Abstract
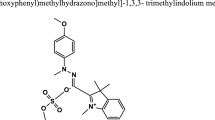
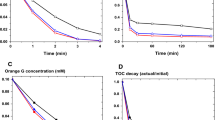
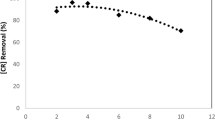
This work investigates oxidative decolorization of two different dyes, Methylene blue and Titan yellow in aqueous solution using an environmentally friendly advanced electro-chemical oxidation (electro-Fenton) process. The effect of operating conditions like H2O2 concentration, current density, initial dye concentration was studied in a batch stirred cell. Individual decolorization decay kinetics for both dyes was investigated. The second-order absolute rate constants (L mol−1 s−1) between hydroxyl radical and dye have been calculated from experimental data by fitting it to the decolorization model. The apparent kinetic constants, k app (s−1) for Methylene blue and Titan yellow dye decolorization were also determined. The experimental data showed a good fit to the theoretical model, which can predict data in a wide range of % dye decolorization. This process also reduces COD of the dye solution, and the unit energy demand (UED) in kWh/kg COD removed for different electrical current has been reported.
Similar content being viewed by others
References
A. K. Gupta, A. Pal and C. Sahoo, Dyes Pigm., 69, 224 (2006).
Q. Z. Dai, M. H. Zhou and L. C. Lei, J. Hazard. Mater., 137, 1870 (2006).
H. Lata, V. K. Garg and R. K. Gupta, Dyes Pigm., 74, 653 (2007).
X. S. Wang, Y. Zhou, Y. Jiang and C. Sun, J. Hazard. Mater., 157, 374 (2008).
V.K. Garg, M. Amita, R. Kumar and R. Gupta, Dyes Pigm., 63, 243 (2004).
T. Robinson, B. Chandran and P. Nigam, Environ. Int., 28, 29 (2002).
S. Wang, Y. Boyjoo and A. Choueib, Chemosphere, 60, 1401 (2005).
O. Hamdaoui, J. Hazard. Mater., 135, 264 (2006).
C. S. Poon, Q. P. Huang and C. Fung, Chemosphere, 38, 1005 (1999).
Y. B. Xie and X. Z. Li, Mater. Chem. Phys., 95, 39 (2006).
K. V. Kumar and A. Kumaran, J. Biochem. Eng., 27, 83 (2005).
D. Ozer, G. Dursun and A. Ozer, J. Hazard. Mater., 144, 171 (2007).
S. Papic, D. Vujevic, N. Koprivanac and D. Sinko, J. Hazard. Mater., 164, 1137 (2009).
Y.H. Huang, S. Chou, M.G. Perng, G. H. Huang and S. S. Cheng, Water Sci. Technol., 39, 145 (1999).
E. Brillas, J. C. Calpe and J. Casado, Water Res., 34, 2253 (2000).
S. H. Lin and C. C. Chang, Water Res., 34, 4243 (2000).
K. Cruz-Gonzalez, O. Torres-Lopez, A. Garcia-Leon, J. L. Guzman-Mar, L. H. Reyes, A. Hernandez-Ramirez and J. M. Peralta-Hernandez, Chem. Eng. J., 160, 199 (2010).
K. Juttner, U. Galla and H. Schmieder, Electrochim. Acta, 45, 2575 (2000).
E. J. Ruiz, C. Arias, E. Brillas, A. Hernandez-Ramirez and J. M. Peralta-Hernandez, Chemosphere, 82, 495 (2011).
C. A. Martinez-Huitle and E. Brillas, Appl. Catal., B, 87, 105 (2009).
G.Q. Zhang, F. L. Yang, M.M. Gao, X.H. Fang and L. F. Liu, Electrochim. Acta, 53, 5155 (2008).
D. Pletcher and F. C. Walsh, Industrial Electrochemistry, 2nd Ed., Chapman and Hall, London, UK (1990).
S. H. Lin and M. L. Chen, Environ. Technol., 16, 693 (1995).
S. H. Gau and F. S. Chang, Wat. Sci. Technol., 34, 455 (1996).
E. Brillas and J. Casado, Chemosphere, 47, 241 (2002).
M. A. Oturan, J. Peiroten, P. Chartrin and A. J. Acher, Environ. Sci. Technol., 34, 3474 (2000).
M. Panizza and G. Cerisola, Water Res., 35, 3987 (2001).
A. Lahkimi, M. A. Oturan and N. Oturan, Environ. Chem. Lett., 5, 35 (2007).
E. Guivarch, S. Trevin, C. Lahitte and M. A. Oturan, Environ. Chem. Lett., 1, 38 (2003).
E. Rosales, M. Pazos, M. A. Longo and M. A. Sanroman, Chem. Eng. J., 155, 62 (2009).
N. Bensalah, M.A. Quiroz Alfaro and C.A. Martnez-Huitle, Chem. Eng. J., 149, 348 (2009).
M. Panizza and G. Cerisola, Water Res., 43, 339 (2009).
L. S. Clesceri, A. E. Greenberg and D. Andrew, Standard methods for the Examination of water and wastewater, 20th Ed., APHA, Washington, DC (1998).
I. Talini and G. K. Anderson, Water Res., 26, 107 (1992).
W. G. Kuo, Water Res., 26, 881 (1992).
P. Ghosh, A.N. Samanta and S. Ray, Can. J. Chem. Eng., 88, 1021 (2010).
M. Zhou, Q. Yu, L. Lei and G. Barton, Sep. Purif. Technol., 57, 380 (2007).
M. Zhou, Q. Yu and L. Lei, Dyes Pigm., 77, 129 (2008).
M. Pera-Titus, V. Garcia-Molina, M.A. Banos, J. Gimenez and S. Esplugas, Appl. Catal. B: Environ., 47, 219 (2004).
B.G. Kwon, D. S. Lee, N. Kang and J. Yoon, Water Res., 33, 2110 (1999).
A. M. El-Dein, J.A. Libra and U. Wiesmann, Water Sci. Technol., 44, 295 (2001).
J. H. Ramirez, F. M. Duarte, F.G. Martins, C. A. Costa and L. M. Madeira, Chem. Eng. J., 148, 394 (2009).
A. R. Khataee, V. Vatanpour and A. R. Amani Ghadim, J. Hazard. Mater., 161, 1225 (2009).
S. Altin, Int. J. Chem. Reactor Eng., 9, A33 (2011).
A. Altin, Sep. Purif. Technol., 61, 391 (2008).
C.T. Wang, J. L. Hu, W. L. Chou and Y.M. Kuo, J. Hazard. Mater., 152, 601 (2008).
E. Brillas, I. Sires and M. A. Oturan, Chem. Rev., 109, 6570 (2009).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghosh, P., Thakur, L.K., Samanta, A.N. et al. Electro-Fenton treatment of synthetic organic dyes: Influence of operational parameters and kinetic study. Korean J. Chem. Eng. 29, 1203–1210 (2012). https://doi.org/10.1007/s11814-012-0011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-012-0011-6