Abstract
Perchlorate (ClO4−), which is a powerful endocrine disruptor affecting iodine fixation in the thyroid gland in humans and in biota, is a pollutant of natural and anthropogenic origin. For this reason, this pollutant must be eliminated from the ecosystems. It has been found in extreme environments such as Antarctica. ClO4− reduction can be achieved with physicochemical treatments in small concentrations and through bacterial degradation. This is a cost-effective method, easy to implement, which makes it a viable method for the removal of perchlorate contamination in ecosystems. This review provides an updated discussion of reducing perchlorate contamination; that includes different perspectives of investigations related to its origin, use, effects on living beings; as well as the technologies used to eliminate this pollutant from the environment; its environmental fate in strategic ecosystems such as Antarctica in particular and astrobiological perspectives.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Perchlorate (ClO4−), an inorganic compound of chlorine, is present in all ecosystems. It is, both, highly toxic and persistent, and it has strong kinetic stability. Its source can be either natural or anthropogenic. The anthropogenic origin of the pollutant is mainly related to its use in the manufacture of ClO4− salts used in rocket fuel, fireworks, ammunition, and flares. It forms naturally in the atmosphere during storms, through reactions between common chlorine species (chlorides) and ozone (O3) (Jiang et al. 2021). ClO4− is also formed in volcanic eruptions. It is present in hypersaline environments, deserts, and Antarctica (Acevedo-Barrios et al. 2022a, b).
ClO4− is easily transferred to groundwater and surface water due to its high solubility, and this compound is considered a pollutant due to its negative effects on human health when ingested in low concentrations (24.5 μg/L drinking water) (Jiang et al. 2021). ClO4− has been found in foods such as vegetables, cereals, and fruits. Similarly, traces of this pollutant are found in human tissues and in breast milk. The negative health impacts include its ability to act as a potent endocrine disruptor by inhibiting iodine fixation in the thyroid gland, which regulates metabolism in adults, and the growth and development of infants (Li et al. 2022).
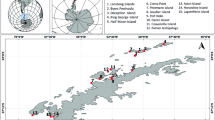
ClO4− is found in remote ecosystems, such as Antarctica, due to atmospheric depositions (Acevedo-Barrios et al. 2022a), and on the Martian surface. The presence of ClO4− in Antarctica has been reported in two points on the South Shetland Islands, and in nine points on the Antarctic peninsula (Acevedo-Barrios et al. 2022a, b).
The presence of humans in Antarctica in recent years has been increasing; research, transportation, and tourism activities have promoted the establishment of stations and transfer systems that could become considerable sources of contamination (Kounaves et al. 2010b). Thus, it is important to increase research and studies related to the presence of perchlorate in Antarctica, to monitor its incidence and possible environmental impacts (Acevedo-Barrios et al. 2022a, 2023).
This review provides an updated discussion of reducing perchlorate contamination; that includes different perspectives of investigations related to its origin, use, and effects on living beings; as well as the technologies used to eliminate this pollutant from the environment; its environmental fate in strategic ecosystems such as Antarctica, in particular, and astrobiological perspectives. This research was carried out in Antarctica, from January 2021 to August 2023
Materials and methodology
Academic texts were compiled and reviewed, using “Antarctica”, “Astrobiology”, “Perchlorate”, and “perchlorate toxicity” as keywords. Search criteria included combinations of keywords in article titles, abstracts, or texts. The title and abstract of each publication were examined to determine if the topic was relevant to this review. Additionally, this information was used to analyze the presence of this contaminant in Antarctica, as described in this research.
Origin and uses of CIO4 −
ClO4− is a chemically stable ion, with toxic effects on humans and biota. It is found in different environmental matrixes (Pleus and Corey 2018), and it is characterized by its persistence in ecosystems (Bardiya and Bae 2011). It has a stable anion in its chemical structure, which is considered as highly oxidizing, surrounded by four oxygen atoms (Murray and Bolger 2014), bringing it a great fugacity that allows it to travel over long distances (Cao et al. 2019).
This pollutant originates, naturally, due to volcanic eruptions and atmospheric processes, and anthropogenically, due to industrial and aerospace activities. Fireworks are one of the largest sources of perchlorate pollution in the environment. This is because fireworks contain a variety of chemicals, such as potassium nitrate, potassium chloride, potassium perchlorate or ammonium perchlorate, carbon, sulfur, manganese, sodium, nitrate, strontium, aluminum, titanate barium, and iron oxalate powder (Wu et al. 2011). In addition to this, a large part of the perchlorate contamination found in groundwater and surface water is associated with military, aerospace, and explosives use, among others (Murray and Bolger 2014; Kumarathilaka et al. 2016).
Electronic waste is considered the type of waste with the greatest flow of exponential growth, and it is related to perchlorate contamination (Eguchi et al. 2014; Parvez et al. 2021). Perchlorate is found in polyvinyl chloride (PVC) and in lithium-ion batteries as a doping material (Interstate Technology Regulatory Council 2005). Therefore, residents and workers at e-waste recycling sites are likely to be exposed to the contaminant. Furthermore, this strong oxidant is used as a cleaning agent during the production of LCD displays (Her et al. 2011).
Ammonium, lithium, magnesium, and potassium perchlorate are used to manufacture herbicides and automobile air bag inflators. The latter has been used in medicine, mainly between 1950 and 1960, to treat hyperthyroidism (Murray and Bolger 2014).
Similarly, perchlorate has been used in the manufacture of dyes, lubricants, rubber, and matches (Interstate Technology Regulatory Council 2005). Likewise, other anthropogenic sources have been identified such as: foundries, flares, drying and etching agents, gunpowder, batteries (Wang et al. 2014), chlorine-based disinfectants, bleaches, cleaners and chemicals for swimming pool chlorination, electronic tubes, paints, enamels, fertilizers, nuclear reactors, and other materials for commercial uses (Maffini et al. 2016; Kumarathilaka et al. 2016). Perchlorate has also been implemented as a growth promoter and thyrostatic drug to increase mass in livestock (Gholamian et al. 2011).
Natural origin
Chlorate (ClO3−) and Perchlorate (ClO4−) are two oxyhalides. These compounds are formed in the atmosphere through the reaction of chlorite (ClO2−), mediated by O3, and, possibly, UV during photochemical processes, or through electrical activity during thunderstorms. (Dasgupta et al. 2005). They can acting as alternative electron acceptors, supporting a range of microbial metabolic processes (Coates and Achenbach 2006).
After being formed in the stratosphere, ClO4− has two ways to enter the soil: wet or dry deposition. In wet deposition, it dissolves in moisture droplets and is brought down to the ground through precipitation. Once on the ground, it can be deposited into the soil. However, because it has a high solubility and low sorption properties, it can easily enter surface water or groundwater sources (Rao et al. 2010). Dry deposition, which can occur through eolian dust, atmospheric aerosols, and gases, is also a possibility. These processes may be responsible for the presence of trace amounts of perchlorate in various environments (Vega et al. 2018).
The high mobility of ClO4− can lead to its accumulation in ecosystems, due to its chemical stability under normal environmental conditions. (Brown and Gu 2006). High concentrations of natural ClO4− are found in specific ecosystems with arid, hyper-arid, and semi-arid soil conditions. Likewise, it can be found in nitrate deposits in the Atacama Desert in Chile, with concentrations of 1000 mg kg−1 of ClO4− (Jackson et al. 2015), as well as in hypersaline environments, volcanic eruptions, and Antarctic soils (Acevedo-Barrios and Olivero-Verbel 2021; Acevedo-Barrios et al. 2022a).
Perchlorate salts present in soils can dissolve and be transported by water which can be used for human consumption, irrigation of crops, or in industrial processes. Consequently, perchlorate originating from a single source can affect various human exposure pathways, leading to an increased intake of ClO4− perchlorate (Murray and Bolger 2014).
The high mobility of perchlorate in water and soil has led to the establishment of regulations to ensure safe levels in drinking water. For instance, the State of California establishes that the maximum level of ClO4− allowed is 6 μg/L, the same level set by the Canadian Environmental Protection Act (Srinivasan and Sorial 2009). On the other hand, in 2009, the US EPA set a recommended value of 15 μg·L−1 for drinking water (USEPA 2008, 2009).
Studies on natural sources of ClO4− that focus on different environmental matrixes have been limited; however, the currently available information allows for proposing different methods for its reduction (Jiang et al. 2021).
However, in the case of Antarctica, the problem could be even greater, since there are several areas with the presence of natural perchlorate (Acevedo-Barrios et al. 2022a), due to the high mobility in water, and because it is used through desalination processes, and for cleaning work on ships and bases; and, in the case of freshwater lakes for human consumption, they must be analyzed for perchlorate. But perchlorate is not one of the aspects considered in water quality evaluations (Dean et al. 2004), and it must be taken into account within the regulations, recognizing its teratogenic potential (Acevedo-Barrios et al. 2018).
ClO4− is distributed in ecosystems through runoff, and it accumulates in food (Murray and Bolger 2014). Processed products like beverages may contain small concentrations of perchloride. Studies show that plants accumulate perchlorate, primarily in the leaves, up to two orders of magnitude of available perchlorate in irrigation water or soil. The fruits of these plants can also accumulate perchlorate, in lower concentrations (Calderón et al. 2017; Estrada et al. 2017). Therefore, crops can accumulate perchlorate if they are irrigated with contaminated water, if they are grown in contaminated agricultural soils, or if fertilizers containing perchlorate are used (Vega et al. 2018).
ClO4− is also present in milk, sauces, instant mixes, meats, fish, tea, sodas, plants, and tobacco products (Andrew Jackson et al. 2005; Zhang et al. 2010; Lee et al. 2012). Another common route of exposure is through domestic inhalation (Kumarathilaka et al. 2016). The estimated daily intake concentrations of ClO4− are shown in Table 1.
Atmospheric origin
Atmospheric production of ClO4− is a known process expressed with the Cl + 2O4 → ClO4− reaction, and this photochemical reaction occurs between chlorine and atmospheric ozone (Trumpolt et al. 2005).
Desert environments
The extreme conditions present in this ecosystem, located at the south pole, range from low temperatures and high salinity, which lead to the natural formation of ClO4− (Calderon et al. 2014). This pollutant is found in the soil and ice of the Antarctic continent at concentrations as high as 1,100 μg/Kg (Kounaves et al. 2010b; Jiang et al. 2013; Acevedo-Barrios and Olivero-Verbel 2021). The Antarctic ecosystem is considered to be a hypersaline environment, and the source of natural ClO4− is the result of atmospheric depositions. This source increases according to the decrease in precipitations, making its permanence in the ecosystem’s indefinite (Acevedo-Barrios et al. 2018). It presents favorable conditions for the development and growth of native bacteria capable of reducing the pollutant due to its extreme conditions. In the desert valleys of Antarctica, ClO4− concentrations are between 91 and 465 ppm, as reported by Acevedo-Barrios et al. 2022a; 2023; 2024.
The accumulation of ClO4− in arid regions is mainly due to the absence of rain and aerobic conditions, which prevent its reduction by bacteria that can use it as an electron acceptor under anaerobic conditions. Microbial reduction of ClO4− occurs via the ClO4− ➔ ClO3− ➔ ClO2− ➔ Cl− + O2 biochemical reaction. Perchlorate reducing organisms can use a wide range of electron donors, and they can be found in various natural settings. These organisms are usually facultatively anaerobic or microaerophilic (Coates and Achenbach 2004). Under relevant environmental conditions, ClO4− is highly conserved and is not transformed by known abiotic processes in toxic environments due to its high solubility. ClO3−, on the other hand, has received less research attention than ClO4−, mainly due to challenges in analyzing environmental samples with low concentrations (Jackson et al. 2012).
Antarctic ecosystem
The presence of ClO4− has been detected in small amounts across all continents, including in Arctic and Antarctic ice sheets, indicating its widespread occurrence in a natural environment (Jiang et al. 2021). The atmosphere deposits environmental pollutants in snow, glaciers, and polar ice caps, which makes it possible to measure the records of the presence of compound environmental contaminants in Antarctica (Du et al. 2019). In polar regions such as Antarctica, Greenland, the Canadian Arctic, North America, and East Asia, increasing ClO4− concentrations have been reported since 1980. Jiang et al. (2016) observed a strong positive correlation between effective stratospheric chlorine equivalent and ClO4− in Antarctic snow, suggesting that increased stratospheric chlorine may have caused the increase in ClO4−. Meanwhile, Cole-Dai et al. (2018) suggest that the increase since 1980 is likely due to increased atmospheric ClO4− production, possibly associated with emissions from anthropogenic sources of ClO4− into the atmosphere in recent years.
Moreover, significant correlations between Cl− and ClO4− fluxes and concentrations in a shallow ice core from Agassiz Ice Cap suggest that ClO4− was predominantly formed from tropospheric Cl− during the 1940–1959 period (Furdui et al. 2018). This increase could be attributed to the emissions of compounds such as methyl chloroform, which is capable of being converted into inorganic species and may contribute to the formation of ClO4− in the stratosphere (Brown and Gu 2006).
The presence of ClO4− is mainly of natural origin, since there are few interactions with human populations in Antarctic snow (Acevedo-Barrios and Olivero-Verbel 2021; Acevedo-Barrios et al. 2022a, b; 2023, 2024). Perchlorate in this region has progressively increased since the 1970s. This trend could be the result of the elevation of atmospheric chlorine levels caused by anthropogenic chlorine emissions (Jiang et al. 2016), because this pollutant is formed when free radicals (Cl⋅) and ozone (O3) react in the stratosphere due to the presence of the ozone hole during the austral summer (Jiang et al. 2021). ClO4− has been found on the Antarctic continent through the ozone hole in late summers due to sunlight, and its concentrations increase in late December when temperatures rise (Crawford et al. 2017). Concentrations of 104.3 ± 33.3 ng/kg of perchlorate have been found in the superficial snow caps of Eastern Antarctica, and this private area presents apparently high concentrations in areas with low accumulation rates (Jiang et al. 2020).
Mars
Antarctica has been highlighted as one of the most suitable places to study surface processes on Mars (Wynn-Williams and Edwards 2000). In Antarctica, three specific areas have been identified as Mars analog sites (Martins et al. 2017). The McMurdo Dry Valleys, which are recognized as the coldest and driest places on planet Earth, with average temperatures of -30 °C in winter and -15 °C in summer. Likewise, abundant presence of microorganisms has been documented in the permafrost and rocks of this region (Friedmann 1982; Gilichinsky et al. 2007). Since the time of the Viking missions, these valleys have been considered the closest climatic analogues to Martian conditions.
The other two Antarctic analogues are the Victoria Land Mountains and Lake Vostok. In the case of Victoria Land, it shares similarities with McMurdo due to the presence of dry valleys, but its higher altitude has favored the existence of microorganisms resistant even to the intense UV radiation present in Antarctica (Meeßen et al. 2015). Lake Vostok has been categorized as an analogue of Enceladus or Europa, due to the presence of microorganisms in a lake covered by a layer of ice several kilometers thick (Shtarkman et al. 2013).
However, an intriguing aspect that deserves attention in the study of astrobiological analogues is the presence of perchlorates on Mars (Kounaves et al. 2010a). Interestingly, perchlorates have also been found in Antarctic marine sediments, with a higher concentration recorded on Deception Island, possibly related to volcanic contributions to the natural formation of perchlorate in Antarctica (Acevedo-Barrios et al. 2022a, b). This connection could be related to the presence of perchlorates on Mars (Kounaves et al. 2014).
The Phoenix Mars lander detected perchlorate anions in amounts close to 0.5% by weight on the Martian surface (Hecht et al. 2009; Kounaves et al. 2010b). The Mars Science Laboratory, using Sample Analysis at Mars, has also detected perchlorate (Archer et al. 2013). Another relevant feature of Mars is the presence of large permafrost layers, which are covered by regolith layers comparable to terrestrial gelisols (Certini et al. 2020).
Having terrestrial perchlorate as an analog has led to some hypotheses suggesting that Martian perchlorate is formed from the oxidizing chemistry of a dry environment. The oxidizing power is in the atmosphere (Hunten 1979). On the other hand, cosmic ray action can produce superoxide (O2−) free radicals (Yen et al. 2000). Chlorine would be of volcanic origin during intense volcanism on Mars (Hartmann and Neukum 2001). Furthermore, due to the red planet’s cold and dry environment, perchlorate would have accumulated on the surface due to photochemical reactions (Kounaves et al. 2010b). These perchlorates represent a fundamental point of convergence between astrobiology and Antarctica as an analog ecosystem of Mars.
Anthropogenic origin
Perchlorate, with its high oxidation potential, has been synthesized for use in various applications, including rocket fuels, explosives, fireworks, airbags, ammunition, and signal flares. This has resulted in its use in military industries, pyrotechnics, and in the manufacture of fertilizers for agricultural use (Cao et al. 2019). The effects of this pollutant is directly proportional to its concentration from anthropogenic sources, where concentrations of 0.1–35.0 μg/L in fresh water (Blount et al. 2010), 0.1–22.1 μg/L in groundwater, 0.0–2,300 μg/L in surface water (Wu et al. 2010), and 1.0–13 μg/kg in soil (Jackson et al. 2010) can be found. The presence of ClO4− in groundwater is considered to be due to runoff via sand or soil.
A considerable percentage of ClO4− in surface water comes from the military industry (Cao et al. 2019). In the same way, the aerospace industry (Murray and Bolger 2014; Kumarathilaka et al. 2016) contributes large concentrations of ClO4− to ecosystems with rocket launches, where the heat generated causes all of the ClO4− to be used in the reaction. Furthermore, various studies report the presence of ClO4− residues from fireworks in surface and underground water (Backus et al. 2005).
The anthropogenic factor could be linked to the use of household detergents in Antarctica, taking into account that the expeditions are carried out on ships or at scientific bases in different countries. Hypochlorite solutions are characterized by having traces of ClO4− (Aranda-Rodriguez et al. 2017).
Effects of CIO4 − on the environment and on health
The extensive application of ClO4− in human activities has distributed it in aquatic and terrestrial ecosystems, and in the food chain (Li et al. 2022). Since it is an emerging pollutant, the information available on its impact on the environment is scarce. Therefore, it is necessary to carry out more studies to determine its effects on biota, considering that its toxicity varies depending on the species (Acevedo-Barrios and Olivero-Verbel 2021).
ClO4− has generated interest among investigators for being a potent endocrine disruptor (Acevedo-Barrios and Olivero-Verbel 2021) that affects thyroid gland function (Li et al. 2022). For instance, the thyroid gland in humans (represented in Fig. 1a) produces two main forms of “iodothyronines”: thyroxine (3,5,3’,5’-tetra-iodo-L-thyronine, T4) and triiodothyronine (3,5,3’-tri-iodo-L-thyronine, T3) (Koibuchi 2018). These hormones are necessary for both adult physiology and appropriate development. Together with distributor proteins (transthyretin and thyroxine binding-protein), these two forms of thyroid hormone circulate in the bloodstream (Zoeller 2021). Then, they are transported to target cells, where they will play a key role in the development and functional maintenance of many organs (Koibuchi and Chin 2000).
(a) Synthesis of thyroid hormones T3 (triiodothyronine) and T4 (thyroxine). a Thyroid gland in humans, b Thyroid follicle, c The accumulation of I- is mediated by the Na + /I- symporter (NIS), driven by the Na + gradient produced by the Na + /K + ATPase. When I- effluxes towards the colloid, thyroid peroxidase (TPO) catalyzes the organization of I- on the thyroglobulin (TG) molecule. The thyroid hormones are released into the bloodstream after the iodinated-TG is proteolyzed following endocytosis. d Endocrine disruption associated with ClO4− in thyrocytes. This compound inhibits the uptake of iodine, thereby blocking its entry into thyroid follicular cells
Thyroid follicles, which are made up of a layer of follicular epithelial cells (sometimes referred to as thyroid follicular cells or thyrocytes) encircling a follicular lumen, are the special structure within the thyroid gland where thyroid hormone is generated (Fig. 1b). The production of thyroid hormone in the follicular lumen, rather than within the follicular epithelial cells, is a noteworthy characteristic of the thyroid hormone synthetic pathway. Outside the cell, no additional hormone is generated (Koibuchi 2018).
The synthesis of T4 and T3 occurs in the follicular cell. Iodide is actively transported into the thyroid follicular cells via the sodium (Na+)/iodide (I−) symporter (NIS) located at the basolateral membrane (Dohán et al. 2003). NIS-mediated I− uptake in the thyroid is the initial step in the production of the iodine-containing thyroid hormones, which are crucial in the early stage of life for suitable Central Nervous System (CNS) formation, with an electrogenic stoichiometry of 2 Na+ per I−. The electrochemical gradient that is required for the iodide transport is produced by the sodium–potassium (Na+/K+) ATPase. After reaching the apical membrane, pendrin, an anion exchanger, and maybe anoctamin, allow for iodide to be released into the follicular lumen (Wémeau and Kopp 2017). Thyroperoxidase (TPO) oxidizes iodide in the follicular lumen when Hydrogen Peroxide (H2O2) is present (Ohye and Sugawara 2010).
Thyroglobulin (TG) primarily forms the colloid in the follicular lumen. Firstly, there is an organification step in which TPO iodinates certain tyrosyl residues on TG to create mono- and diiodotyrosines (MIT, DIT; respectively). TPO, then, couples the iodotyrosines to form T4 and T3. Thyroid hormones are released into the bloodstream, at least in part, through the thyroid hormone-transporting channel MCT8, after TG is internalized by pinocytosis from the follicular lumen into the follicular cell 6 (Visser et al. 2011). T4 makes up over 80% of the hormone released, whereas T3 makes up 20%. The liberated iodide is then recycled back into the follicular lumen for the creation of thyroid hormones (Moreno et al. 2008) (Fig. 1c).
The presence of ClO4− can cause disruption of the thyroid gland, since this compound can obstruct or compete with iodine for transport through NIS into the thyroid; effectively blocking iodine from entering the thyroid follicular cells through circulation. This may inhibit the thyroid's ability to absorb iodine and lower the production of thyroid hormones, both of which have been related to hypothyroidism in organism (Kirk 2006). Iodide transport is inhibited at low ClO4− concentrations by altering the stoichiometry of iodide transfer, which reduces the driving force (Kumar et al. 2023).
Electrophysiological analyses showed that perchlorate ions attach to a sodium symporter allosteric region that is non-transport, hindering the binding of Na+/ I− ions (Llorente-Esteban et al. 2020) (Fig. 1d). In addition to the thyroid tissues, the cerebrovascular plexus, eyes, lactate, and salivary glands all have sodium/iodide symporters. NIS is also found in the heart, kidneys, and lungs in trace levels (Dohán et al. 2007). These tissues, in contrast to the thyroid, do not metabolize iodine; instead, it is reabsorbed into the bloodstream, milk, and saliva (Spitzweg et al. 1999). Other effects associated with perchlorate in humans and other species will be detailed further below.
Effects on human health
Human beings intake contaminated water and food allowing ClO4− to enter into the digestive system; this is the main route of ClO4− exposure (Ali et al. 2021). It can also enter the respiratory system if it is inhaled from dust contaminated with ClO4− (Li et al. 2022). After it enters the body, it is absorbed by the digestive system and sent to the bloodstream, where it inhibits the fixation of iodine in the thyroid gland. Changes in this process hinder what causes hypothyroidism (Li et al. 2022). Numerous studies have investigated the association between exposure to ClO4− in the environment and alterations in the production of T3, T4, and TSH thyroid hormones. In addition, ClO4− increases the risk of cardiovascular diseases; it also affects the nervous, reproductive, and immune systems (Pleus and Corey 2018). ClO4− is a neurotoxic capable of inducing teratogenesis in pregnant women.
The presence of ClO4− has been detected in adult and infant biological samples such as urine, saliva, breast milk, maternal blood, and in the umbilical cord (Pleus and Corey 2018; Ali et al. 2021; Li et al. 2022).
ClO4− can induce teratogenesis during the first trimester of pregnancy (Acevedo-Barrios et al. 2018; Acevedo-Barrios and Olivero-Verbel 2021). High concentrations of ClO4− can affect the metabolism, development, and reproduction of unborn babies, as it causes a decrease in the transfer of thyroid hormones from the placenta to the fetus (Ali et al. 2021). Damage to the DNA of testicular tissues occurs in men, which leads to a reduction in testicular spermatogenesis (Ali et al. 2021). It can damage the skeletal system and the central nervous system of newborns and children, leading to learning problems, intellectual deficiencies, and physical disabilities (Ye et al. 2012; Acevedo-Barrios and Olivero-Verbel 2021).
Effects on the biota
ClO4− enters living beings through trophic transfer, passing from soil to higher organisms through plants, due to the application of chlorine-based fertilizers or irrigation water contaminated with ClO4− (Constantinou et al. 2019). The majority of vascular plants are able to rapidly absorb and accumulate ClO4− in their plant tissues, mainly in their leaves, affecting their normal development and growth (Acevedo-Barrios and Olivero-Verbel 2021; Oze et al. 2021).
Furthermore, ClO4− reduces the plant’s leaf area and levels of dry shoot biomass, directly due to its toxicity, or indirectly by modifying the release and/or availability of soil nutrients (Oze et al. 2021). A study by He et al. (2013) found that ClO4− affects the root systems and the chlorophyll content of the Acorus calamus, Canna indica, Thalia dealbata, and Eichhornia crassipes species (He et al. 2013). It can also alter the biomass ratio in different organs of Alternanthera philoxeroides, thus increasing its proportion in the stem, and decreasing it in the leaf (Xie et al. 2009).
Potassium perchlorate (KClO4−), on the other hand, affects the bioluminescence of the Vibrio fischeri bacterium, alters the homeostasis of the Pseudokirchneriella subcapitata algae, and can cause endocrine disruption in the Daphnia magna crustacean. In the Eisenia fetida earthworm, it causes severe weight loss, alterations in the central nervous system, teratogenesis, and mucus production (Acevedo-Barrios et al. 2018). In pregnant female rats, ClO4− causes hypothyroidism, and, in male rats, it causes DNA damage and reduced sperm production (Yu et al. 2019). It decreases the number of spawned eggs in the Oryzias latipes fish, and it causes functional hermaphrodism in Gasterosteus aculeatus females. The effects in fish, in general, include affectation of the normal development of their lateral plates, decrease in swimming, reduction in pigmentation, and slow growth rates (Acevedo-Barrios and Olivero-Verbel 2021).
Concentrations (1.6 ± 1.1 μg/kg) of ClO4− have been found on the Fildes Peninsula in Antarctica. This peninsula has high levels of wildlife activity, especially colonies of penguins, skuas, and seals (Jiang et al. 2021). The presence of this pollutant has also been determined in seawater, algae, and Antarctic fish, considering that they function as food for wildlife. They could have the pollutant in their body (Jiang et al. 2021). However, to date, there are no studies on the effect of ClO4− on Antarctic fauna (Acevedo-Barrios and Olivero-Verbel 2021). Table 2 outlines the effects associated with some organisms.
Physicochemical methods to determine the origin of CIO4 −
The determination of the origin of ClO4− is associated with the sources of exposure; therefore, the methods to identify ClO4− show whether it is present in the sample to be evaluated. The origin is studied by evaluating the possible exposure to this pollutant, which can be present both in food and in drinking water, as well as in other environmental matrixes (Dong et al. 2019).
Selective ClO4 − electrode
An ion-selective membrane (ISM) is used for this technique, which contains the ionophore responsible for the selectivity of the electrode (Itterheimová et al. 2022). One of the most frequently used inophores is Dodecabenzylbambusuril (Bn12BU[6]), because it binds very strongly to the ion ClO4− ion [stability constant ~ 1010 /M]), due to the good ratios between the size of the ion and the receptor cavity (Itterheimová et al. 2022). The electrode is rinsed with distilled water before use and calibrated depending on the quantities to be measured in ppm. Likewise, it is rinsed between measurements, to avoid dragging and contamination of the samples (Acevedo-Barrios et al. 2022a, b).
Liquid chromatography tandem mass spectrometry (LC–MS/MS)
This technique has an analytical power that combines chromatography— either liquid or gas—as a separation method, and mass spectrometry as a method of detection, identification, and quantification of compounds. MS is characterized by being highly sensitive; it uses the ionization and separation of molecules, and it records the ions produced in a vacuum system. MS has also been used for the quantification of trace amounts of ClO4− in aqueous matrixes: in human urine and saliva samples, in food, soil, and water. (Kannan et al. 2009; Kumarathilaka et al. 2016).
UHPLC-Q-orbitrap HRMS (ultra high-performance LC-Q-orbitrap high resolution mass spectrometry) method
This method has been used for the nutritional investigation of the quality of goat’s milk contaminated with sodium perchlorate (NaClO4−). This untargeted metabolomics and proteomics method based on UHPLC-Q-Orbitrap HRMS for metabolic pathways and biological processes involved in animal enzymes is used to study the composition and concentrations of the metabolite molecules, with the aim of determining the presence of the pollutant. Furthermore, statistical methods are used to analyze the samples, where the significant metabolites become discriminated by a sample treatment process, in such a way that the amount of milk contaminated with NaClO4− can be determined (Jia et al. 2022).
Technologies for the remediation of CIO4 −
ClO4− is not easily degradable in the environment; therefore, its use in fertilizers, irrigation, and water treatment allows leads to its persistence in food and drinking water (Xie et al. 2018). Over the years, several important technologies have been introduced to remove or reduce ClO4− in water and soil (Ye et al. 2012). These methods include filtration by membrane, biodegradation, chemical reduction, electrochemical reduction, bioelectrochemical reduction, and ion exchange (IX) (Ye et al. 2012). ClO4− remediation technologies are classified into four groups: Physical–chemical treatments, chemical treatments, integrated or combined technologies, and biological treatments (Ye et al. 2012; Xie et al. 2018; Acevedo-Barrios and Olivero-Verbel 2021).
Halophilic bacteria isolated from hypersaline soils have the Perchlorate reductase and Superoxide chlorite enzymes. The perchlorate reductase enzyme is responsible for reducing perchlorate to chlorate, and chlorate to chlorite, while the superoxide chlorite enzyme reduces chlorite to Cl− and molecular oxygen. Figure 2 describes the perchlorate degradation pathway (Acevedo-Barrios et al. 2019, 2022b). The biological reduction of perchlorate is regulated by environmental factors such as pH, temperature, salt concentration, presence of metabolic inhibitors or electron acceptors, nutritional conditions, and contact time; also due to physiological factors, such as type and physiological age of the bacteria, concentration of cellular biomass, and mutations (Ting et al. 2008; Acevedo-Barrios and Olivero-Verbel 2021). Phenotypic characterization studies have shown that perchlorate-reducing bacteria exhibit a wide range of metabolic capabilities and can thrive in diverse and extreme conditions. Although these technologies are effective, it is necessary to continue carrying out studies that allow for improving the reduction and complete elimination of ClO4− in ecosystems (Ye et al. 2012).
Physicochemical methods
These are the most widely used technologies for the elimination of ClO4−. They include treatments such as activated carbon, membrane filtration, ion exchange (IX), precipitation, and chemical and electrochemical reduction (Ye et al. 2012; Xie et al. 2018). When using these methods, it must be considered that, although physicochemical treatments have advantages such as low cost, easy operation, and high treatment efficiency, they have the disadvantage of being non-selective. They can generate regenerative brines or reject streams with high concentrations of ClO4− that can affect the effectiveness of the process (Xie et al. 2018; Acevedo-Barrios and Olivero-Verbel 2021).
Activated carbon
Powdered or granulated activated carbon is the oldest and most widely used adsorbent (Xie et al. 2018). Its adsorption mechanism is based on surface complexation and electrostatic forces (Ye et al. 2012). The capacity of activated carbon to adsorb ClO4− is limited by the nature of the material, the pH of the contaminated water, and other inorganic/organic components with which it comes into contact, thus greatly affecting the chemistry of the surface and the porous structure of the adsorbent (Ye et al. 2012; Xie et al. 2018). The high efficiency of activated carbon is due to the presence of oxygenated functional groups such as: carboxylic, lactonic, and phenolic (Ye et al. 2012; Acevedo-Barrios and Olivero-Verbel 2021). In addition, its simplicity of operation, profitability, and regenerative nature of the adsorbents (Baimenov et al. 2020), which can be of various types (cellulose nanofibers, biocarbon, carbon nanotubes, modified activated carbon, among others) (Shahrokhi-Shahraki et al. 2021). It is important to mention that of the adsorbents, activated carbon has distinctive characteristics (high pore volume and large surface area) that make it a valuable adsorbent for the removal of many contaminants, such as heavy metals (Shahrokhi-Shahraki et al. 2021), it also increases the efficiency in the absorption of perchlorate and other types of endocrine disrupting contaminants (Shahrokhi-Shahraki et al. 2021).
ClO4− is a typical anion, and it is considered to be an emerging contaminant. Among the strategies for its removal from the environment, especially from water, is the use of activated carbon, which can be modified with various functional groups. In research carried out on the adsorption of ClO4− on raw and oxidized carbon nanotubes, to elucidate the affinity mechanism of these with anionic contaminants, it was demonstrated that the adsorption of ClO4− was promoted by oxidized double-walled carbon nanotubes (DWCNT), due to the introduction of more oxygen-containing functional groups, which served as additional adsorption sites, thus, the adsorption mechanism of ClO4− depends on the pH and on the adsorbent (mainly on the oxygen- and nitrogen-containing groups) (Fang and Chen 2012).
Other studies suggest that the adsorption of ClO4− occurs, at least partially, through specific interactions of ClO4− with surface functional groups, so that specific chemical interactions between these, in combination with electrostatic forces, are the main mechanism for the adsorption of ClO4− on activated carbon (Mahmudov and Huang 2010). Furthermore, it has been shown that treating granular activated carbon with acids can improve its ClO4− removal capacity in water. Several studies have examined the characteristics of acid-treated granular activated carbons, which have made it possible to define the effects of various adsorption parameters on their efficiency, taking into account contact time, the initial concentration of ClO4−, the pH of the solution, temperature, and the presence of coexisting anions on its efficiency (Krishnan G. et al. 2017). These findings highlight the importance of different functional groups in activated carbon in elucidating the adsorption mechanisms of environmental pollutants (Mahmudov and Huang 2010; Byrne et al. 2014; Yao et al. 2017).
Membrane filtration
Membrane filtration one of the most widely used methods— it can be divided into reverse osmosis (RO), which is based on the use of a semipermeable filtering membrane that generates this phenomenon in order to retain all the total dissolved solids TDS existing in the water; purified water collection and storage tanks; ultrafiltration (UF) and nanofiltration (NF), techniques activated through the use of pressure, in which solutes of different molecular weights are separated from the solution (Muñi et al. 2005); and, finally, electrodialysis, a separation process that uses selective ionic membranes, enabling the separation of charged species present in an electrolyte solution by applying an electric current in a direction perpendicular to the membranes (Mier López et al. 2004; Ye et al. 2012; Xie et al. 2018; Hu et al. 2021).
In RO, NF, and UL, the ClO4− is selectively eliminated through a membrane, and, in the case of electrodialysis, the ions that are dissolved in water move to both ends of the polymeric membrane, while they are under the effect of an electric field (Hu et al. 2021). The diffusion of this pollutant by any of the membrane filtration methods increases with decreasing pH, mainly because the charge on the membrane surface increases its negativity, which in turn increases the electrostatic repulsion dynamics that occur between ClO4− and these electronegative membranes (Ye et al. 2012). Therefore, the optimum pH to carry out the reduction or elimination of ClO4− is 4.3, with which it is possible to obtain removal results of approximately 92% with adsorbent doses and ClO4− concentrations of 0.8 g/L and 10 mg/L, respectively (Hu et al. 2021). Reverse osmosis treatment is essential to eliminate chlorates and perchlorates in water for food use, which guarantees the absence of salts. However, to improve the effectiveness of this technology, it is necessary to pre-treat the water that includes filtration, ultrafiltration, dosing system, using a filter with a diameter of less than 5 microns (Eisenberg and Middlebrooks 2013); which will improve the percentage reduction of perchlorate in the treated water (Ye et al. 2012).
Ion exchange
One of the most widely used technologies for the removal of ClO4− in drinking water is ion exchange (IX), which is one of the most effective technologies when working with small amounts (Xie et al. 2018). The method is based on anion exchanges that are achieved through the loading of an immobilized functional group (Ye et al. 2012). The efficient removal of the pollutant with this method is affected by factors such as cation exchange capacity, regeneration capacity, and stability, apart from the choice of resin (Hu et al. 2021). Conventional resins, such as IRA-900, have a slow removal rate. On the other hand, if highly selective resins are used, this removal/reduction rate is increased, and the advantage is that the chemistry of the water is not changed by adding nutrients or chemical compounds during the treatment process (Ye et al. 2012).
Additionally, conventional resins are usually composed of a polymer matrix to which a large number of polar radicals, acids, or bases have been attached, while highly selective resins can be chelating resins or hybrid technologies (Hu et al. 2021). Conventional ones may have a larger particle size, while highly selective resins may have a more homogeneous structure with only micropores within the sites. This can influence the kinetics and operating capacity of the resins (Alexandratos 2009). Therefore, conventional resins are more suitable for general applications, while highly selective resins are more specialized and can adapt to different types of water and wastewater (Ye et al. 2012).
Precipitation
Several analytical chemistry techniques have been used for reduction of the ClO4− anion with large organic cations. Among them, we find the use of tetraphenylarsonium chloride combined with conductimetry, potentiometry, and gravimetry, for which it became an important method for the preparation of various porous oxide materials, with good results (Lu et al. 2013). However, the ClO4− present in aqueous solutions is reduced because, in this technique, concentrating it in others has disadvantages, such as an increase in costs due to the implementation of filters, the production of moisture and sludge, and an increase in waste in need of disposal (Acevedo-Barrios and Olivero-Verbel 2021).
Chemical reduction
Metals, such as iron, are used for this method. At high temperatures and pressures, and an acidic pH, they can reduce considerable concentrations of ClO4− to chloride. Titanium and transition metals, such as molybdenum, ruthenium, and vanadium, are used in the same way. Chemical reduction poses a problem because no typical reducer is capable of reducing ClO4− up to typical environmental concentrations, the reduction reaction is slow, and the implementation of this technique is limited by economic and technical factors (Acevedo-Barrios and Olivero-Verbel 2021).
Electrochemical reduction
Electrochemical reduction reactions can be conducted without catalysts (Ye et al. 2012), where the ClO4− ions that are dissolved in acidic aqueous solutions are reduced to Cl − ions. The preferred materials for electrodes are ruthenium, tungsten, iridium, platinum, rhodium, technetium, and rhenium (Acevedo-Barrios and Olivero-Verbel 2021). The main disadvantage of this technology is electrode erosion (Ye et al. 2012), in addition, the reactions require large surfaces and are very slow, apart from being affected by the presence of other substances in the solution (Acevedo-Barrios and Olivero-Verbel 2021).
Biological treatment of ClO4 −
Biodegradation is possible because native soil and groundwater bacteria have special enzymes that can lower the activation energy required for the biological reduction of ClO4− (Ye et al. 2012; Song et al. 2017). This pollutant manages to be microbiologically reduced to chloride (Cl −) under anaerobic conditions, because its high reduction potential (E° = 1.287 V) makes it a good electron acceptor (Ye et al. 2012; Acevedo-Barrios et al. 2022b).
The key factors to conduct bioremediation or biodegradation of the soil are the absence of oxygen, the presence of an electron donor, an optimal pH value (~ 7), and, when an unsaturated zone is to be remedied, enough liquid content to promote the growth of bacteria (Xie et al. 2018). However, these conditions are difficult to maintain in deep unsaturated zones or when performing in situ soil remediation. These techniques include phytoremediation, which involves the use of plants and trees to accumulate and remove perchlorate from groundwater and soils (Sijimol et al. 2015), where ClO4− is degraded through its absorption and accumulation in plants and microorganisms in their rhizosphere (Xie et al. 2018).
The cost required to implement these technologies is relatively low, and they are suitable for treating large-scale wastewater and soil areas highly contaminated with ClO4−. However, the bioremediation rate is relatively slow, the metabolic products of microorganisms can lead to secondary contamination, and, to be used in drinking water treatment, they must have very strict operating conditions and a somewhat long operating cycle, without considering the health effects associated with these microorganisms (Ye et al. 2012; Xie et al. 2018).
Antarctic ClO4 − Reducing Bacteria
ClO4− can be present in various environmental matrixes thanks to its high solubility and stability (Acevedo-Barrios et al. 2022a, b). In arid and semi-arid areas, such as Antarctica or the desert of Atacama in Chile (Jackson et al. 2015), traces of the pollutant can be found in soils and their sediments, or in surface and groundwaters (Jia et al. 2022). The presence of ClO4− in marine sediments, seawater, Antarctic soil, and other areas, has allowed and increased the growth of bacteria that can naturally tolerate and degrade this pollutant (Acevedo-Barrios et al. 2022b).
Characteristics of the Bacteria
These bacteria with the potential to reduce ClO4− are anaerobic or facultative microaerophilic. They are phylogenetically diverse, and the most detected class is Betaproteobacteria; Gammaproteobacteria, Deltaproteobacteria, Alphaproteobacteria (Acevedo-Barrios and Olivero-Verbel 2021), and Psychobacteria, as can be seen in Table 3 (Acevedo-Barrios et al. 2022b). The bacteria collected from Deception Island can grow in heterotrophic aerobic conditions at 4 °C, and presented positive catalase and negative oxidase activity, apart from having positive reactions for N-acetylglucosaminidase and nitrate reduction capacity (Acevedo-Barrios et al. 2022b).
Habitat of the Bacteria
The habitat of these microorganisms is extreme, with limited bacterial diversity. They have high salt concentrations, low nutrient availability, and a variable ionic composition (González Díaz and Mira Peinado 2021). Reducing perchlorate bacteria can grow in different ecosystems, including extreme conditions, such as hypersaline and hyperthermophilic soils, saline lakes, and even hot springs (Acevedo-Barrios et al. 2022b). They develop in environments with neutral pH, and one of their energy sources is yeast, which stimulates their growth. Their nutrition is variable due to their presence in different ecosystems, and the temperature zones in which they can develop are mesophilic and thermophilic (González Díaz and Mira Peinado 2021; Acevedo-Barrios et al. 2022b).
Metabolism of the Bacteria
These microorganisms use two enzymes that can lower the activation energy required to reduce ClO4− (Acevedo-Barrios et al. 2019), making it a valuable electron acceptor for different types of bacteria. The mechanism used by these microorganisms to reduce the concentration of the pollutant depends on its reduction to chlorite (ClO2−) in the bacterial periplasm (Melnyk and Coates 2015). Using the perchlorate reductase enzyme that reduces perchlorate to chlorate and chlorate to chlorite, then using the chlorite superoxide enzyme (Acevedo-Barrios et al. 2019), it is dismutated from chlorite to chloride and molecular oxygen. This biogenic oxygen produced as an intermediate is not released, but it is rather reduced by the microorganism itself (Melnyk and Coates 2015). The biological reduction route of perchlorate can be seen in Fig. 2 (Acevedo-Barrios et al. 2022b).
The main application of these extremophilic bacteria is for biotransformation, and, with this method, the ClO4− is reduced in a less toxic way and helps with the sanitation of environments contaminated with it. With this method, the “in-situ” and “ex-situ” bioremediation of ecosystems are carried out, and, in this sense, it can be used to treat unsaturated areas and soils (Acevedo-Barrios and Olivero-Verbel 2021).
Integrated or combined technologies
The combinations of different technologies provide greater flexibility and degradation efficiency during the reduction or elimination of ClO4− (Xie et al. 2018). An example of these integrated techniques is the chemical reduction of ClO4− using H2 by heterogeneous catalysis, combined with activated carbon powder, which has good adsorption capacity and is used as a support medium (Ye et al. 2012). This technique requires further research before being applied because it is in the exploration stage (Xie et al. 2018).
Environmental fate
The course of ClO4− is directly associated with its source, and it is understood that this would be a factor of variability when identifying its environmental destination. Stable isotope techniques (180/160 and 170/160; 32Cl/35Cl) help identify natural ClO4− and anthropogenic ClO4− (Kumarathilaka et al. 2016).
Most of the natural sources of ClO4− are found in arid and desert areas on Earth, which is related to the fact that the mobilization of groundwater is restricted by the net infiltration of precipitation in these environments, together with a higher percentage of evapotranspiration, which results in a greater accumulation of ClO4−. An example of this is the Atacama Desert in Chile, which is also known as one of the largest sources of nitrate, where electrostatic discharges with oxidants such as ozone O3, electrostatic discharges, and gas–solid reactions with oxidants followed by dry deposition led to the presence of ClO4− in this ecosystem, as cited in Kumarathilaka et al. (2016). This same scheme is also suggested for other arid zones.
The atmospheric concentration of perchlorate and chlorate found in Antarctic lakes is 1,100 ug/kg, and perchlorate salts have been reported in kelp forests and Antarctica (Kounaves et al. 2010b; Kumarathilaka et al. 2016). Furthermore, the concentration of perchlorate in different ecosystems is associated with anthropogenic sources, which causes the environmental fate to vary or be different from the perchlorate cycle shown in Fig. 3. In the cycle of ClO4− formation through the atmospheric photochemistry in the Atacama Desert, the difference is mainly in the origin of the source, which changes the environmental fate cycle of ClO4− (Fig. 4).
Astrobiological perspectives
The presence of perchlorates, possible liquid water, low temperatures, and the ability of some terrestrial microorganisms that resist these conditions make Mars a potentially habitable planet (Schirmack et al. 2015). Considering that various Antarctic organisms, such as Halorubrum lacusprofundi, are tolerant to perchlorate, being inhibited only at higher concentrations than those detected on Mars, then, the development of organisms similar to this haloarchaea in the Martian environment would be possible (Laye and DasSarma 2018).
Haloarchaea are relevant extremophiles in astrobiology, as they survive in different planetary environments and are critical to detecting life in situ. Their study addresses the origin of life, biochemical capabilities, and pigments as possible biosignatures. Investigation of these extremophiles in laboratories, and by future space telescopes, could provide valuable answers on the existence of life in the universe (DasSarma et al. 2020). In addition, research on other perchlorate-reducing microorganisms, such as Azospirillum brasilense USD2, provides insights into Mars's terraforming and future astronauts’ well-being (Sunilkumar and Lal 2021). These findings open new questions on the habitability of other planets and life in the universe, particularly considering that the presence of perchlorates can form cryo-brins, making water able to remain liquid due to the presence of salts (Cottin et al. 2017).
The incidence of cosmic rays on the Martian surface, with the presence of perchlorates, could release oxygen (O2) (Quinn et al. 2013). Reaching, in calcium and magnesium perchlorate brines, even levels close to those currently found in Earth's oceans, being the possible concentration of O2 on Mars, today, higher than the one that was present in the great moment of oxidation and allowed aerobic life on Earth. Perchlorate is an opportunity for the potential habitability of aerobic life on Mars (Stamenković et al. 2018).
Conclusion
ClO4− is a toxic pollutant for humans, biota, and the environment, found in different ecosystems, and its origin can be anthropogenic or natural (Acevedo-Barrios et al. 2022a). Because of its high solubility, its presence in groundwater or surface water classifies it as a pollutant of increasing relevance (Cao et al. 2019). Its main effect on health is its potential as an endocrine disruptor, since it attacks the thyroid cells (Ali et al. 2021). It is imperative to continue delving into the mechanism of endocrine disruption at the molecular level of ClO4− and its toxicity in other unstudied biological models.
There are many types of technologies for reducing ClO4−, which are classified into four groups: Physical–chemical treatments, chemical treatments, integrated or combined technologies, and biological treatments (Ye et al. 2012; Xie et al. 2018; Acevedo-Barrios and Olivero-Verbel 2021). The four groups (physical–chemical treatments, chemical treatments, integrated or combined technologies, and biological treatments) can be effectively combined to treat perchlorate pollution in Antarctica and worldwide; generating hybrid technologies, to obtain a balance between their cost and efficiency (Fang and Naidu 2023).
However, biological methods are the most promising, because it does not generate waste that could be environmentally dangerous, and, therefore, do not generate cost overruns (Acevedo-Barrios and Olivero-Verbel 2021). The extremophile bacteria used in this method belong to the Betaproteobacteria, Gammaproteobacteria, Deltaproteobacteria, Alphaproteobacteria, and Psychobacteria classes (Acevedo-Barrios and Olivero-Verbel 2021; Acevedo-Barrios et al. 2022b).
Within environmental fate specifications, it has been determined that it can vary according to the perchlorate’s sources of origin, which can be natural or anthropogenic (even combined). Likewise, this pollutant has a natural environmental fate regulated by the photochemical formation of the atmosphere and an anthropogenic environmental fate, because of industrial and domestic activities.
In conclusion, Antarctica is an excellent ecosystem to isolate perchlorate-reducing bacteria, which may also have great astrobiological potential. However, studies and existing bibliographic information are limited, so it is necessary to conduct more research to elucidate the biological reduction mechanisms of this pollutant in strategic ecosystems such as Antarctica (Acevedo-Barrios et al. 2022b). Research points to the development of new technologies to eliminate perchlorate contamination from ecosystems, which requires the combination of various technologies with ionic exchange and bioremediation, to remove perchlorate from the environment (Acevedo-Barrios and Olivero-Verbel 2021).
Data availability
Due to the nature of the research, the authors confirm that the data supporting the fundings of this study are available within the article.
References
Acevedo-Barrios R, Sabater-Marco C, Olivero-Verbel J (2018) Ecotoxicological assessment of perchlorate using in vitro and in vivo assays. Environ Sci Pollut Res 25:13697–13708. https://doi.org/10.1007/s11356-018-1565-6
Acevedo-Barrios R, Bertel-Sevilla A, Alonso-Molina J, Olivero-Verbel J (2019) Perchlorate-Reducing bacteria from hypersaline soils of the colombian caribbean. Int J Microbiol 2019:1–13. https://doi.org/10.1155/2019/6981865
Acevedo-Barrios R, Rubiano-Labrador C, Miranda-Castro W (2022a) Presence of perchlorate in marine sediments from Antarctica during 2017–2020. Environ Monit Assess 194:102. https://doi.org/10.1007/s10661-022-09765-4
Acevedo-Barrios R, Rubiano-Labrador C, Navarro-Narvaez D et al (2022b) Perchlorate-reducing bacteria from Antarctic marine sediments. Environ Monit Assess 194:654. https://doi.org/10.1007/s10661-022-10328-w
Acevedo-Barrios R, Olivero-Verbel J (2021) Perchlorate Contamination: Sources, Effects, and Technologies for Remediation. In: de Voogt P (ed) Reviews of Environmental Contamination and Toxicology Volume 256. Springer International Publishing, Cham, pp 103–120. https://doi.org/10.1007/398_2021_66
Acevedo-Barrios RL, Hernández Rocha I, Puentes Martinez D, et al (2023) Psychrobacter sp: perchlorate reducing bacteria, isolated from marine sediments from Margarita Bay, Antarctica. LACCEI 1. https://doi.org/10.18687/LACCEI2023.1.1.995
Acevedo-Barrios R, Tirado-Ballestas I, Bertel-Sevilla A, et al (2024) Bioprospecting of extremophilic perchlorate-reducing bacteria: report of promising Bacillus spp. isolated from sediments of the bay of Cartagena, Colombia. Biodegradation. https://doi.org/10.1007/s10532-024-10079-0
Alexandratos SD (2009) Ion-exchange resins: a retrospective from industrial and engineering chemistry research. Ind Eng Chem Res 48:388–398. https://doi.org/10.1021/ie801242v
Ali MM, Khater SA, Fayed AA et al (2021) Apoptotic endocrinal toxic effects of perchlorate in human placental cells. Toxicol Rep 8:863–870. https://doi.org/10.1016/j.toxrep.2021.04.002
Andrew Jackson W, Kumar Anandam S, Anderson T et al (2005) Perchlorate occurrence in the Texas Southern High Plains Aquifer System. Groundw Monit Remediat 25:137–149. https://doi.org/10.1111/j.1745-6592.2005.0009.x
Aranda-Rodriguez R, Lemieux F, Jin Z et al (2017) (Yet more) challenges for water treatment plants: potential contribution of hypochlorite solutions to bromate, chlorate, chlorite and perchlorate in drinking water. J Water Supply Res Technol-Aqua 66:621–631. https://doi.org/10.2166/aqua.2017.147
Archer PD, Sutter B, Ming DW, et al (2013) Possible Detection of Perchlorates by Evolved Gas Analysis of Rocknest Soils: Global Implication. The Woodlands, TX. https://ntrs.nasa.gov/citations/20130009133
Backus SM, Klawuun P, Brown S et al (2005) Determination of perchlorate in selected surface waters in the Great Lakes Basin by HPLC/MS/MS. Chemosphere 61:834–843. https://doi.org/10.1016/j.chemosphere.2005.04.054
Baimenov A, Berillo DA, Poulopoulos SG, Inglezakis VJ (2020) A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv Colloid Interface Sci 276:102088. https://doi.org/10.1016/j.cis.2019.102088
Bardiya N, Bae JH (2011) Dissimilatory perchlorate reduction: a review. Microbiol Res 166:237–254. https://doi.org/10.1016/j.micres.2010.11.005
Blount BC, Özpinar A, Alwis KU et al (2008) Perchlorate, nitrate, thiocyanate, and iodide levels in chicken feed, water, and eggs from three farms. J Agric Food Chem 56:10709–10715. https://doi.org/10.1021/jf8018326
Blount BC, Alwis KU, Jain RB et al (2010) Perchlorate, nitrate, and iodide intake through tap water. Environ Sci Technol 44:9564–9570. https://doi.org/10.1021/es1025195
Brown GM, Gu B (2006) The chemistry of perchlorate in the environment. Perchlorate Environ Occur Interact Treat 17–47. https://doi.org/10.1007/0-387-31113-0_2
Bulaeva E, Lanctôt C, Reynolds L et al (2015) Sodium perchlorate disrupts development and affects metamorphosis- and growth-related gene expression in tadpoles of the wood frog (Lithobates sylvaticus). Gen Comp Endocrinol 222:33–43. https://doi.org/10.1016/j.ygcen.2015.01.012
Byrne TM, Gu X, Hou P et al (2014) Quaternary nitrogen activated carbons for removal of perchlorate with electrochemical regeneration. Carbon 73:1–12. https://doi.org/10.1016/j.carbon.2014.02.020
Calderon R, Palma P, Parker D et al (2014) Perchlorate levels in soil and waters from the Atacama Desert. Arch Environ Contam Toxicol 66:155–161. https://doi.org/10.1007/s00244-013-9960-y
Calderón R, Godoy F, Escudey M, Palma P (2017) A review of perchlorate (ClO4−) occurrence in fruits and vegetables. Environ Monit Assess 189:82. https://doi.org/10.1007/s10661-017-5793-x
Cao F, Jaunat J, Sturchio N et al (2019) Worldwide occurrence and origin of perchlorate ion in waters: A review. Sci Total Environ 661:737–749. https://doi.org/10.1016/j.scitotenv.2019.01.107
Certini G, Karunatillake S, Zhao Y-YS et al (2020) Disambiguating the soils of Mars. Planet Space Sci 186:104922. https://doi.org/10.1016/j.pss.2020.104922
Coates JD, Achenbach LA (2004) Microbial perchlorate reduction: rocket-fueled metabolism. Nat Rev Microbiol 2:569–580. https://doi.org/10.1038/nrmicro926
Coates JD, Achenbach LA (2006) The Microbiology of Perchlorate Reduction and its Bioremediative Application. In: Gu B, Coates JD (eds) Perchlorate: Environmental Occurrence, Interactions and Treatment. Springer US, Boston, MA, pp 279–295. https://doi.org/10.1007/0-387-31113-0_12
Cole-Dai J, Peterson KM, Kennedy JA et al (2018) Evidence of influence of human activities and volcanic eruptions on environmental perchlorate from a 300-year greenland ice core record. Environ Sci Technol 52:8373–8380. https://doi.org/10.1021/acs.est.8b01890
Constantinou P, Louca-Christodoulou D, Agapiou A (2019) LC-ESI-MS/MS determination of oxyhalides (chlorate, perchlorate and bromate) in food and water samples, and chlorate on household water treatment devices along with perchlorate in plants. Chemosphere 235:757–766. https://doi.org/10.1016/j.chemosphere.2019.06.180
Cottin H, Kotler JM, Bartik K et al (2017) Astrobiology and the Possibility of Life on Earth and Elsewhere…. Space Sci Rev 209:1–42. https://doi.org/10.1007/s11214-015-0196-1
Crawford TZ, Kub AD, Peterson KM et al (2017) Reduced perchlorate in West Antarctica snow during stratospheric ozone hole. Antarct Sci 29:292–296. https://doi.org/10.1017/S0954102016000705
Dasgupta PK, Martinelango PK, Jackson WA et al (2005) The origin of naturally occurring perchlorate: the role of atmospheric processes. Environ Sci Technol 39:1569–1575. https://doi.org/10.1021/es048612x
DasSarma S, DasSarma P, Laye VJ, Schwieterman EW (2020) Extremophilic models for astrobiology: haloarchaeal survival strategies and pigments for remote sensing. Extremophiles 24:31–41. https://doi.org/10.1007/s00792-019-01126-3
Dean KE, Palachek RM, Noel JM et al (2004) Development of freshwater water-quality criteria for perchlorate. Environ Toxicol Chem 23:1441–1451. https://doi.org/10.1897/02-648
Dohán O, De la Vieja A, Paroder V et al (2003) The Sodium/Iodide Symporter (NIS): Characterization, Regulation, and Medical Significance. Endocr Rev 24:48–77. https://doi.org/10.1210/er.2001-0029
Dohán O, Portulano C, Basquin C et al (2007) The Na+/I symporter (NIS) mediates electroneutral active transport of the environmental pollutant perchlorate. Proc Natl Acad Sci U S A 104:20250–20255. https://doi.org/10.1073/pnas.0707207104
Dong H, Xiao K, Xian Y et al (2019) A novel approach for simultaneous analysis of perchlorate (ClO4−) and bromate (Br O3−) in fruits and vegetables using modified QuEChERS combined with ultrahigh performance liquid chromatography-tandem mass spectrometry. Food Chem 270:196–203. https://doi.org/10.1016/j.foodchem.2018.07.091
Du Z, Xiao C, Furdui VI, Zhang W (2019) The perchlorate record during 1956–2004 from Tienshan ice core, East Asia. Sci Total Environ 656:1121–1132. https://doi.org/10.1016/j.scitotenv.2018.11.456
Eguchi A, Kunisue T, Wu Q et al (2014) Occurrence of perchlorate and thiocyanate in human serum from e-waste recycling and reference sites in Vietnam: association with thyroid hormone and iodide levels. Arch Environ Contam Toxicol 67:29–41. https://doi.org/10.1007/s00244-014-0021-y
Eisenberg TN, Middlebrooks EJ (2013) Reverse Osmosis Treatment of Drinking Water. Butterworth Publishers, Elsevier, Tennessee Technological University Cookeville, Tennessee
Estrada NL, Böhlke JK, Sturchio NC et al (2017) Stable isotopic composition of perchlorate and nitrate accumulated in plants: Hydroponic experiments and field data. Sci Total Environ 595:556–566. https://doi.org/10.1016/j.scitotenv.2017.03.223
Fang Q, Chen B (2012) Adsorption of perchlorate onto raw and oxidized carbon nanotubes in aqueous solution. Carbon 50:2209–2219. https://doi.org/10.1016/j.carbon.2012.01.036
Fang C, Naidu R (2023) A review of perchlorate contamination: Analysis and remediation strategies. Chemosphere 338:139562. https://doi.org/10.1016/j.chemosphere.2023.139562
Friedmann EI (1982) Endolithic Microorganisms in the Antarctic Cold Desert. Science 215:1045–1053. https://doi.org/10.1126/science.215.4536.1045
Furdui VI, Zheng J, Furdui A (2018) Anthropogenic Perchlorate Increases since 1980 in the Canadian High Arctic. Environ Sci Technol 52:972–981. https://doi.org/10.1021/acs.est.7b03132
Gholamian F, Sheikh-Mohseni MA, Salavati-Niasari M (2011) Highly selective determination of perchlorate by a novel potentiometric sensor based on a synthesized complex of copper. Mater Sci Eng C 31:1688–1691. https://doi.org/10.1016/j.msec.2011.07.017
Gilichinsky D, Wilson G, Friedmann E et al (2007) Microbial Populations in Antarctic Permafrost: Biodiversity, State, Age, and Implication for Astrobiology. Astrobiology 7:275–311. https://doi.org/10.1089/ast.2006.0012
González Díaz DE, Mira Peinado SC (2021) Caracterización de bacterias tolerantes a la sal reductoras de perclorato provenientes de la isla Media Luna, Antártida. https://utb.alma.exlibrisgroup.com/view/delivery/57UTB_INST/1217376980005731
Hartmann WK, Neukum G (2001) Cratering Chronology and the Evolution of Mars. In: Kallenbach R, Geiss J, Hartmann WK (eds) Chronology and Evolution of Mars. Springer Netherlands, Dordrecht, pp 165–194. https://doi.org/10.1007/978-94-017-1035-0_6
He H, Gao H, Chen G et al (2013) Effects of perchlorate on growth of four wetland plants and its accumulation in plant tissues. Environ Sci Pollut Res Int 20:7301–7308. https://doi.org/10.1007/s11356-013-1744-4
Hecht MH, Kounaves SP, Quinn RC et al (2009) Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix lander site. Science 325:64–67. https://doi.org/10.1126/science.1172466
Her N, Kim J, Yoon Y (2010) Perchlorate in dairy milk and milk-based powdered infant formula in South Korea. Chemosphere 81:732–737. https://doi.org/10.1016/j.chemosphere.2010.07.031
Her N, Jeong H, Kim J, Yoon Y (2011) Occurrence of perchlorate in drinking water and seawater in South Korea. Arch Environ Contam Toxicol 61:166–172. https://doi.org/10.1007/s00244-010-9616-0
Hu J, Xian Y, Wu Y et al (2021) Perchlorate occurrence in foodstuffs and water: Analytical methods and techniques for removal from water – A review. Food Chem 360:130146. https://doi.org/10.1016/j.foodchem.2021.130146
Hunten DM (1979) Possible oxidant sources in the atmosphere and surface of Mars. J Mol Evol 14:71–78. https://doi.org/10.1007/BF01732369
Interstate Technology Regulatory Council (2005) Perchlorate: Overview of Issues, Status, and Remedial Options. Washington, DC. https://itrcweb.org/viewdocument/perchlorate-overview-of-issues-st?CommunityKey=0c358b0a-a5b9-4fd7-a832-11888551a153&tab=librarydocuments
Itterheimová P, Bobacka J, Šindelář V, Lubal P (2022) Perchlorate Solid-Contact Ion-Selective Electrode Based on Dodecabenzylbambus[6]uril. Chemosensors 10:115. https://doi.org/10.3390/chemosensors10030115
Jackson WA, Böhlke JK, Gu B et al (2010) Isotopic composition and origin of indigenous natural perchlorate and co-occurring nitrate in the southwestern United States. Environ Sci Technol 44:4869–4876. https://doi.org/10.1021/es903802j
Jackson WA, Davila AF, Estrada N et al (2012) Perchlorate and chlorate biogeochemistry in ice-covered lakes of the McMurdo Dry Valleys, Antarctica. Geochim Cosmochim Acta 98:19–30. https://doi.org/10.1016/j.gca.2012.09.014
Jackson WA, Böhlke JK, Andraski BJ et al (2015) Global patterns and environmental controls of perchlorate and nitrate co-occurrence in arid and semi-arid environments. Geochim Cosmochim Acta 164:502–522. https://doi.org/10.1016/j.gca.2015.05.016
Jia W, Wang X, Wu X, Shi L (2022) Monitoring contamination of perchlorate migrating along the food chain to dairy products poses risks to human health. Food Chem 374:131633. https://doi.org/10.1016/j.foodchem.2021.131633
Jiang S, Li Y-S, Sun B (2013) Determination of trace level of perchlorate in Antarctic snow and ice by ion chromatography coupled with tandem mass spectrometry using an automated sample on-line preconcentration method. Chin Chem Lett 24:311–314. https://doi.org/10.1016/j.cclet.2013.02.011
Jiang S, Cox TS, Cole-Dai J et al (2016) Trends of perchlorate in Antarctic snow: Implications for atmospheric production and preservation in snow. Geophys Res Lett 43:9913–9919. https://doi.org/10.1002/2016GL070203
Jiang S, Cole-dai J, An C et al (2020) Spatial variability of perchlorate in East Antarctic surface snow : Implications for atmospheric production. Atmos Environ 238:117743. https://doi.org/10.1016/j.atmosenv.2020.117743
Jiang S, Shi G, Cole-Dai J et al (2021) Occurrence, latitudinal gradient and potential sources of perchlorate in the atmosphere across the hemispheres (31°N to 80°S). Environ Int 156:106611. https://doi.org/10.1016/j.envint.2021.106611
Kannan K, Praamsma ML, Oldi JF et al (2009) Occurrence of perchlorate in drinking water, groundwater, surface water and human saliva from India. Chemosphere 76:22–26. https://doi.org/10.1016/j.chemosphere.2009.02.054
Kim DH, Yoon Y, Baek K et al (2014) Occurrence of perchlorate in rice from different areas in the Republic of Korea. Environ Sci Pollut Res Int 21:1251–1257. https://doi.org/10.1007/s11356-013-2013-2
Kirk AB (2006) Environmental perchlorate: Why it matters. Anal Chim Acta 567:4–12. https://doi.org/10.1016/j.aca.2006.03.047
Kirk AB, Smith EE, Tian K et al (2003) Perchlorate in Milk. Environ Sci Technol 37:4979–4981. https://doi.org/10.1021/es034735q
Koibuchi N, Chin WW (2000) Thyroid Hormone Action and Brain Development. Trends Endocrinol Metab 11:123–128. https://doi.org/10.1016/S1043-2760(00)00238-1
Koibuchi N (2018) Molecular Mechanisms of Thyroid Hormone Synthesis and Secretion. In: Belfiore A, LeRoith D (eds) Principles of Endocrinology and Hormone Action. Springer International Publishing, Cham, pp 73–81. https://doi.org/10.1007/978-3-319-44675-2_5
Kounaves SP, Stroble ST, Anderson RM et al (2010b) Discovery of Natural Perchlorate in the Antarctic Dry Valleys and Its Global Implications. Environ Sci Technol 44:2360–2364. https://doi.org/10.1021/es9033606
Kounaves SP, Chaniotakis NA, Chevrier VF et al (2014) Identification of the perchlorate parent salts at the Phoenix Mars landing site and possible implications. Icarus 232:226–231. https://doi.org/10.1016/j.icarus.2014.01.016
Kounaves SP, Hecht MH, Kapit J, et al (2010a) Soluble sulfate in the martian soil at the Phoenix landing site. Geophys Res Lett 37:. https://doi.org/10.1029/2010GL042613
Krishnan GR, Radhika R, Jayalatha T et al (2017) Removal of perchlorate from drinking water using granular activated carbon modified by acidic functional group: Adsorption kinetics and equilibrium studies. Process Saf Environ Prot 109:158–171. https://doi.org/10.1016/j.psep.2017.03.014
Kumar KS, Kavitha S, Parameswari K et al (2023) Environmental occurrence, toxicity and remediation of perchlorate – A review. Chemosphere 311:137017. https://doi.org/10.1016/j.chemosphere.2022.137017
Kumarathilaka P, Oze C, Indraratne SP, Vithanage M (2016) Perchlorate as an emerging contaminant in soil, water and food. Chemosphere 150:667–677. https://doi.org/10.1016/j.chemosphere.2016.01.109
Laye VJ, DasSarma S (2018) An Antarctic Extreme Halophile and Its Polyextremophilic Enzyme: Effects of Perchlorate Salts. Astrobiology 18:412–418. https://doi.org/10.1089/ast.2017.1766
Lee J-W, Oh S-H, Oh J-E (2012) Monitoring of perchlorate in diverse foods and its estimated dietary exposure for Korea populations. J Hazard Mater 243:52–58. https://doi.org/10.1016/j.jhazmat.2012.09.037
Li M, Xiao M, Xiao Q et al (2022) Perchlorate and chlorate in breast milk, infant formulas, baby supplementary food and the implications for infant exposure. Environ Int 158:106939. https://doi.org/10.1016/j.envint.2021.106939
Li X, Li J, Li K et al (2023) Effects of perchlorate and exogenous T4 exposures on body condition and endochondral ossification of Rana chensinensis tadpoles. Aquat Toxicol 265:106767. https://doi.org/10.1016/j.aquatox.2023.106767
Llorente-Esteban A, Manville RW, Reyna-Neyra A et al (2020) Allosteric regulation of mammalian Na+/I− symporter activity by perchlorate. Nat Struct Mol Biol 27:533–539. https://doi.org/10.1038/s41594-020-0417-5
Lu S, Jing X, Liu J et al (2013) Synthesis of porous sheet-like Co3O4 microstructure by precipitation method and its potential applications in the thermal decomposition of ammonium perchlorate. J Solid State Chem 197:345–351. https://doi.org/10.1016/j.jssc.2012.09.020
Maffini MV, Trasande L, Neltner TG (2016) Perchlorate and Diet: Human Exposures, Risks, and Mitigation Strategies. Curr Environ Health Rep 3:107–117. https://doi.org/10.1007/s40572-016-0090-3
Mahmudov R, Huang CP (2010) Perchlorate removal by activated carbon adsorption. Sep Purif Technol 70:329–337. https://doi.org/10.1016/j.seppur.2009.10.016
Martins Z, Cottin H, Kotler JM et al (2017) Earth as a Tool for Astrobiology—A European Perspective. Space Sci Rev 209:43–81. https://doi.org/10.1007/s11214-017-0369-1
Meeßen J, Wuthenow P, Schille P et al (2015) Resistance of the Lichen Buellia frigida to Simulated Space Conditions during the Preflight Tests for BIOMEX—Viability Assay and Morphological Stability. Astrobiology 15:601–615. https://doi.org/10.1089/ast.2015.1281
Melnyk RA, Coates JD (2015) The Perchlorate Reduction Genomic Island: Mechanisms and Pathways of Evolution by Horizontal Gene Transfer. BMC Genomics 16:862. https://doi.org/10.1186/s12864-015-2011-5
Mier López M, Ibáñez Mendizábal R, Ortiz Uribe I, Rivero Martínez MJ (2004) Electrodiálisis con membranas bipolares: fundamentos y aplicaciones. Ing Quím 166–182. https://dialnet.unirioja.es/servlet/articulo?codigo=1066023
Minicozzi MR, von Hippel FA, Furin CG, Buck CL (2019) Sodium perchlorate induces non-alcoholic fatty liver disease in developing stickleback. Environ Pollut 251:390–399. https://doi.org/10.1016/j.envpol.2019.05.001
Moreno JC, Klootwijk W, van Toor H et al (2008) Mutations in the Iodotyrosine Deiodinase Gene and Hypothyroidism. N Engl J Med 358:1811–1818. https://doi.org/10.1056/NEJMoa0706819
Muñi A, Páez G, Faría J, et al (2005) Eficiencia de un sistema de ultrafiltración/nanofiltración tangencial en serie para el fraccionamiento y concentración del lactosuero. Rev Científica XV:361–367. https://www.redalyc.org/articulo.oa?id=95915410
Murray CW, Bolger PM (2014) Environmental Contaminants: Perchlorate. In: Motarjemi Y (ed) Encyclopedia of Food Safety. Academic Press, Waltham, pp 337–341. https://doi.org/10.1016/B978-0-12-378612-8.00200-6
Ohye H, Sugawara M (2010) Dual oxidase, hydrogen peroxide and thyroid diseases. Exp Biol Med 235:424–433. https://doi.org/10.1258/ebm.2009.009241
Oze C, Beisel J, Dabsys E et al (2021) Perchlorate and Agriculture on Mars. Soil Syst 5:37. https://doi.org/10.3390/soilsystems5030037
Parvez SM, Jahan F, Brune M-N et al (2021) Health consequences of exposure to e-waste: an updated systematic review. Lancet Planet Health 5:e905–e920. https://doi.org/10.1016/S2542-5196(21)00263-1
Petersen AM, Dillon D, Bernhardt RR et al (2015) Perchlorate Disrupts Embryonic Androgen Synthesis and Reproductive Development in Threespine Stickleback without Changing Whole-Body Levels of Thyroid Hormone. General and Comparative Endocrinology 210:130–144. https://doi.org/10.1016/j.ygcen.2014.10.015
Pleus RC, Corey LM (2018) Environmental exposure to perchlorate: A review of toxicology and human health. Toxicol Appl Pharmacol 358:102–109. https://doi.org/10.1016/j.taap.2018.09.001
Quinn RC, Martucci HFH, Miller SR et al (2013) Perchlorate Radiolysis on Mars and the Origin of Martian Soil Reactivity. Astrobiology 13:515–520. https://doi.org/10.1089/ast.2013.0999
Rao B, Anderson TA, Redder A, Jackson WA (2010) Perchlorate Formation by Ozone Oxidation of Aqueous Chlorine/Oxy-Chlorine Species: Role of ClxOy Radicals. Environ Sci Technol 44:2961–2967. https://doi.org/10.1021/es903065f
Reh B, Wang X, Feng Y, Bhandari RK (2022) Potassium perchlorate effects on primordial germ cells of developing medaka larvae. Aquat Toxicol 251:106283. https://doi.org/10.1016/j.aquatox.2022.106283
Sanchez-Hernandez JC (2006) Earthworm biomarkers in ecological risk assessment. Rev Environ Contam Toxicol 188:85–126. https://doi.org/10.1007/978-0-387-32964-2_3
Schirmack J, Alawi M, Wagner D (2015) Influence of Martian regolith analogs on the activity and growth of methanogenic archaea, with special regard to long-term desiccation. Front Microbiol 6:210. https://doi.org/10.3389/fmicb.2015.00210
Shahrokhi-Shahraki R, Benally C, El-Din MG, Park J (2021) High efficiency removal of heavy metals using tire-derived activated carbon vs commercial activated carbon: Insights into the adsorption mechanisms. Chemosphere 264:128455. https://doi.org/10.1016/j.chemosphere.2020.128455
Shtarkman YM, Koçer ZA, Edgar R et al (2013) Subglacial Lake Vostok (Antarctica) Accretion Ice Contains a Diverse Set of Sequences from Aquatic, Marine and Sediment-Inhabiting Bacteria and Eukarya. PLoS ONE 8:e67221. https://doi.org/10.1371/journal.pone.0067221
Sijimol MR, Jyothy S, Pradeepkumar AP et al (2015) Review on Fate, Toxicity, and Remediation of Perchlorate. Environ Forensics 16:125–134. https://doi.org/10.1080/15275922.2015.1022914
Song W, Gao B, Wang H et al (2017) The rapid adsorption-microbial reduction of perchlorate from aqueous solution by novel amine-crosslinked magnetic biopolymer resin. Bioresour Technol 240:68–76. https://doi.org/10.1016/j.biortech.2017.03.064
Spitzweg C, Joba W, Schriever K et al (1999) Analysis of human sodium iodide symporter immunoreactivity in human exocrine glands. J Clin Endocrinol Metab 84:4178–4184. https://doi.org/10.1210/jcem.84.11.6117
Srinivasan R, Sorial GA (2009) Treatment of perchlorate in drinking water: A critical review. Sep Purif Technol 69:7–21. https://doi.org/10.1016/j.seppur.2009.06.025
Stamenković V, Ward LM, Mischna M, Fischer WW (2018) O2 solubility in Martian near-surface environments and implications for aerobic life. Nat Geosci 11:905–909. https://doi.org/10.1038/s41561-018-0243-0
Sunilkumar U, Lal S (2021) Perchlorate Reducing Bacteria And Their Insight Towards Astrobiology. IJRAR 8:1. https://doi.org/10.13140/RG.2.2.26997.70880
Ting W-P, Lu M-C, Huang Y-H (2008) The reactor design and comparison of Fenton, electro-Fenton and photoelectro-Fenton processes for mineralization of benzene sulfonic acid (BSA). J Hazard Mater 156:421–427. https://doi.org/10.1016/j.jhazmat.2007.12.031
Trumpolt CW, Crain M, Cullison GD et al (2005) Perchlorate: Sources, uses, and occurrences in the environment. Remediat J 16:65–89. https://doi.org/10.1002/rem.20071
United States Environmental Protection Agency (USEPA) (2008) Interim Drinking Water Health Advisory For Perchlorate. Health and Ecological Criteria Division Office of Science and Technology Office of Water; U.S. Environmental Protection Agency Washington, Washington, D. C. https://www.epa.gov/sites/default/files/2019-03/documents/interim-dw-perchlorate.pdf
United States Environmental Protection Agency (USEPA) (2009) Revised Assessment Guidance for Perchlorate. Office of solid waste and emergency response, Washington, D. C. https://www.epa.gov/fedfac/revised-assessment-guidance-perchlorate
Vega M, Nerenberg R, Vargas IT (2018) Perchlorate contamination in Chile: Legacy, challenges, and potential solutions. Environ Res 164:316–326. https://doi.org/10.1016/j.envres.2018.02.034
Visser WE, Friesema ECH, Visser TJ (2011) Minireview: Thyroid Hormone Transporters: The Knowns and the Unknowns. Mol Endocrinol 25:1–14. https://doi.org/10.1210/me.2010-0095
Wang Z, Forsyth D, Lau BP et al (2009) Estimated dietary exposure of Canadians to perchlorate through the consumption of fruits and vegetables available in Ottawa markets. J Agric Food Chem 57:9250–9255. https://doi.org/10.1021/jf901910x
Wang Z, Gao M, Zhang Y et al (2014) Perchlorate reduction by hydrogen autotrophic bacteria in a bioelectrochemical reactor. J Environ Manage 142:10–16. https://doi.org/10.1016/j.jenvman.2014.04.003
Wémeau J-L, Kopp P (2017) Pendred syndrome. Best Pract Res Clin Endocrinol Metab 31:213–224. https://doi.org/10.1016/j.beem.2017.04.011
Wu Q, Zhang T, Sun H, Kannan K (2010) Perchlorate in tap water, groundwater, surface waters, and bottled water from China and its association with other inorganic anions and with disinfection byproducts. Arch Environ Contam Toxicol 58:543–550. https://doi.org/10.1007/s00244-010-9485-6
Wu Q, Oldi JF, Kannan K (2011) Fate of perchlorate in a man-made reflecting pond following a fireworks display in Albany, New York, USA. Environ Toxicol Chem 30:2449–2455. https://doi.org/10.1002/etc.648
Wynn-Williams DD, Edwards HGM (2000) Antarctic ecosystems as models for extraterrestrial surface habitats. Planet Space Sci 48:1065–1075. https://doi.org/10.1016/S0032-0633(00)00080-5
Xie Y, Ren L, Zhu X et al (2018) Physical and chemical treatments for removal of perchlorate from water–A review. Process Saf Environ Prot 116:180–198. https://doi.org/10.1016/j.psep.2018.02.009
Xie Y, Cai X, Liu W, Deng W (2009) [Effects of perchlorate on growth and chlorophyll fluorescence parameters of Alternanthera philoxeroides]. Huan Jing Ke Xue Huanjing Kexue 30:2425–2431. https://europepmc.org/article/med/19799312
Yang M, Her N (2011) Perchlorate in Soybean Sprouts (Glycine max L. Merr.), Water Dropwort (Oenanthe stolonifera DC.), and Lotus (Nelumbo nucifera Gaertn.) Root in South Korea. J Agric Food Chem 59:7490–7495. https://doi.org/10.1021/jf2009638
Yao F, Zhong Y, Yang Q et al (2017) Effective adsorption/electrocatalytic degradation of perchlorate using Pd/Pt supported on N-doped activated carbon fiber cathode. J Hazard Mater 323:602–610. https://doi.org/10.1016/j.jhazmat.2016.08.052
Ye L, You H, Yao J, Su H (2012) Water treatment technologies for perchlorate: A review. Desalination 298:1–12. https://doi.org/10.1016/j.desal.2012.05.006
Yen AS, Kim SS, Hecht MH et al (2000) Evidence That the Reactivity of the Martian Soil Is Due to Superoxide Ions. Science 289:1909–1912. https://doi.org/10.1126/science.289.5486.1909
Yu J, Dong H-W, Shi L-T et al (2019) Reproductive toxicity of perchlorate in rats. Food Chem Toxicol 128:212–222. https://doi.org/10.1016/j.fct.2019.04.014
Zhang T, Wu Q, Sun HW et al (2010) Perchlorate and Iodide in Whole Blood Samples from Infants, Children, and Adults in Nanchang, China. Environ Sci Technol 44:6947–6953. https://doi.org/10.1021/es101354g
Zoeller RT (2021) Chapter Ten - Endocrine disrupting chemicals and thyroid hormone action. In: Vandenberg LN, Turgeon JL (eds) Advances in Pharmacology. Academic Press, pp 401–417. https://doi.org/10.1016/bs.apha.2021.05.002
Acknowledgements
The authors thank the Universidad Tecnológica de Bolívar for financing the expeditions to the white continent [Grant number: CI2021P01].
Funding
Open Access funding provided by Colombia Consortium. Support was provided by Universidad Tecnológica de Bolívar.
Author information
Authors and Affiliations
Contributions
R. Acevedo-Barrios, D. A. Puentes Martínez, I. O. Hernández Rocha, C. Rubiano-Labrador, A. C. De la Parra-Guerra, L. Carranza-López, A. Monroy-Licht, M. A. Leal and D. Tovar were in charge of conceptualization, investigation, visualization; writing, reviewing, and editing the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Consent for publication
Publication consent not applicable.
Consent to participate
Participate consent not applicable.
Human or animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Code availability
Not applicable.
Informed consent
Informed consent not applicable.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Acevedo-Barrios, R., Puentes Martínez, D.A., Hernández Rocha, I.O. et al. Perchlorate in antarctica, origin, effects, treatments, environmental fate, and astrobiological perspectives: a review. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-06004-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-06004-w