaterials for regenerative therapies. Curr Org Chem. 2014;18:2299–314.
Brown XQ, Ookawa K, Wong JY. Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials. 2005;26:3123–9.
Jun I, Han HS, Edwards J, Jeon H. Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci. 2018;19:745.
Berkovitch Y, Seliktar D. Semi-synthetic hydrogel composition and stiffness regulate neuronal morphogenesis. Int J Pharm. 2017;523:545–55.
Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromol. 2000;1:31–8.
Wei D, Xiao W, Sun J, Zhong M, Guo L, Fan H, et al. A biocompatible hydrogel with improved stiffness and hydrophilicity for modular tissue engineering assembly. J Mater ChemB. 2015;3:2753–63.
Costantini M, Testa S, Fornetti E, Barbetta A, Trombetta M, Cannata SM, et al. Engineering muscle networks in 3D gelatin methacryloyl hydrogels: influence of mechanical stiffness and geometrical confinement. Front Bioeng Biotechnol. 2017;5:22.
Pepelanova I, Kruppa K, Scheper T, Lavrentieva A. Gelatin-Methacryloyl (GelMA) hydrogels with defined degree of functionalization as a versatile toolkit for 3D cell culture and extrusion bioprinting. Bioengineering (Basel). 2018;5:55.
Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, et al. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv Func Mater. 2016;26:2809–19.
Eke G, Mangir N, Hasirci N, MacNeil S, Hasirci V. Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials. 2017;129:188–98.
Zhang X, Li J, Ye P, Gao G, Hubbell K, Cui X. Coculture of mesenchymal stem cells and endothelial cells enhances host tissue integration and epidermis maturation through AKT activation in gelatin methacryloyl hydrogel-based skin model. Acta Biomater. 2017;59:317–26.
Kerscher P, Kaczmarek JA, Head SE, Ellis ME, Seeto WJ, Kim J, et al. Direct production of human cardiac tissues by pluripotent stem cell encapsulation in gelatin methacryloyl. ACS Biomater Sci Eng. 2016;3:1499–509.
Visser J, Gawlitta D, Benders KE, Toma SM, Pouran B, van Weeren R, et al. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials. 2015;37:174–82.
Ebrahimi M, Ostrovidov S, Salehi S, Kim SB, Bae H, Khademhosseini A. Enhanced skeletal muscle formation on microfluidic spun gelatin methacryloyl (GelMA) fibres using surface patterning and agrin treatment. J Tissue Eng Regen Med. 2018;12:2151–63.
Lin RZ, Chen YC, Moreno-Luna R, Khademhosseini A, Melero-Martin JM. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:6785–96.
Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: a key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–54.
Donato DM, Ryzhova LM, Meenderink LM, Kaverina I, Hanks SK. Dynamics and mechanism of p130Cas localization to focal adhesions. J Biol Chem. 2010;285:20769–79.
Ciobanasu C, Faivre B, Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol. 2013;92:339–48.
Janoštiak R, Pataki AC, Brábek J, Rösel D. Mechanosensors in integrin signaling: the emerging role of p130Cas. Eur J Cell Biol. 2014;93:445–54.
Elosegui-Artola A, Andreu I, Beedle AE, Lezamiz A, Uroz M, Kosmalska AJ, et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell. 2017;171:1397–410.
Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–62.
Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–12.
Gumbiner B. Signal transduction by B-catenin. Curr Opin Cell Biol. 1995;7:634–40.
Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R. Nuclear localization of b-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10.
Spadaro D, Le S, Laroche T, Mean I, Jond L, Yan J, et al. Tension-dependent stretching activates ZO-1 to control the junctional localization of its interactors. Curr Biol. 2017;27:3783–95.
Lewis JM, Baskaran R, Taagepera S, Schwartz MA, Wang JY. Integrin regulation of c-Abl tyrosine kinase activity and cytoplasmic-nuclear transport. Proc Natl Acad Sci U S A. 1996;93:15174–9.
Speight P, Kofler M, Szászi K, Kapus A. Context-dependent switch in chemo/mechanotransduction via multilevel crosstalk among cytoskeleton-regulated MRTF and TAZ and TGFβ-regulated Smad3. Nat Commun. 2016;7:1–17.
Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-kappaB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. The FASEB J. 2003;17:386–96.
Escoll M, Lastra D, Pajares M, Robledinos-Antón N, Rojo AI, Fernández-Ginés R, et al. Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol. 2020;30:101425.
Li Y, Chu JS, Kurpinski K, Li X, Bautista DM, Yang L, et al. Biophysical regulation of histone acetylation in mesenchymal stem cells. Biophys J. 2011;100:1902–9.
Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, et al. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–81.
Driscoll T, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J. 2015;108:2783–93.
Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, et al. Characterization of the mammalian YAP (Yes-associated protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem. 1995;270:14733–41.
Kanai F, Marignani A, Sarbassova D, Yagi R, Hall RA, Donowitz M, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. The EMBO J. 2000;19:6778–91.
Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–93.
Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–70.
Hansen CG, Moroishi T, Guan KL. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 2015;25:499–513.
Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63.
Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–70.
Shao Y, Sang J, Fu J. On human pluripotent stem cell control: the rise of 3D bioengineering and mechanobiology. Biomaterials. 2015;52:26–43.
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, et al. YAP/TAZ are mechanoregulators of TGF-β-Smad signaling and renal fibrogenesis. J Am Soc Nephrol. 2016;27:3117–28.
Kim MK, Jang JW, Bae SC. DNA binding partners of YAP/TAZ. BMB Rep. 2018;51:126–33.
Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates Cell proliferation apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34.
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61.
Zhang X, Milton CC, Humbert O, Harvey KF. Transcriptional output of the Salvador/warts/hippo pathway is controlled in distinct fashions in Drosophila melanogaster and mammalian cell lines. Cancer Res. 2009;69:6033–41.
Liu C, Huang W, Lei Q. Regulation and function of the TAZ transcription co-activator. Int J Biochem Mol Bio. 2011;2:247.
Sun C, De Mello V, Mohamed A, Ortuste Quiroga HP, Garcia-Munoz A, Al Bloshi A, et al. Common and distinctive functions of the hippo effectors Taz and Yap in Skeletal Muscle stem cell function. Stem Cells. 2017;35:1958–72.
Plouffe SW, Lin KC, Moore JL 3rd, Tan FE, Ma S, Ye Z, et al. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J Biol Chem. 2018;293:11230–40.
Finch-Edmondson ML, Strauss R, Passman AM, Sudol M, Yeoh GC, Callus BA. TAZ protein accumulation is negatively regulated by YAP abundance in mammalian cells. J Biol Chem. 2015;290:27928–38.
Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18.
Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, et al. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87.
Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, et al. TAZ promotes PC2 degradation through a SCFβ-Trcp E3 ligase complex. Mol Cell Biol. 2007;27:6383–95.
Hossain Z, Ali SM, Ko HL, Xu J, Ng C, Guo K, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A. 2007;104:1631–6.
Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol-Renal. 2008;294:F542–53.
Brenan L. Elucidating the role of YAP in directing mesenchymal stem cell fate. Master of Arts thesis, Boston University. 2015.
Nie P, Li Y, Suo H, Jiang N, Yu D, Fang B. Dasatinib promotes chondrogenic differentiation of human mesenchymal stem cells via the Src/Hippo-YAP signaling pathway. ACS Biomater Sci Eng. 2019;5:5255–65.
Qin H, Hejna M, Liu Y, Percharde M, Wossidlo M, Blouin L, et al. YAP induces human naive pluripotency. Cell Rep. 2016;14:2301–12.
Hsu HT, Estarás C, Huang L, Jones KA. Specifying the anterior primitive streak by modulating YAP1 levels in human pluripotent stem cells. Stem cell Rep. 2018;11:1357–64.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803.
Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10.
Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67.
Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60.
Debaugnies M, Sánchez-Danés A, Rorive S, Raphaël M, Liagre M, Parent MA, et al. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 2018;19:e45809.
Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83.
Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signalling and beyond. Physiol Rev. 2014;94:1287–312.
Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016;18:540–8.
Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–59.
Smith Q, Chan XY, Carmo AM, Trempel M, Saunders M, Gerecht S. Compliant substratum guides endothelial commitment from human pluripotent stem cells. Sci Adv. 2017;3:e1602883.
Price AJ, Huang EY, Sebastiano V, Dunn AR. A semi-interpenetrating network of polyacrylamide and recombinant basement membrane allows pluripotent cell culture in a soft, ligand-rich microenvironment. Biomaterials. 2017;121:179–92.
Wang HB, Dembo M, Hanks SK, Wang YL. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98:11295–300.
Hadden WJ, Young JL, Holle AW, McFetridge ML, Kim DY, Wijesinghe P, et al. Stem cell migration and mechanotransduction on linear stiffness gradient hydrogels. Proc Natl Acad Sci U S A. 2017;114:5647–52.
Lachowski D, Cortes E, Robinson B, Rice A, Rombouts K, Del Río Hernández AE. FAK controls the mechanical activation of YAP, a transcriptional regulator required for durotaxis. The FASEB J. 2017;32:1099–107.
Ong CS, Yesantharao P, Huang CY, Mattson G, Boktor J, Fukunishi T, et al. 3D bioprinting using stem cells. Pediatr Res. 2018;83:223–31.
Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, Alsberg E. Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries. Mater Horiz. 2019;6:1625–31.
Gershovich JG, Buravkova LB. Morphofunctional status and osteogenic differentiation potential of human mesenchymal stromal precursor cells during in vitro modeling of microgravity effects. Bull Exp Biol Med. 2007;144:608–13.
Baio J, Martinez AF, Silva I, Hoehn CV, Countryman S, Bailey L, et al. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. NPJ Microgravity. 2018;4:13.
Acknowledgements
We would to acknowledge Sunandan Divatia School of Science, NMIMS (deemed to-be University) and Symbiosis Centre for Stem Cell Research (SCSCR), Symbiosis International University, for providing the necessary resources and infrastructure. The work was funded by Department of Biotechnology (DBT), Govt of India, sanction order BT/PR28474/MED/31/393/2018. JKV was provided Senior Research Fellowship by Council of Scientific and Industrial Research (CSIR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Virdi, J.K., Pethe, P. Biomaterials Regulate Mechanosensors YAP/TAZ in Stem Cell Growth and Differentiation. Tissue Eng Regen Med 18, 199–215 (2021). https://doi.org/10.1007/s13770-020-00301-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-020-00301-4