Abstract
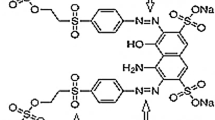
Textile effluents are toxic and carcinogenic materials that exist in the aquatic environment. In this study, the degradation efficiency of commercially available scarlet red dye investigated on TSA-SS Electro Fenton process (EFP) was reported. It is of great interest in the field of environmental engineering to remove dyes from aquatic environment. The influence of operating parameters such as pH (2–9), current density (0.1–0.5 mA/cm2), concentration of dye (0.1–0.5 g/L), H2O2 (0.1–0.5 g/L) concentration and Fe2+ concentration (0.01–0.03 g/L) were analyzed by batch system. The optimum degradation conditions were determined as pH—3, current density—0.4 mA/cm2, concentration of dye—0.4 g/L, H2O2 concentration—0.5 g/L and Fe2+ concentration—0.025 g/L. These results indicated that the degradation efficiency of scarlet red dye by EFP depends on solution pH and Fenton reagent concentration and a low pH value was favorable for the dye degradation. It has been demonstrated that more than 94% dye removal was obtained at 50 min. Electro Fenton process was also investigated by cyclic voltammetry technologies.






Similar content being viewed by others
References
Oller I, Malato S, Sánchez-Pérez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination: a review. J Sci Total Environ 409:4141–4166
Matilainen A, Sillanpää M (2010) Removal of natural organic matter from drinking water by advanced oxidation processes. Chemos J 80:351–365
Reddy PM, Raju BR, Karuppiah J, Reddy EL, Subrahmanyam C (2013) Degradation and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem Eng J 217:41–47
Gua L, Nie JY, Zhua N, Wangc L, Yuana HP, Shoua Z (2012) Enhanced Fenton’s degradation of real naphthalene dye intermediate wastewater containing 6-nitro-1-diazo-2-naphthol-4-sulfonic acid: a pilot scale study. Chem Eng J 189–190:108–116
Panda N, Sahoo H, Mohapatra S (2011) Decolourization of methyl orange using fenton-like mesoporous Fe2O3–SiO2 composite. J Hazard Mater 185:359–365
Whebi DJ, Hafez HM, El Masri MH, El Jamal MM (2010) Influence of certain inorganic ions and ligands on degradation of methyl red by Fenton’s reagent. J Univ Chem Technol Metall 45(3):303–312
Cruz-González K, Torres-López O, García-León A, Guzmán-Mar JL, Reyes LH, Hernández-Ramírez A, Peralta-Hernández JM (2010) Determination of optimum operating parameters for Acid Yellow 36 decolorization by electro-Fenton process using BDD cathode. Chem Eng J 160:199–206
Rahmani AR, Nematollahi D, Azarian G, Godini K, Berizi Z (2015) Activated sludge treatment by electro-Fenton process: parameter optimization and degradation mechanism. Korean J Chem Eng 32(8):1570–1577
Samarghandi MR, Shabanloo A, Shamsi K, Mehralipour J, Poureshgh Y (2014) Performance of Electrofenton process to remove cyanide from aquatic environments in presence of interfering humic acids. J Health 4(4):293–303
Panizza M, Mehmet A, Oturan (2011) Degradation of Alizarin Red by electro-Fenton process using a graphite-felt cathode. Electrochim Acta 56(20):7084–7087
Babuponnusami A, Muthukumar K (2012) Removal of phenol by heterogenous photo electro Fenton-like process using nano-zero valent iron. J Sep Purif Technol 98:130–135
Rosales E, Pazos M, Sanromán MA (2012) Advances in the electro-Fenton process for remediation of recalcitrant organic compounds. J Chem Eng Technol 35(4):609–617
Zhen GY, Lu XQ, Wang BY, Zhao YC, Chai XL, Niu DJ, Zhao TT (2014) Enhanced dewatering characteristics of waste activated sludge with Fenton pretreatment: effectiveness and statistical optimization. Front Environ Sci Eng 8(2):267–276
Zhou L, Zhongxin H, Zhang C, Bi Z, Jin T, Zhou M (2013) Electro generation of hydrogen peroxide for electro-Fenton system by oxygen reduction using chemically modified graphite felt cathode. J Sep Purif Technol 111:131–136
Moussavi G, Bagheri A, Khavanin A (2012) The investigation of degradation and mineralization of high concentrations of formaldehyde in an electro-Fenton process combined with the biodegradation. J Hazard Mater 237–238:147–152
Sevimli MF, Deliktaş E, Şahinkaya S, Güçlü D (2014) A comparative study for treatment of white liquor by different applications of Fenton process. Arab J Chem 7(6):1116–1123
Moussavi G, Aqanaghad M (2015) Performance evaluation of electro-Fenton process for pretreatment and biodegradability improvement of a pesticide manufacturing plant effluent. J Sustain Environ Res 25:249–254
Isarain-Cha`vez E, Garrido JA, Rodriguez RM, Centellas F, Arias C, Cabot PL, Brillas E (2011) Mineralization of metoprolol by electro-Fenton and photoelectro-Fenton processes. J Phys Chem 115:1234–1242
Azizi A, Moghaddam MRA, Maknoon R, Kowsari E (2016) Investigation of enhanced Fenton process (EFP) in color and COD removal of wastewater containing Acid Red 18 by response surface methodology: evaluation of EFP as post treatment. J Desalination Water Treat 57:14083–14092
Ibhadon AO, Fitzpatrick P (2013) Heterogeneous photocatalysis: recent advances and applications. J Catal. 3:189–218
Ghosh P, Thakur LK, Samanta AN, Ray S (2012) Electro-Fenton treatment of synthetic organic dyes: influence of operational parameters and kinetic study. Korean J Chem Eng 29(9):1203–1210
De Luna MD, Veciana ML, Su CC, Lu MC (2012) Acetaminophen degradation by electro-Fenton and photo electro-Fenton using a double cathode electrochemical cell. J Hazard Mater. 217–218:200–207
Xu X-R, Li X-Z (2010) Degradation of azo dye Orange G in aqueous solutions by persulfate with ferrous ion. J Sep Purif Technol 72(1):105–111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gomathi, E., Balraj, B. & Kumaraguru, K. Electrochemical degradation of scarlet red dye from aqueous environment by titanium-based dimensionally stable anodes with SS electrodes. Appl Biol Chem 61, 289–293 (2018). https://doi.org/10.1007/s13765-018-0357-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-018-0357-5