Abstract
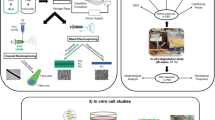
We investigated the use of Polycaprolactone (PCL)/ β-tricalcium phosphate (β-TCP) composites with applied mechanical stimulation as scaffold for bone tissue engineering. PCL-based three-dimensional (3D) structures were fabricated in a solvent-free process using a 3D-printing technique. The mass fraction of β-TCP was varied in the range 0–30%, and the structure and compressive modulus of the specimens was characterized. The shape and interconnectivity of the pores was found to be satisfactory, and the compressive modulus of the specimens was comparable with that of human trabecular bone. Human mesenchymal stem cells were seeded on the composites, and various biological evaluations were performed over 9 days. With a mass fraction of β-TCP of 30%, differentiation began earlier; however, the cell proliferation rate was lower. Through the use of mechanical stimulation, however, the proliferation rate recovered, and was comparable with that of the other groups. This stimulation effect was also observed in ECM generation and other biological assays. With mechanical stimulation, expression of osteogenic markers was lower on samples with a β-TCP content of 10 wt% than without β-TCP; however, with mechanical stimulation, the sample with a β-TCP content of 30 wt% exhibited significantly greater expression of those markers than the other samples. We found that mechanical stimulation and the addition of β-TCP interacted closely, and that a mass fraction of β-TCP of 30% was particularly useful as a bone tissue scaffold when accompanied by mechanical stimulation.








Similar content being viewed by others
References
O’Brien FJ. Biomaterials and scaffolds for tissue engineering. Materialstoday. 2011;14:88–95.
Park SA, Lee JB, Kim YE, Kim JE, Lee JH, Shin J, et al. Fabrication of biomimetic PCL scaffold using rapid prototyping for bone tissue engineering. Macromol Res. 2014;22:882–7.
Liao H, Chen J, Lee M. Bone tissue engineering with adipose-derived stem cells in bioactive composites of laser-sintered porous polycaprolactone scaffolds and platelet-rich plasma. Materials. 2013;6:4911–29.
Su J, Chen L, Li L. Characterization of polycaprolactone and starch blends for potential application within the biomaterials field. Afr J Biotechnol. 2012;11:694–701.
Aunoble S, Clément D, Frayssinet P, Harmand MF, Le Huec JC. Biological performance of a new beta–TCP/PLLA composite material for applications in spine surgery: in vitro and in vivo studies. J Biomed Mater Res A. 2006;78:416–22.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–31.
Gao C, Deng Y, Feng P, Mao Z, Li P, Yang B, et al. Current progress in bioactive ceramic scaffolds for bone repair and regeneration. Int J Mol Sci. 2014;15:4714–32.
Tripathi G, Basu B. A porous hydroxyapatite scaffold for bone tissue engineering: physico-mechanical and biological evaluations. Ceram Int. 2012;38:341–9.
Yeo MG, Kim GH. Preparation and characterization of 3D composite Scaffolds based on rapid-prototyped PCL/β-TCP struts and electrospun PCL coated with collagen and HA for bone regeneration. Chem Mater. 2012;24:903–13.
Lu L, Zhang Q, Wootton D, Chiou R, Li D, Lu B, et al. Biocompatibility and biodegradation studies of PCL/β-TCP bone tissue scaffold fabricated by structural porogen method. J Mater Sci Mater Med. 2012;23:2217–26.
Cao H, Kuboyama N. biodegradable porous composite scaffold of PGA/β-TCP for bone tissue engineering. Bone. 2010;46:386–95.
Liu F. Synthesis of bioceramic scaffolds for bone tissue engineering by rapid prototyping technique. Key Eng Mater. 2012;64:704–10.
Yamachika E, Iidab S. Bone regeneration from mesenchymal stem cells (MSCs) and compact bone-derived MSCs as an animal model. Jpn Dent Sci Rev. 2013;49:35–44.
Huang C, Ogawa R. Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Eng Part A. 2012;18:2106–13.
Hess R, Douglas T, Myers KA, Rentsch B, Rentsch C, Worch H, et al. Hydrostatic pressure stimulation of human mesenchymal stem cells seeded on collagen-based artificial extracellular matrices. J Biomech Eng. 2010;132:021001.
Zhao Y, Lv X, Liu Y, Zhao Y, Li Q, Chen Y, et al. Hydrostatic pressure promotes the proliferation and osteogenic/chondrogenic differentiation of mesenchymal stem cells: the roles of RhoA and Rac1. Stem Cell Res. 2015;14:283–96.
Liu J, Zhao Z, Li J, Zou L, Shuler C, Zou Y, et al. Hydrostatic pressures promote initial osteodifferentiation with ERK1/2 Not p38 MAPK signaling involved. J Cell Biochem. 2009;107:224–32.
Jeong J, Park S, Shin J, Kang Y, Han K, Shin J. Effects of intermittent hydrostatic pressure magnitude on the chondrogenesis of MSCs without biochemical agents under 3D co-culture. J Mater Sci Mater Med. 2012;23:2773–81.
Sharaf B, Faris CB, Abukawa H, Susarla SM, Vacanti JP, Kaban LB, et al. Three-dimensionally printed polycaprolactone and β–Tricalcium phosphate scaffolds for bone tissue engineering: an in vitro study. J Oral Maxillofac Surg. 2012;70:647–56.
Jayabalan M, Shalumon KT, Mitha MK. Injectable biomaterials for minimally invasive orthopedic treatments. J Mater Sci Mater Med. 2009;20:1379–87.
Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6:605–17.
Erisken C, Kalyon DM, Wang H. Functionally graded electrospun polycaprolactone and b-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials. 2008;29:4065–73.
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420–30.
Nishimura I, Hisanaga R, Sato T, Arano T, Nomoto S, Ikada Y, et al. Effect of osteogenic differentiation medium on proliferation and differentiation of human mesenchymal stem cells in three-dimensional culture with radial flow bioreactor. Regen Ther. 2015;2:24–31.
Zhang D, Weinbaum S, Cowin SC. Estimates of the peak pressures in bone pore water. J Biomech Eng. 1998;120:697–703.
Kim YJ, Park S, Lee YJ, Shin JW, Kim DH, Heo SJ, et al. Effects of intermittent hydrostatic pressure on cell adhesive forces and other related parameters under various resting periods. J Biomed Mater Res B Appl Biomater. 2008;85:353–60.
Shim JH, Huh JB, Park JY, Jeon YC, Kang SS, Kim JY, et al. Fabrication of blended polycaprolactone/poly (lactic-co-glycolic acid)/β-tricalcium phosphate thin membrane using solid freeform fabrication technology for guided bone regeneration. Tissue Eng Part A. 2013;19:317–28.
Acknowledgements
This work was supported by the grants of the Korea Health Technology R&D Project through the KHIDI (HI16C0362, the Ministry of Health & Welfare, ROK) and the National Research Foundation of Korea (NRF) Grant (NRF-2014K2A2A7066637).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no financial conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Rights and permissions
About this article
Cite this article
Park, S.H., Park, S.A., Kang, Y.G. et al. PCL/β-TCP Composite Scaffolds Exhibit Positive Osteogenic Differentiation with Mechanical Stimulation. Tissue Eng Regen Med 14, 349–358 (2017). https://doi.org/10.1007/s13770-017-0022-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0022-9