Abstract
Recent developments in nanotechnology and nanoscience in all phases of human life have radically altered the diagnosis, treatment, and prevention of numerous diseases. Silver nanoparticles (Ag-NPs) have been among the most dynamic, and Several metallic nanoparticles developed for biological uses include some beautiful nanomaterials. Ag-NPs have played a pivotal role in nanotechnology and nanoscience, mostly nanomedicine. In this work, Ficus carica leaf extract was utilized to create silver nanoparticles using a straightforward procedure. This study explored the reduction of silver ions by F. carica leaf extract in the solution. UV–Vis spectroscopy, XRD analysis, and STEM characterize prepared biogenic Ag-NPs. Afterward, antibacterial and antibiofilm properties were investigated. The images obtained from STEM analysis surveyed that the NP sizes ranged from 11 to 20 nm. Ag-NPs demonstrated antibacterial and antibiofilm activities against all tested Gram-positive and Gram-negative bacteria strains. It has been indicated that Ag-NPs have inhibitory potency against collagenase activity. Ag-NPs exhibited anticancer activity in the HeLa cell line, and the IC50 value was calculated as 8.403 μg/mL. In this study, it has been shown that the synthesis method is environmentally friendly, fast, safe, and easy to use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introductıon
Nanoparticles (NPs) involve atom clusters in the size range of 1–100 nm, and several of their syntheses and uses have been carried out in recent years (Santhoshkumar et al. 2019). Nanotechnology designs and synthesizes nanotubes, nanowires, and nanorings by processing known molecules with various atoms and molecules (Korkmaz et al. 2020c). To create nanoparticles, physical and chemical processes are used. However, because of the toxicological action of chemically synthesized NPs, the green synthesis method has appeared as an alternative method in recent years. Nanoparticles, especially those under 100 nm, are used effectively in medicine, biotechnology, biomedicine, cosmetology, material engineering, and chemistry due to their superior properties to volumetric materials (Singh et al. 2008; Korkmaz 2019; Saravanakumar et al. 2018; Belachew 2023). Among the known nanomaterials, silver nanomaterials are widely researched and used as antibacterial agents (Du et al. 2023). The antibacterial property of silver has been known for centuries and has been used in many fields (Arora et al. 2008; Yang et al. 2012). Silver ions (Ag +) have been indicated in the literature for their antimicrobial activities on all known types of microorganisms. The processes of binding and entry of Ag NPs into bacterial cells, release of silver ions, disruption of cell walls and membranes, and increased oxidative stress involve antibacterial action (Du et al. 2023; Miller et al. 2015). Besides their antibacterial effect, Ag+ has no known harmful effects on human health (Korkmaz et al. 2020c; Singh et al. 2008; Sengottaiyan et al. 2016). Ag NPs are primarily used in the cosmetics and detergent industry. Skin products containing Ag+ both refresh the skin and form an antimicrobial surface. Ag-NPs are also used in products alternative to detergents using Ag+ ion (Singh et al. 2008). Apart from these, Ag+ is used for food shelf-life extension and packaging, medical and biomedical purposes, and the purification of drinking water (Pal et al. 2007).
Several articles on the antibacterial and anticancer properties of Ag-NPs made using green synthesis exist. However, the activity of the NPs varies due to the differences in the reduction potential of the plant used and the size, surface area, application dose, and duration of the NPs (Pal et al. 2007; P.T.C et al. 2018; Morones et al. 2005; Tian et al. 2007). Ag-NPs have been reported to be non-toxic to healthy cells in studies on cell culture and wound healing (Pallavicini et al. 2017). During Ag-NP synthesis, controlling the particle size and experiment conditions is crucial. Especially in the past few years, several different procedures have evolved to maintain the size and shape of NPs synthesized in the solution medium. In one of these studies, it has been reported that plants and herbal products are economical, renewable, and non-toxic materials for NP production (Nematollahi 2015). Because there is no need for toxic chemicals to reduce metals in NP synthesis. There are no obstacles to NPs in terms of their use in human health products (Song and Kim 2009). Many studies have been conducted on synthesizing Ag-NPs (to obtain Ag, Ag2O, or an Ag-Ag2O mixture) using different plant extracts (Korkmaz et al. 2020c; Korkmaz 2019, 2020; Saravanakumar et al. 2018; Sengottaiyan et al. 2016; Sengottaiyan et al. 2016; Pal et al. 2007; P. T.C et al. 2018; Morones et al. 2005; Tian et al. 2007; Pallavicini et al. 2017; Nematollahi 2015; Song and Kim 2009; Logeswari et al. 2015; Ahmed et al. 2016). However, no published paper on the synthesis of NPs using Ficus carica leaf extract has been reported.
Türkiye is one of the leading countries in fig production globally (Hasdemir 2023). In other words, fig leaves are in the position of agricultural waste. This study aims to contribute to the waste management system by evaluating this agricultural waste, which is abundant in Türkiye. Since fig is mainly used as a fruit, their leaves are usually discarded as garbage. In the current study, eco-friendly NPs were produced from fig leaves, which are herbal waste, and the characterization and antibacterial and anticancer properties of Ag-NPs were analyzed. This study obtained Ag NPs with superior antimicrobial and anticancer properties by green synthesis, an economical and rapid method using fig leaves.
Materials and methods
Preparation of Ficus carica extracts
F. carica leaves, which have completed their physical maturity, were collected from the field for plant extract. It was washed with distilled water to remove dust and other foreign materials and dried thoroughly in an environment without direct sunlight and with good air circulation. 100 g of the dried plant was weighed, and 1 L of distilled water was added to it and heated at 80 °C for 8 h. It was filtered once it had reached room temperature. The obtained extract was kept at + 4° for NP synthesis (Korkmaz 2019).
Synthesis of biogenic Ag-NPs
20 mL of freshly prepared fig leaves extract was added to the prepared solution of 80 mL AgNO3 (0.01 M) and mixed for 1 day at 50 °C. The formation of Ag-NPs in the solution was discerned by a color change from a colorless clear solution to reddish brown when monitored spectrophotometrically. Silver concentration was optimized since each plant has a different capacity to reduce phytochemicals. The next day, it waited until it reached room temperature, and the NPs were precipitated by centrifugation. For this study, the synthesis method given in the literature was used (Namasivayam and Chitrakala 2011; Jo et al. 2009). At the end of approximately two days, Ag NPs were synthesized quickly, cost-effectively, and safely according to physical, chemical, and other synthesis methods in the literature (Iravani et al. 2014).
UV–visible spectroscopy
UV–visible spectroscopy, a primary method for characterizing nanoparticles, is one of the main characterization steps. Ag-NPs have optical properties at specific wavelengths attributed to them (Vilchis-Nestor et al. 2008). UV–Vis analysis was performed using a Thermo Scientific spectrophotometer. At 25 °C, 1 mL of sample and distilled water were taken into a quartz cuvette and analyzed in UV–Vis. The stability of biogenically prepared Ag-NPs was observed for more than 1 year.
XRD (X-ray diffraction) analysis
Structural analysis of Ag-NPs was performed with Panalytical brand, Empyrean Advance model X-ray diffractometer. XRD analysis is used to qualitatively identify samples' crystal degree, isomorphic substitutions, and chemical types of molecules.
Scanning transmission electron microscopy (STEM)
Surface morphology, dimensional properties, and shapes of silver nanoparticles were analyzed using STEM microscopy. Like UV–Vis analysis, STEM analysis is one of the most widely used techniques for characterizing NPs. It is a surface imaging technique on the morphology of NPs at nano and micro scales (Ahmed et al. 2016; Vilchis-Nestor et al. 2008).
Antimicrobial assay
To test the antimicrobial effect of Ag-NPs obtained from F. carica leaves, two Gram ( +) (Staphylococcus epidermidis DSMZ 20044, Staphylococcus aureus ATCC 25923) and two Gram (−) (Enterobacter aerogenes ATCC 13048, Salmonella infantis) bacteria were analyzed by minimum inhibition concentration (MIC) assay. Stock bacteria strains were incubated until 0.5 McFarland turbidity (1.5 × 108 CFU/mL). Two-fold dilutions of Ag-NPs were performed with concentrations varying from 2500 to 78.125 µg/mL using a 96-well plate incubated at 37 °C for 24 h. The MIC of Ag-NPs was detected by UV–Vis spectrophotometer at 600 nm, and it was figured out that minimum concentration inhibited microorganism growth. Then, a minimum bactericidal concentration (MBC) assay was applied to demonstrate bacterial inhibition in the wells. After that, it was filtered and allowed to cool to ambient temperature. Inoculating the questionable wells onto petri plates, they were incubated at 37 °C for 24 h (Andrews 2001).
Antibiofilm test
Sterile medium and Ag-NPs serially diluted and bacterial culture were inoculated into a 96-well microplate and then incubated at 37 °C for 48 h for a biofilm inhibition test. After incubation, all wells were drained and desiccated. 95% methanol was put into wells and then removed for incubation. 0.1% crystalline violet solution was loaded in each well and kept at room temperature. Following staining, 33% glacial acetic acid and 95% ethanol were added to the wells of Gram ( +) and Gram (−) bacteria for 15 min and measured at 600 nm (Merritt et al. 2011), which is the optical density of organic molecules and bacterial growth (Kumar and Yadav 2009). The activity of Ag-NPs on the antibiofilm layer was computed by the following formula (Donlan and Costerton 2002):
In vitro anticancer of biosynthesized Ag-NPs
HeLa cells were purchased from ATCC. HeLa cells were cultured at 37 °C in a moistened atmosphere, including 5% CO2 in the air, and 1 × 104 HeLa cells were seeded onto 96-well plates, and the plates were then incubated for 24 h. Biosynthesized Ag-NPs treated HeLa cells at doses ranging from 0.5 to 64 g/mL. Plates were performed to MTT assay. It was made at a 5 mg/mL concentration of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Each well received 100 L of MTT, then incubated for 4 h. After purple-colored formazone crystals appeared, 200 mL of dimethyl sulphoxide was used to dissolve them (DMSO). These crystals were seen in a multi-well plate reader at 570 nm.
Collagenase ınhibition assay
The inhibition assay for collagenase was performed based on spectrophotometric methods, as reported earlier in the literature with FALGPA (N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala), with some modifications for use in a microplate reader (Van Wart and Steinbrink 1981) . A solution of tricine buffer containing Clostridium histolyticum collagenase (EC.3.4.23.3) was prepared. The reaction mixture in each well had 50 mM tricine buffer (pH = 7.5 with 10 mM CaCI2 and 400 mM NaCI), 1 mM FALGPA, 0.1-unit ChC solution, and different concentrations of Ag-NPs. The reaction plates were pre-incubated with Ag-NPs at 25 °C for 15 min using a suitable thermostatted Multiskan GO spectrophotometer (Thermo Scientific). The reaction was initiated by adding the substrate, and the hydrolysis of FALGPA (ɛfalgpa: 0.53).was recorded at 345 nm for 5 min in 96-well plates.
Results and discussion
UV–visible spectroscopy
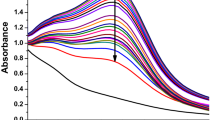
The addition of F. carica leaf extract to AgNO3 solution caused a reddish-brownish visual discoloration of the colorless solution, which showed the reduction of Ag+ to Ag° within 5 min due to the collective release of free-conducting electrons generated by the electromagnetic field by the surface plasmon resonance (SPR) stimulation. The formation of Ag-NPs was confirmed by a characteristic SPR band UV–Vis spectrum at 419 nm of the colloidal solution (Fig. 1). Depending on the size and shape of the particles, the maximal UV–Vis absorption band changes (Korkmaz et al. 2020c). It is possible to say that the not-very-wide absorbance peak at 419 nm, which we obtained as a result of the synthesis, represents the formation of smaller NPs (Philip et al. 2011).
Previous articles have reported that the maximum absorbance values of Ag NPs between 420 and 450 nm vary depending on the chemical environment, plant extract concentration, reaction conditions (pH, temperature, etc.), and particle size (Kalishwaralal et al. 2010; Tang and Zheng 2018; Erdogan et al. 2019; Sangiliyandi 2010; Wani et al. 2011).
X-ray diffraction (XRD) analysis
The crystal behavior of the obtained powder material was analyzed by X-ray diffraction. Figure 2 shows the XRD model of Ag-NPs prepared using F. carica leaf extracts. A series of solid diffraction peaks were observed corresponding to the five principal peak planes with values of 32.47°, 38.27°, 54.98°, 64.69°, and 67.80° (Fig. 2). The spectra corresponding to (111), (200), (220), (311), and (222), which can match well with the cubic phase structure, match well with the card number JCPDS:(01-076-1393). It is possible to confirm with the findings of other sources in the literature that the other peaks observed belong to the organic phytochemical residues in the plant extract (Korkmaz 2019; Ahmed et al. 2016; Sangiliyandi 2010). Expansion in diffraction peaks indicated that a small crystal size was obtained (Sheny et al. 2011). The other peak in the spectrum belongs to pure Ag. As a result of the study, it was found that the synthesized material was a mixture of Ag-Ag2O.
The Debye–Scherrer equation was used to get the mean particle size:
Here, the particle size is denoted by D; the X-ray wavelength used is expressed in λ; θ, Bragg angle; β is the half-height of the XRD vertex, and k is the shape factor constant (k = 0.9) (Annamalai et al. 2011). The mean particle size of Ag-NPs from the Debye Scherer formula was around 22.6 nm.
Some previous studies have reported smaller Ag-NPs than those synthesized in this study (Namasivayam and Chitrakala 2011; Kalishwaralal et al. 2010), while some have reported ones close in size (Jo et al. 2009; Korkmaz et al. 2020a, 2020b; Ivask et al. 2014).
Although the synthesis is easy with the green synthesis method, the product yield is meager. Therefore, the experiment was repeated at least four times to obtain a sufficient product for all studies. The same synthesis conditions were created in each repetition of the synthesis, and the same results were obtained in UV–Vis and XRD analysis results. This is essential for the repeatability of the experiment.
Scanning transmission electron microscopy (STEM)
In STEM analysis, it is possible to acquire high-resolution images if the sample material is well distributed before the analysis. Figure 3 shows an excellent example of this. When the non-agglomerated structure and morphology of Ag-NPs are examined, it is seen that NPs have spherical geometry. STEM images of Ag-NPs taken at 500kx magnification show NPs of 20 nm and smaller sizes.
Ag-NPs with similar shapes and sizes synthesized from different plants in the literature are listed in Table 1 below.
Antimicrobial assay
Silver has been extensively utilized in cosmetic, textile, food storage, pharmaceutical, dye reduction, and environmental applications because of its beneficial antibacterial effect. (Korkmaz 2019; Sondi and Salopek-Sondi 2004). To clarify the antibacterial characteristic of silver, many papers have been carried out by scientists (Korkmaz 2020). The antibacterial properties of Ag-NPs bio-synthesized from F. carica leaves were applied against Staphylococcus aureus, Staphylococcus epidermidis, and Enterobacter aerogenes, Salmonella infantis by minimum inhibition concentration and minimum bactericidal concentration methods. The maximum antibacterial activity was reported against S. epidermidis at 156.25 µg/mL. The MIC and MBC activity were 625 µg/mL and 1250 µg/mL against two Gram (−) bacteria, E. aerogenes and S. infantis. Besides, the MIC and MBC values against Gram ( +) bacteria were recorded at 1250 µg/mL and 2500 µg/mL against S. aureus and 156.25 µg/mL and 312.5 µg/mL against S. epidermidis, respectively (Figs. 4 and 5). The antibacterial effect of Ag-NPs obtained from F. carica leaves was influential and significant in the current paper. The most sensitive (S. epidermis) and the most resistant (S. aureus) to applied Ag-NPs occurred against Gram ( +) bacteria. The impact of Ag-NPs alters depending on differences in particle size, capping ligand, and surface charges of Ag-NPs (Philip et al. 2011). According to MIC results of EUCAST (European Committee on Antimicrobial Susceptibility Testing), breakpoints results (EUCAST 2023), S. aureus and S. epidermidis against Ciproflaxocin and S. infantis against Ampicillin has MIC value 1000 and 2000 µg/mL. When comparing this paper's results with EUCAST, it is seen that Ag-NPs give favorable results, especially against S. epidermidis and S. infantis. Silver ions can efficiently react with a substance that involves phosphorous and sulfur. Therefore, biomolecules like cell membranes and DNA proteins readily interact with Ag-NPs since they contain these substances. It was observed that transmission electron microscopy showed that Ag-NPs aggregated in the cell wall, cell membrane, and organelles brought about disruptions and dysfunctions (Erdogan et al. 2019; Wani et al. 2011; Velsankar et al. 2020a, 2022, 2020b, 2020; AshaRani et al. 2009). In short, it was observed that Ag-NPs inhibit bacterial growth and kill bacteria, thus providing further evidence for the antibacterial feature of Ag-NPs.
Antibiofilm test
The layer called a biofilm is created when microbes attach proteins, polysaccharides, and carbohydrates to live or dead surfaces. Biofilm is an obstacle in several fields, such as the food industry, water supply network, and water purification systems, which can directly influence human health and the environment (Korkmaz 2019). Ag-NPs presented an efficient biofilm inhibition percentage against B. subtilis and S. infantis by 38.7% and 36.3%, respectively. A former study reported that B. subtilis biofilm inhibition was obtained at the highest percentage at 2500 µg/mL (Korkmaz et al. 2020a). However, the current study's highest biofilm inhibition percentage achieved was at a lower concentration of 156.25 µg/mL (Fig. 6). Severe issues generated by biofilm endanger community health care and habitat in various areas such as the food sector, wastewater treatment plant, water supply network, and paper industry. The solution to this issue is complicated because the biofilm layer can be devastated by using chemicals that are in danger of harming human health. (Korkmaz et al. 2020a). It would be suitable to use eco-friendly Ag-NPs synthesized from F. carica leaves rather than chemical agents that adversely impact human health and the environment.
Anticancer activity of biosynthesized Ag-NPs
In HeLa cell lines, the produced nanoparticles were examined for cytotoxic activities. After 24 h incubation with various doses (0.5, 1, 2, 4.8, 16, 32, 64 µg/mL), they investigated cytotoxic activity well. As predicted, the rising concentration of nanoparticles reduced the viability of cells. As a result, Fig. 7 illustrates the IC50 values for the HeLa cell line were 8.403 g/mL, and in confluent cultures, the nanoparticles' anticancer impact was dose-dependent.
It is clear that a rise in the concentration of Ag-NPs reduces cell survival or inhibits cell proliferation. Asharani et al. (2009) informed that the proliferation of HeLa cells was decreased after treatment with silver nanoparticles. In the literature research, cytotoxicity tests were performed with silver nanoparticles on the HeLa cell line, and IC50 values were determined in various studies. In these studies, IC50 values were 2.94 μg/mL in the study with Vitex agnus-castus L. (Ekici et al. 2023), 5 µg/mL in the study with Nepeta deflersiana (Al-Sheddi et al. 2018), 11.8 μg/mL in the study with Elaeagnus angustifolia (Erdoğan et al. 2021), and 28 μg/mL in the study Ephorbia antiquorum study found values of Rajkuberan et al. (2017). A significant inhibitory effect was observed when a higher dose of silver nanoparticles was used. Dose-dependent cytotoxicity was noticed in HeLa cells treated with Ag-NPs, and an increase in Ag-NP concentration indicated an increase in cytotoxicity in HeLa cells (Sukirtha et al. 2012).
Collagenase ınhibitory activity
The inhibitory effect of silver nanoparticles was examined on collagenase using substrate FALGPA. To determine IC50 and Ki values, different substrate and Ag-NPs (200–2000 µg/mL) concentrations were used, and the control enzyme activity without Ag-NPs was plotted at 100%. The used Ag-NPs had IC50 and Ki values of 14.83 ± 0.97 mM (r2: 0.978) and 1.17 ± 0.06 mM, respectively. The effects of metal ions on different enzyme activities have been discussed in previously reported studies (Kucuk and Gulcin 2016; Kırıcı et al. 1843; Caglayan et al. 2020). Lineweaver–Burk plots were used to determine the type of collagenase activity inhibition that Ag-NPs caused, and it indicated a non-competitive inhibition of collagenase (Fig. 8). Kang et al. (2005) investigated the effect of monovalent and divalent cations on a collagenolytic enzyme. They reported that Cu+2 strongly inhibited the enzyme with 3% relative activity, although other metal ions (Fe+2, Co+2, Mn+2, and K+) also had the potential to inhibit the enzyme (Kang et al. 2005). Similarly, it has been reported in some previous studies that heavy metal ions such as Hg2+, Cu2+, Pb2+, Zn2+, Ni2+, Fe2+, and Ag+ inhibited collagenase (Hanada et al. 1973; Chakraborty and Chandra 1986). The results demonstrated that the nanoparticles have inhibition potency on ChC activity, offering further insight into the inhibition of the enzyme.
Conclusion
According to 2023 data from the official website of the Agricultural Economics and Policy Development Institute (AEPDI), Turkey has become the country that produces the most figs in the world, with 320 thousand tons of fig production. The leaves of figs made in Turkey are considered agricultural waste in the country, and this study has been an excellent example of how waste can be evaluated, contributing to scientific studies and bringing it into the economy. This study reported a simple, one-step, environmentally friendly synthesis of Ag-NPs using F. carica leaf extract. Reduction of Ag+ ions to Ag-NPs phytochemicals such as flavonoids, phenolic compounds, and tannins found in F. carica leaf extract served as reducing agents. While Ag-NPs synthesized with the help of STEM were observed to have a spherical structure and different sizes, powder XRD analysis revealed that the average particle size was approximately 23 nm. Experiments have shown that Ag-NPs are effective on pathogenic bacteria such as Staphylococcus aureus, Staphylococcus epidermidis, and Enterobacter aerogenes, Salmonella infantis. Biosynthesized Ag-NPs from F. carica displayed crucial antibacterial properties, as observed by their MIC, MBC values, and antibiofilm activity. Therefore, they could be applied in pomade and plaster, including Ag-NPs and an eco-friendly antibacterial agent in other fields. Ag-NPs have been extensively studied in the clinic as components of advanced anticancer agents against cancer. The Ag-NPs synthesized in this study showed anticancer properties in the HeLa cell lines and produced similar results to the literature. The emergence of new areas of use of nanomaterials has led scientists to find different synthesis methods in this field. The biosynthesis method is the best alternative for chemical and other physical methods due to its easy synthesis, low cost, and easy availability. Nanoparticles can be used in many fields, such as agriculture, textile, and material science. Ag-NPs have unique optical, electrical, and thermal properties. Due to their antibacterial and antibiofilm properties, Ag-NPs obtained by the biosynthesis method can be used in medical applications, the paint industry, electronics, wastewater treatment plants, and filter membranes of drinking water tanks.
References
Ahmed S, Saifullah M, Ahmad BL, Swami SI (2016) Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J Radiat Res Appl Sci 9:1–7. https://doi.org/10.1016/j.jrras.2015.06.006
Ajitha B, Reddy YAK, Reddy PS (2015) Biosynthesis of silver nanoparticles using momordica charantia leaf broth: evaluation of their innate antimicrobial and catalytic activities. J Photochem Photobiol B 146:1–9
Ajitha B, Reddy YAK, Rajesh KM, Reddy PS (2016) Sesbania grandiflora leaf extract assisted green synthesis of silver nanoparticles: antimicrobial activity. Mater Today: Proc 3(6):1977–1984
Al-Sheddi ES, Farshori NN, Al-Oqail MM, et al (2018) Anticancer potential of green synthesized silver nanoparticles using extract of nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg Chem Appl
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. https://doi.org/10.1093/jac/48.suppl_1.5
Annamalai A, Vishnudas D, Mitra B, Sant SB (2011) Drug invention today green-synthesis and characterization of silver nanoparticles by aqueous leaf extracts of Cardiospermum helicacabum L. Drug Invention Today 4:340–344
Arora S, Jain J, Rajwade JM, Paknikar KM (2008) Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett 179:93–100. https://doi.org/10.1016/j.toxlet.2008.04.009
AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3(2):279–290. https://doi.org/10.1021/nn800596w
Belachew GT (2023) Silver nanoparticle for disease treatment: a review. Biomed J Sci Tech Res 49(1):40262–40267
Biswas A, Vanlalveni C, Adhikari PP, Lalfakzuala R, Rokhum L (2019) Biosynthesis, characterisation and antibacterial activity of Mikania micrantha leaf extract-mediated AgNPs. Micro Nano Lett 14(7):799–803
Caglayan C, Taslimi P, Türk C, Gülcin İ, Kandemir FM, Demir Y, Beydemir Ş (2020) Inhibition effects of some pesticides and heavy metals on carbonic anhydrase enzyme activity purified from horse mackerel (Trachurus trachurus) gill tissues. Environ Sci Pollut Res 27:10607–10616
Chakraborty R, Chandra AL (1986) Purification and characterization of a streptomycete collagenase. J Appl Bacteriol 61:331–337
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. https://doi.org/10.1128/CMR.15.2.167-193.2002
Du J, Cong Y, Wang X, Kang Y, Zhang P, Li L (2023) Green synthesis of antimicrobial peptide-protected silver nanoclusters with regulated antibacterial behavior. ACS Appl Bio Mater 6:3919–3926. https://doi.org/10.1021/acsabm.3c00646
Ekici S, Bozkaya E, Bozkaya O, Cerci NA, Aluc Y, Ekici H (2023) Vitex Agnus-Castus L. nanoparticles: preparation, characterization and assessment of antimicrobial and anticancer activity. ChemistrySelect 8(32):e202302102
Elemike EE, Onwudiwe DC, Fayemi OE, Ekennia AC, Ebenso EE, Tiedt LR (2017) Biosynthesis, electrochemical, antimicrobial and antioxidant studies of silver nanoparticles mediated by Talinum triangulare aqueous leaf extract. J Cluster Sci 28:309–330
Erdogan O, Abbak M, Demirbolat GM, Birtekocak F, Aksel M, Pasa S, Cevik O (2019) Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: the characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 14:e0216496. https://doi.org/10.1371/journal.pone.0216496
Erdoğan Ö, Paşa S, Cevik O (2021) Green synthesis and characterization of anticancer effected silver nanoparticles with silverberry (elaeagnus angustifolia) fruit aqueous extract. Int J Pure Appl Sci 7(3):391–400
EUCAST (2023) The European committee on antimicrobial susceptibility testing. breakpoint tables for interpretation of MICs and zone diameters. Version 13.1, https://www.eucast.org/mic_and_zone_distributions_and_ecoffs
Goswami SR, Sahareen T, Singh M, Kumar S (2015) Role of biogenic silver nanoparticles in disruption of cell–cell adhesion in Staphylococcus aureus and Escherichia coli biofilm. J Ind Eng Chem 26:73–80
Hanada K, Mizutani T, Yamagishi M, Tsuji H, Misaki T, Sawada J (1973) The isolation of collagenase and its enzymological and physioco-chemical properties. Agr Biol Chem 37(8):1771–1781
Hasdemir M (2023) Agricultural economics and policy development institute, 1–2
Huang W, Yan M, Duan H, Bi Y, Cheng X, Yu H (2020) Synergistic antifungal activity of green synthesized silver nanoparticles and epoxiconazole against Setosphaeria turcica. J Nanomater 2020:1–7
Iravani S, Korbekandi H, Mirmohammadi SV, Zolfaghari B (2014) Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci 9(6):385
Ivask A, ElBadawy A, Kaweeteerawat C, Boren D, Fischer H, Ji Z, Chang CH, Liu R, Tolaymat T, Telesca D, Zink JI, Cohen Y, Holden PA, Godwin HA (2014) Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. ACS Nano 8:374–386. https://doi.org/10.1021/nn4044047
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi. Plant Dis 93:1037–1043. https://doi.org/10.1094/PDIS-93-10-1037
Kalishwaralal K, BarathManiKanth S, Pandian SRK, Deepak V, Gurunathan S (2010) Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces 79:340–344. https://doi.org/10.1016/j.colsurfb.2010.04.014
Kang S, Jang YB, Choi YJ, Kong JY (2005) Purification and properties of a collagenolytic protease produce by marine bacterium Vibirio vulnificus CYK279H. Biotechnol Bioprocess Eng 10:593–598
Kırıcı M, Kırıcı M, Beydemir Ş, Atamanalp M (2016) Purification of carbonic anhydrase from Capoeta umbla (Heckel, 1843) gills and toxicological effects of some metals on enzyme activity. Turk J Fish Aquatic Sci 16:169–175
Korkmaz N (2019) Antibacterial activity and biofilm property of silver nanoparticles synthesized by using saintpaulia aqueous leaf extract. J Inst Sci Technol 9:2225–2235. https://doi.org/10.21597/jist.561197
Korkmaz N (2020) Bioreduction: the biological activity, characterization, and synthesis of silver nanoparticles. Turk J Chem 44:325–334. https://doi.org/10.3906/kim-1910-8
Korkmaz N, Ceylan Y, Hamid A, Karadağ A, Bülbül AS, Aftab MN, Çevik Ö, Şen F (2020a) Biogenic silver nanoparticles synthesized via Mimusops elengi fruit extract, a study on antibiofilm, antibacterial, and anticancer activities. J Drug Deliv Sci Technol 59:101864. https://doi.org/10.1016/j.jddst.2020.101864
Korkmaz N, Ceylan Y, Taslimi P, Karadağ A, Bülbül AS, Şen F (2020b) Biogenic nano silver: synthesis, characterization, antibacterial, antibiofilms, and enzymatic activity. Adv Powder Technol 31:2942–2950. https://doi.org/10.1016/j.apt.2020.05.020
Korkmaz N, Ceylan Y, Karadag A, Bülbül AS, Nauman MA, Saygılı S, Şen F (2020c) Biogenic silver nanoparticles synthesized from Rhododendron ponticum and their antibacterial, antibiofilm and cytotoxic activities. J Pharm Biomed Anal 179:112993. https://doi.org/10.1016/j.jpba.2019.112993
Kucuk M, Gulcin İ (2016) Purification and characterization of the carbonic anhydrase enzyme from Black Sea trout (Salmo trutta Labrax Coruhensis) kidney and inhibition effects of some metal ions on enzyme activity. Environ Toxicol Pharmacol 44:134–139
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157. https://doi.org/10.1002/jctb.2023
Logeswari P, Silambarasan S, Abraham J (2015) Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J Saudi Chem Soc 19:311–317. https://doi.org/10.1016/j.jscs.2012.04.007
Merritt JH, Kadouri DE, O’Toole GA (2011) Growing and analyzing static biofilms. Curr Protoc Microbiol. https://doi.org/10.1002/9780471729259.mc01b01s22
Miller KP, Wang L, Benicewicz BC, Decho AW (2015) Inorganic nanoparticles engineered to attack bacteria. Chem Soc Rev 44:7787–7807. https://doi.org/10.1039/C5CS00041F
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, Yacaman MJ (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353. https://doi.org/10.1088/0957-4484/16/10/059
Namasivayam SKR, Chitrakala K (2011) Ecotoxicological effect of Lecanicillium lecanii (Ascomycota: Hypocreales) based silver nanoparticles on growth parameters of economically important plants. J Biopestic 4:97–101
Nematollahi F (2015) Silver nanoparticles green synthesis using aqueous extract of Salvia limbata C.A. Mey. Int J Biosci 6:30–35
Pal S, Tak YK, Song JM (2007) Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl Environ Microbiol 73:1712–1720. https://doi.org/10.1128/AEM.02218-06
Pallavicini P, Arciola CR, Bertoglio F, Curtosi S, Dacarro G, D’Agostino A, Ferrari F, Merli D, Milanese C, Rossi S, Taglietti A, Tenci M, Visai L (2017) Silver nanoparticles synthesized and coated with pectin: an ideal compromise for anti-bacterial and anti-biofilm action combined with wound-healing properties. J Colloid Interface Sci 498:271–281. https://doi.org/10.1016/j.jcis.2017.03.062
Philip D, Unni C, Aromal SA, Vidhu VK (2011) Murraya koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc 78:899–904. https://doi.org/10.1016/j.saa.2010.12.060
Prathna TC, Sharma SK, Kennedy M (2018) Nanoparticles in household level water treatment: an overview. Sep Purif Technol 199:260–270
Rajkuberan C, Prabukumar S, Sathishkumar G, Wilson A, Ravindran K, Sivaramakrishnan S (2017) Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J Saudi Chem Soc 21(8):911–919
Ramadan MA, Shawkey AE, Rabeh MA, Abdellatif AO (2020) Promising antimicrobial activities of oil and silver nanoparticles obtained from Melaleuca alternifolia leaves against selected skin-infecting pathogens. J Herbal Med 20:100289
Sangiliyandi G (2010) Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomed. https://doi.org/10.2147/IJN.S11727
Santhoshkumar J, Agarwal H, Menon S, Rajeshkumar S, Venkat Kumar S (2019) A biological synthesis of copper nanoparticles and its potential applications, içinde. Green synthesis, characterization and applications of nanoparticles. Elsevier, pp 199–221. https://doi.org/10.1016/B978-0-08-102579-6.00009-5
Saravanakumar K, Chelliah R, Shanmugam S, Varukattu NB, Oh D-H, Kathiresan K, Wang M-H (2018) Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J Photochem Photobiol B Biol 185:126–135. https://doi.org/10.1016/j.jphotobiol.2018.05.032
Sengottaiyan A, Mythili R, Selvankumar T, Aravinthan A, Kamala-Kannan S, Manoharan K, Thiyagarajan P, Govarthanan M, Kim J-H (2016) Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res Chem Intermed 42:3095–3103. https://doi.org/10.1007/s11164-015-2199-7
Sheny DS, Mathew J, Philip D (2011) Phytosynthesis of Au, Ag and Au-Ag bimetallic nanoparticles using aqueous extract and dried leaf of Anacardium occidentale. Spectrochim Acta Part A Mol Biomol Spectrosc 79:254–262. https://doi.org/10.1016/j.saa.2011.02.051
Singh M, Singh S, Prasad S, Gambhir IS (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig J Nanomater Biostruct 3:115–122
Soman S, Ray JG (2016) Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: characterization and assessment of antibacterial activity. J Photochem Photobiol b: Biol 163:391–402
Sondi I, Salopek-Sondi B (2004) Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J Colloid Interface Sci 275:177–182. https://doi.org/10.1016/j.jcis.2004.02.012
Song JY, Kim BS (2009) Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst Eng 32:79–84. https://doi.org/10.1007/s00449-008-0224-6
Sukirtha R, Priyanka KM, Antony JJ, Kamalakkannan S, Thangam R, Gunasekaran P, Krishnan M, Achiraman S (2012) Cytotoxic effect of green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem 47:273–279. https://doi.org/10.1016/j.procbio.2011.11.003
Tang S, Zheng J (2018) Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater 7:1701503. https://doi.org/10.1002/adhm.201701503
Tian J, Wong KKY, Ho C-M, Lok C-N, Yu W-Y, Che C-M, Chiu J-F, Tam PKH (2007) Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2:129–136. https://doi.org/10.1002/cmdc.200600171
Vanlalveni C, Lallianrawna S, Biswas A, Selvaraj M, Changmai B, Rokhum SL (2021) Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv 11(5):2804–2837
Van Wart H, Steinbrink DR (1981) A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal Biochem 113(2):356–365. https://doi.org/10.1016/0003-2697(81)90089-0
Velmurugan P, Cho M, Lim SS, Seo SK, Myung H, Bang KS, Oh BT (2015) Phytosynthesis of silver nanoparticles by Prunus yedoensis leaf extract and their antimicrobial activity. Mater Lett 138:272–275
Velsankar K, Aswin Kumar RM, Preethi R, Muthulakshmi V, Sudhahar S (2020) Green synthesis of CuO nanoparticles via Allium sativum extract and its characterizations on antimicrobial, antioxidant, antilarvicidal activities. J Environ Chem Eng 8:104123. https://doi.org/10.1016/j.jece.2020.104123
Velsankar K, Vinothini V, Sudhahar S, Kumar MK, Mohandoss S (2020a) Green synthesis of CuO nanoparticles via Plectranthus amboinicus leaves extract with its characterization on structural, morphological, and biological properties. Appl Nanosci 10:3953–3971. https://doi.org/10.1007/s13204-020-01504-w
Velsankar K, Preethi R, Ram PSJ, Ramesh M, Sudhahar S (2020b) Evaluations of biosynthesized Ag nanoparticles via Allium Sativum flower extract in biological applications. Appl Nanosci 10:3675–3691. https://doi.org/10.1007/s13204-020-01463-2
Velsankar K, Parvathy G, Mohandoss S, Krishna Kumar M, Sudhahar S (2022) Celosia argentea leaf extract-mediated green synthesized iron oxide nanoparticles for bio-applications. J Nanostruct Chem 12:625–640. https://doi.org/10.1007/s40097-021-00434-5
Vilchis-Nestor AR, Sánchez-Mendieta V, Camacho-López MA, Gómez-Espinosa RM, Camacho-López MA, Arenas-Alatorre JA (2008) Solventless synthesis and optical properties of Au and Ag nanoparticles using Camellia sinensis extract. Mater Lett 62:3103–3105. https://doi.org/10.1016/j.matlet.2008.01.138
Wani IA, Ganguly A, Ahmed J, Ahmad T (2011) Silver nanoparticles: ultrasonic wave assisted synthesis, optical characterization and surface area studies. Mater Lett 65:520–522. https://doi.org/10.1016/j.matlet.2010.11.003
Yang G, Xie J, Deng Y, Bian Y, Hong F (2012) Hydrothermal synthesis of bacterial cellulose/AgNPs composite: a “green” route for antibacterial application. Carbohydr Polym 87:2482–2487. https://doi.org/10.1016/j.carbpol.2011.11.017
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Korkmaz, N., Ceylan, Y., İmamoğlu, R. et al. Eco-friendly biogenic silver nanoparticles: synthesis, characterization and biological applications. Int. J. Environ. Sci. Technol. (2024). https://doi.org/10.1007/s13762-024-05860-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13762-024-05860-w