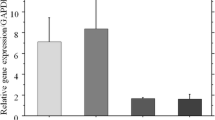
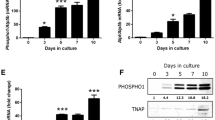
Abstract
Tissue-nonspecific alkaline phosphatase (TNAP) plays a key role in mineralization by degrading inorganic pyrophosphate and providing free inorganic phosphate. We have previously reported that TNAP is induced by β-glycerophosphate and NaH2PO4 in short-term cultures of SaOS-2 human osteoblast-like cells and that PHEX (phosphate-regulating gene with homologies to endopeptidase on the X chromosome) mRNA is also induced after TNAP induction. In the present study, we have investigated the effects of levamisole, a TNAP inhibitor, and phosphonoformic acid (PFA), a type III sodium-phosphate cotransporter inhibitor, on the phosphate-induced expression of TNAP and mineralization. Levamisole inhibited β-glycerophosphate-induced mineralization, TNAP and PHEX expression, and the increase in enzymatic activity of NPP1 (5′-nucleotide pyrophosphatase phosphodiesterase 1), but did not inhibit NaH2PO4-induced mineralization. PFA completely inhibited NaH2PO4-induced mineralization and NPP1 enzymatic activation, and partly inhibited β-glycerophosphate-induced mineralization, but did not affect the increase in TNAP activity. These results suggest that phosphate derived from TNAP-induced hydrolysis of β-glycerophosphate yields signals that induce TNAP expression and mineralization, and that PHEX expression may be linked to TNAP expression. However, luciferase assays failed to detect any transcriptional activation of the promoter region of the human TNAP gene by β-glycerophosphate or NaH2PO4, suggesting that the effects of these phosphates may be indirect.








Similar content being viewed by others
References
Harris H (1989) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186:133–150. doi:10.1016/0009-8981(90)90031-M
Millán JL (2006) Mammalian alkaline phosphatases. Wiley-VCH, Weinheim
Weiss MJ, Ray K, Henthorn PS et al (1988) Structure of the human liver/bone/kidney alkaline phosphatase gene. J Biol Chem 263:12002–12010
Hui M, Tenenbaum HC (1998) New face of an old enzyme: alkaline phosphatases may contribute to human tissue aging by inducing tissue hardening and calcification. Anat Rec 253:91–94. doi:10.1002/(SICI)1097-0185(199806)253:3<91::AID-AR5>3.0.CO;2-H
Anderson HC, Sipe JB, Hessle L et al (2004) Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol 164:841–847
Anderson HC (1995) Molecular biology of matrix vesicles. Clin Orthop Relat Res 314:266–280
Johnson K, Moffa A, Chen Y (1999) Matrix vesicle plasma cell membrane glycoprotein-1 regulates mineralization by murine osteoblastic MC3T3 cells. J Bone Miner Res 14:883–892. doi:10.1359/jbmr.1999.14.6.883
Ho AM, Johnson MD, Kingsley DM (2000) Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289:265–270. doi:10.1126/science.289.5477.265
Terkeltaub RA (2001) Inorganic pyrophosphate generation and disposition on pathophysiology. Am J Physiol 281:C1–C11
Hessle L, Johnson KA, Anderson HC et al (2002) Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci USA 99:9445–9449. doi:10.1073/pnas.142063399
Harmey D, Hessle L, Narisawa S et al (2004) Concerted regulation of inorganic pyrophosphate and osteopontin by Akp2, Enpp1, and Ank. Am J Pathol 164:1199–1209
Weiss MJ, Cole DEC, Ray K et al (1988) A missense mutation in the human liver/bone/kidney alkaline phosphatase gene causing a lethal form of hypophosphatasia. Proc Natl Acad Sci USA 85:7666–7669. doi:10.1073/pnas.85.20.7666
Henthorn PS, Raducha M, Fedde KN et al (1992) Different missense mutations at the tissue-nonspecific alkaline phosphatase gene locus in autosomal recessively inherited forms of mild and severe hypophosphatasia. Proc Natl Acad Sci USA 89:9924–9928. doi:10.1073/pnas.89.20.9924
Orimo H, Hayashi Z, Watanabe A et al (1994) Novel missense and frameshift mutations in the tissue-nonspecific alkaline phosphatase gene in a Japanese patients with hypophosphatasia. Hum Mol Genet 3:1683–1684. doi:10.1093/hmg/3.9.1683
Orimo H, Goseki-Sone M, Sato S et al (1997) Detection of deletion 1154-1156 hypophosphatasia mutation using TNSALP exon amplification. Genomics 42:364–366. doi:10.1006/geno.1997.4733
Mornet E (2000) Hypophosphatasia: the mutations in the tissue-nonspecific alkaline phosphatase gene. Hum Mutat 15:309–315. doi:10.1002/(SICI)1098-1004(200004)15:4<309::AID-HUMU2>3.0.CO;2-C
Orimo H, Girschick HJ, Goseki-Sone M et al (2001) Mutational analysis and functional correlation with phenotype in German patients with childhood-type hypophosphatasia. J Bone Miner Res 16:2313–2319. doi:10.1359/jbmr.2001.16.12.2313
Waymire KG, Mahunen JD, Jaje JM et al (1995) Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat Genet 11:45–50. doi:10.1038/ng0995-45
Fedde KN, Blair L, Silverstein J et al (1999) Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Miner Res 14:2015–2026. doi:10.1359/jbmr.1999.14.12.2015
Whyte MP (2001) Hypophosphatasia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease, 8th edn., vol 4. McGraw-Hill, New York, pp 5313-5329
Wennberg C, Hessle L, Lundberg P et al (2000) Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res 15:1879–1888. doi:10.1359/jbmr.2000.15.10.1879
Goseki-Sone M, Sogabe N, Fukushi-Irie M et al (2005) Functional analysis of the single nucleotide polymorphism (787T>C) in the tissue-nonspecific alkaline phosphatase gene associated with BMD. J Bone Miner Res 20:773–782. doi:10.1359/JBMR.041229
Orimo H, Shimada T (2006) Effects of phosphates on the expression of tissue-nonspecific alkaline phosphatase gene and phosphate-regulating genes in short-term cultures of human osteosarcoma cell lines. Mol Cell Biochem 282:101–108. doi:10.1007/s11010-006-1520-6
Quarles LD (2003) FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol 285:E1–E9
Liu S, Zhou J, Tang W et al (2006) Pathogenic role of Fgf23 in Hyp mice. Am J Physiol 291:E38–E46
Rowe PSN, Kumagai Y, Gutierrez G et al (2004) MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone 34:303–319
Collins JF, Bai L, Ghishan FK (2004) The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch-Eur J Physiol 447:647–652
Guicheux J, Palmer G, Shukunami C et al (2000) A novel in vitro culture system for analysis of functional role of phosphate transport in endochondral ossification. Bone 27:69–74
Johnson K, Hashimoto S, Lotz M et al (2001) Up-regulated expression of the phosphodiesterase nucleotide pyrophosphatase family member PC-1 is a marker and pathogenic factor for knee meniscal cartilage matrix calcification. Arthritis Rheum 44:1071–1081
Orimo H, Shimada T (2005) Regulation of the human tissue-nonspecific alkaline phosphatase gene expression by all-trans-retinoic acid in SaOS-2 osteosarcoma cell line. Bone 36:866–876
Rodan SB, Imai Y, Thiede MA et al (1987) Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Res 47:4961–4966
Fedde KN (1992) Human osteosarcoma cells spontaneously release matrix-vesicle-like structures with the capacity to mineralize. Bone Miner 17:145–151
Stinson RA, Thacker JD, Lin CC (1993) Expression and nature of the alkaline phosphatase gene in cultured osteosarcoma cells. Clin Chim Acta 221:105–114
McQuillan DJ, Richardson MD, Bateman JF (1995) Matrix deposition by a calcifying human osteogenic sarcoma cell line (SAOS-2). Bone 16:415–426
Boskey AL (1998) Biomineralization: conflicts, challenges, and opportunities. J Cell Biochem Suppl 30/31:83–91
Beck GR Jr (2003) Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem 90:234–243
Garimella R, Bi X, Anderson HC et al (2006) Nature of phosphate substrate as a major determinant of mineral type formed in matrix vesicle-mediated in vitro mineralization: an FTIR imaging study. Bone 38:811–817
Nakano Y, Addison WN, Kaartinen MT (2007) ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone 41:549–561
Adams CS, Mansfield K, Perlot RL et al (2001) Matrix regulation of skeletal cell apoptosis: role of calcium and phosphate ions. J Biol Chem 276:20316–20322
Tenenbaum HC (1987) Levamisole and inorganic pyrophosphate inhibit beta-glycerophosphate induced mineralization of bone formed in vitro. Bone Miner 3:13–26
Thomas JT, Boot-Handford RP, Grant ME (1990) Modulation of type X collagen gene expression by calcium β-glycerophosphate and levamisole: implications for a possible role for type X collagen in endochondral bone formation. J Cell Sci 95:639–648
Van Bell H (1976) Alkaline phosphatase. I. Kinetics and inhibition by levamisole of purified isoenzymes from humans. Clin Chem 22:972–976
Szczepanska-Konkel M, Yusufi ANK, Van Scoy M et al (1986) Phosphonocarboxylic acids as specific inhibitors of Na+-dependent transport of phosphate across renal brush border membrane. J Biol Chem 261:6375–6383
Palmer G, Bonjour J-P, Caverzasio J (1997) Expression of a newly identified phosphate transporter/retrovirus receptor in human SaOS-2 osteoblast-like cells and its regulation by insulin-like growth factor I. Endocrinology 138:5202–5209
Yoshiko Y, Candeliere GA, Maeda N et al (2007) Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol 27:4465–4474
Addison WN, Azari F, Sørensen ES et al (2007) Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 282:15872–15883
Johnson KA, Hessle L, Vaingankar S et al (2000) Osteoblast tissue-nonspecific alkaline phosphatase antagonizes and regulates PC-1. Am J Physiol 279:R1365–R1377
Bielesz B, Klaushofer K, Oberbauer R (2004) Renal phosphate loss in hereditary and acquired disorders of bone mineralization. Bone 35:1229–1239
The HYP Consortium (1995) A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11:130–136
Ecarot B, Desbarats M (1999) 1,25-(OH)2D3 down-regulates expression of Phex, a marker of the mature osteoblast. Endocrinology 140:1192–1199
Orimo H, Goseki-Sone M, Hosoi T et al (2008) Functional assay of the mutant tissue-nonspecific alkaline phosphatase gene using U2OS osteoblast-like cells. Mol Genet Metab. doi:10.1016/j.ymgme.2008.03.015
Orimo H, Shimada T (2006) Posttranscriptional modulation of the human tissue-nonspecific alkaline phosphatase gene expression by 1,25-dihydroxyvitamin D3 in MG-63 osteoblastic osteosarcoma cells. Nutr Res 26:227–234
Brisson D, Vahl M-C, St-Pierre J et al (2001) Glycerol: a neglected variable in metabolic processes? BioEssays 23:534–542
Beck GR Jr, Knecht N (2003) Osteopontin regulation by inorganic phosphate is ERK1/2-, protein kinase C-, and proteasome-dependent. J Biol Chem 278:41921–41929
Julien M, Magne D, Masson M et al (2007) Phosphate stimulates matrix Gla protein expression in chondrocytes through the extracellular signal regulated kinase signaling pathway. Endocrinology 148:530–537
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orimo, H., Shimada, T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem 315, 51–60 (2008). https://doi.org/10.1007/s11010-008-9788-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9788-3