Abstract
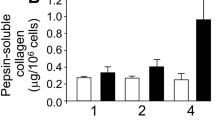
Three-dimensional (3D) cultures are known to promote cell differentiation. Previously, we investigated the differentiation of rat dermal fibroblasts to α-smooth muscle actin (α-SMA)-positive myofibroblasts through transforming growth factor (TGF)-ß production using a 3D culture model. Here, we investigated the phenotypic change from dermal mesenchymal cells (mostly fibroblasts) to osteoblast-like cells, being inspired by the roles of smooth muscle cells or fibroblasts during vascular calcification. Spindle-shaped cells that grew in heterologous populations out of dermal explants from 2-day-old Wistar rats were cultured within a collagen matrix. α-SMA and alkaline phosphatase (ALP) meßsenger RNA (mRNA) levels initially increased, followed by a rise in Runx2 and osteocalcin (OCN) mRNA levels without calcification. Calcium deposits were produced in the presence of a high concentration of inorganic phosphate (2.1 mM) or ß-glycerophosphate (ßGP, 10 mM) after 2 weeks of culture, and both were sensitive to an inhibitor of type III phosphate transporters. An ALP inhibitor decreased only ßGP-induced calcification. Inhibition of TGF-ß type-I receptors attenuated ALP mRNA levels and ßGP-induced calcification, suggesting that endogenous TGF-ß stimulates ALP activity and then ßGP breakdown. An increase in the number of cells embedded in the collagen gel enhanced the mRNA levels of Runx2 and OCN, but not of ALP. Collectively, several factors are likely to promote the differentiation of dermal mesenchymal cells into osteoblast-like cells and ectopic calcification in a 3D collagen matrix, implying the utility of these cells as a potential autologous cell source for tissue engineering.
Similar content being viewed by others
References
Buttery L, Bielby R, Howard D, Shakesheff K. Osteogenic differentiation of embryonic stem cells in 2D and 3D culture. Methods Mol Biol 2011;695:281–308.
Matthews BG, Naot D, Callon KE, Musson DS, Locklin R, Hulley PA, et al. Enhanced osteoblastogenesis in three-dimensional collagen gels. Bonekey Rep 2014;3:560.
Hata S, Okamura K, Hatta M, Ishikawa H, Yamazaki J. Proteolytic and non-proteolytic activation of keratinocyte-derived latent TGF-ß1 induces fibroblast differentiation in a wound-healing model using rat skin. J Pharmacol Sci 2014;124:230–243.
Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–550.
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363.
Lorenz K, Sicker M, Schmelzer E, Rupf T, Salvetter J, Schulz-Siegmund M, et al. Multilineage differentiation potential of human dermal skinderived fibroblasts. Exp Dermatol 2008;17:925–932.
Alt E, Yan Y, Gehmert S, Song YH, Altman A, Gehmert S, et al. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell 2011;103:197–208.
Cheng SL, Shao JS, Charlton-Kachigian N, Loewy AP, Towler DA. MSX2 promotes osteogenesis and suppresses adipogenic differentiation of multipotent mesenchymal progenitors. J Biol Chem 2003;278:45969–45977.
Shao JS, Cai J, Towler DA. Molecular mechanisms of vascular calcification:lessons learned from the aorta. Arterioscler Thromb Vasc Biol 2006;26:1423–1430.
Simionescu A, Simionescu DT, Vyavahare NR. Osteogenic responses in fibroblasts activated by elastin degradation products and transforming growth factor-beta1:role of myofibroblasts in vascular calcification. Am J Pathol 2007;171:116–123.
Casser-Bette M, Murray AB, Closs EI, Erfle V, Schmidt J. Bone formation by osteoblast-like cells in a three-dimensional cell culture. Calcif Tissue Int 1990;46:46–56.
Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000;87:E10–E17.
Wang N, Wang X, Xing C, Sun B, Yu X, Hu J, et al. Role of TGF-beta1 in bone matrix production in vascular smooth muscle cells induced by a high-phosphate environment. Nephron Exp Nephrol 2010;115:e60–e68.
Shi C, Cheng T. Effects of acute wound environment on neonatal rat dermal multipotent cells. Cells Tissues Organs 2003;175:177–185.
Hasebe Y, Hasegawa S, Hashimoto N, Toyoda M, Matsumoto K, Umezawa A, et al. Analysis of cell characterization using cell surface markers in the dermis. J Dermatol Sci 2011;62:98–106.
Loghman-Adham M, Szczepanska-Konkel M, Yusufi AN, Van Scoy M, Dousa TP. Inhibition of Na+-Pi cotransporter in small gut brush border by phosphonocarboxylic acids. Am J Physiol 1987;252(2 Pt 1):G244–G249.
Montessuit C, Caverzasio J, Bonjour JP. Characterization of a Pi transport system in cartilage matrix vesicles. Potential role in the calcification process. J Biol Chem 1991;266:17791–17797.
Peng SB, Yan L, Xia X, Watkins SA, Brooks HB, Beight D, et al. Kinetic characterization of novel pyrazole TGF-beta receptor I kinase inhibitors and their blockade of the epithelial-mesenchymal transition. Biochemistry 2005;44:2293–2304.
Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol 2015;25:92–99.
Janssens K, ten Dijke P, Janssens S, Van Hul W. Transforming growth factor-beta1 to the bone. Endocr Rev 2005;26:743–774.
Caverzasio J, Bonjour JP. Characteristics and regulation of Pi transport in osteogenic cells for bone metabolism. Kidney Int 1996;49:975–980.
Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A 1994;91:7071–7075.
Yoshiko Y, Candeliere GA, Maeda N, Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 2007;27:4465–4474.
Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 2006;98:905–912.
Orimo H, Shimada T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem 2008;315:51–60.
Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and ß-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 2013;4:117.
Nakano Y, Addison WN, Kaartinen MT. ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone 2007;41:549–561.
Franceschi RT, Ge C, Xiao G, Roca H, Jiang D. Transcriptional regulation of osteoblasts. Ann N Y Acad Sci 2007;1116:196–207.
Hosaka N, Mizobuchi M, Ogata H, Kumata C, Kondo F, Koiwa F, et al. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif Tissue Int 2009;85:523–529.
Bitar M, Brown RA, Salih V, Kidane AG, Knowles JC, Nazhat SN. Effect of cell density on osteoblastic differentiation and matrix degradation of biomimetic dense collagen scaffolds. Biomacromolecules 2008;9:129–135.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suyama, T., Hatta, M., Hata, S. et al. Differentiation of rat dermal mesenchymal cells and calcification in three-dimensional cultures. Tissue Eng Regen Med 13, 527–537 (2016). https://doi.org/10.1007/s13770-016-9124-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-016-9124-z