Abstract
The application of membrane technology in wastewater treatment for reuse is one of the alternatives to reduce the demand of water in domestic, agricultural and industrial sectors. This study attempts to improve wastewater quality effluent, prior to disinfection, for re-use. Clarifier effluent from a biological nutrient removal (BNR) treatment plant was diverted to a nonwoven membrane pilot filtration unit. It was thus positioned to further enhance the clarifier effluent as a polishing arrangement. Water Quality Index (WQI), Single Factor Pollution Index (Pi), Water Use License (WUL) and removal efficiency methods were used to interpret the water quality data for BNR clarifier effluent and nonwoven membrane permeate. The results showed that this nonwoven microfilter membrane is able to improve wastewater quality from BNR clarifier effluent. Escherichia coli (E. coli) reduction was the highest with a removal efficiency of 90%, followed by chemical oxygen demand 25%, NH4 + 22%, NO3− 12.6%, PO4 7.8%, suspended solids 6.3%. The lowest reduction was electrical conductivity of 5.2%. Permeate results indicated that physiochemical and microbial parameters were complying with the limits of WUL. Pi has revealed that BNR clarifier effluent water quality is medium polluted versus slightly polluted permeate. WQI results for BNR clarifier effluent showed good water quality and the water can be reused for domestic, irrigation and industrial purposes, while permeate WQI results indicated excellent water quality and the water can be reused for drinking, domestic, irrigation and industrial purposes. Outstanding permeate water quality improvement was observed on E.coli counts improving from 4974.48 to 294.33 counts/L. The standard of E.coli according to the WUL at this wastewater care works is 500 counts/L. Results indicate that nonwoven membrane filtration can improve microbial contamination and decrease the demand of chlorine for disinfection of wastewater final effluent. The nonwoven membrane filtration can decrease the water scarcity gap in South Africa for direct water reclamation by improving effluent wastewater.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
South Africa has been walking a thin line with water scarcity for decades now. Currently, the water usage trends are not sustainable. The example of Cape Town’s, South Africa recent drought exposed this gap in the water sector. It is therefore essential to consider alternative sources for water (Donnenfeld 2018). Furthermore, depleting ground water sources by farming activities together with present usage of potable water to flush toilets and irrigate household and inner-city gardens is not sustainable (Howard 2015). According to the 2014 green drop audit close to a quarter of South Africa’s wastewater treatment facilities are in a critical state and the report concluded that these treatment facilities are defined as high risk, requiring urgent intervention (Donnenfeld et al. 2018). Reclamation from these poor functioning treatment facilities will thus be challenging. Wastewater reuse and recycling are important alternatives to reduce demand in domestic, agricultural and industrial sectors (Kesari et al. 2021). South Africa’s limited water resources will limit economic and social development. For efficiency improvement and expansion, it is necessary to develop improved wastewater treatment (WWT) technologies appropriate for rural areas and or decentralized residential areas in emerging countries (Donnenfeld et al. 2018). For wastewater treatment, membrane filtration is among the technologies that show excellent improvement of biological nutrient removal (BNR) clarifier effluent. Membrane filtration is increasingly being specified as a possible alternative for pollution reduction and reclamation of wastewater for reuse (Chang et al. 2007). Considering the wide applications of membrane filtration, microfiltration (MF) membranes have been extensively used for improvement of wastewater effluent (Anspach et al. 1999; Zhu et al. 2012). Methods in which MF can be operated are dead-end mode and cross-flow mode. In dead end membrane operation, the pumped feed moves perpendicular to the surface of the membrane. Water passes through the filters and it is recovered as permeate. Fouling layer or cake is formed when solids accumulate on the filter surfaces. This decreases the flow through filters, known as the flux or flow through the filter per meter squared per hour. Flux reduction to extend where membranes are blocked or fouled, is a major challenge. In cross-flow membrane operation, the feed is pumped indirectly on the surface of the membrane and solids accrued on the filters to form cake. Different membrane configurations and wastewater treatment operating procedures have been developed by suppliers for ultrafiltration and microfiltration membranes (Tchobanoglous et al. 1998). The microfilters that were used in this study consist of nonwoven polyacrylonitrile fiber needlefelt membranes. Nonwovens are composed of a random network of overlap fibers creating multiple connected pores through which liquids can flow. It has controllable pore size distribution and high fiber surface area per unit weight-volume. Treatment cost can be drastically reduced with use of low-cost membranes which are constructed of inexpensive fibers. Limited information was found in the literature on the use of nonwoven material for membrane filtration processes (Ren et al. 2010). Currently, the study has not finalized on treating effluent quality for wastewater and additional parameters such as reduction in chemicals of emerging concern are evaluated. Currently in South Africa there is less than five water reclamation plant, thus it means most of the water is returned to the environment, while it can be further treated to supplement water supply. Membrane process has attracted increasing attention in recent years as an alternative treatment for municipal wastewater (Van Nieuwenhuijzen et al. 2008). Various studies have compared membrane technology with convention-activated sludge treatment (Munz et al. 2008; Otton et al. 2006; Pauwels et al. 2006). Nonwoven membranes due to their good mechanical properties and high porosities could be used as the filter material for domestic wastewater treatment coupled with activated sludge system (Kiyak et al. 2019). There is a growing interest in nonwovens because of their low cost of manufacturing and it is one of the most promising wastewater treatment technologies (Fujii et al. 2002). The main objective of this research was to apply the nonwoven microfiltration membrane on activated sludge final effluent for improving wastewater quality for re-use. This was attempted by a nonwoven filtration media together with aeration to improve physical performance of the membrane with respect to flux.
Materials and methods
Materials
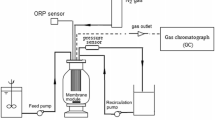
The research was conducted out by using BNR secondary clarifier effluent from a wastewater care works in the east of Gauteng, South Africa. Figure 1 illustrates the schematic diagram of the membrane system that was adopted in this study. The influent (secondary clarifier effluent) was pumped firstly to an outside tank which is named (pilot tank) for this purpose, samples collected from this pilot tank are also compared to the final permeate samples and influent stream. The pilot tank could act as a control environment for comparison of the treatment capability of the microfilter.
The experimental setup consisted of a pilot tank and filter unit. Influent was pumped into the tank unit that supplied the needlefelt microfilter arrangement. The filtrate pump was used to pump the permeate out of the filtrate unit, and the recycle pump was used to pump some of the nitrate-loaded filtrate from filter unit back to the pilot tank to allow denitrification to take place. The recycle pump also functioned as a waste pathway by redirecting it away from the tank unit. Air was supplied in the filter unit by an oil-less compressor for cleaning and ensuring dissolved oxygen of above 4 mg/L. Making this process aerobic, thus providing additional nitrification. (Patent 2019/07569). BNR secondary clarifier effluent passed through the filters at flow rate 3.0 L/min at atmospheric pressure. The membrane was operated in dead end mode. The average flux was calculated at 12 L /m2/h. The membrane module was aerated continuously to reduce membrane fouling and the DO level was measured to be 7.4 mg/L. Although the influent supplied to the experimental setup originated from the outflow of BNR secondary clarifier, residual microbes accumulated as attached biomass on the filter surfaces in a similar manner as seen on rotating biological contactors in Figs. 2 and 3. This biomass acted and enabled the additional treatment such as reduction in E.coli as seen in the experimental results (Aybar et al. 2019). Table 1 illustrates polyacrylonitrile (PAN) nonwoven membrane specifications.
Cleaning procedure
Membrane fouling results in reduction in filtrate production causing flux to decrease over time under continuous trans-membrane operation. Cleaning of the membrane was achieved by an increase in aeration. This air scouring action restored the membrane flux. Membrane cleaning was done only once during the study and for a duration 15 min.
Analytical methods
The secondary clarifier effluent and permeate samples were analyzed for ammonium nitrogen (NH4+), nitrates (NO3−), phosphate (PO4), chemical oxygen demand (COD), potential hydrogen (pH), electrical conductivity (EC), suspended solids (SS) and Escherichia coli (E.coli). All parameters were analyzed according to the standard methods of water and wastewater (Alpha, 2017). The collected data were analyzed from the laboratory, further comparison with of water use license standard was performed (see Table 2).
Removal efficiency of permeate was calculated to determine how efficient the pilot plant is in removing targeted water quality parameters. The removal efficiency was calculated using Eq. (1):
where: CA1 is the mass concentration in BNR clarifier effluent at the system input (mg/L) and CA2 is the mass concentration of the permeate at the system output (mg/L) and EA is the concentration increment of pollution in percentage (mg/L).
Single factor pollution index was used to determine the extent of pollution for BNR clarifier effluent discharged to the surface and also permeate. The single factor analysis was calculated using Eq. (2):
where Pi is the pollution index of i units pollutant. Ci is the measured concentration of i units pollutant (mg/L), Si the III level water quality standard category value of i units pollutant according to “Environmental Quality Standards for Surface Water. Table 3 shows the category of single factor pollution index in determination of water quality status and pollution.
Water quality index was also used to determine the suitability for the BNR clarifier effluent and the permeate. Water Quality Index calculated using Eq. (3):
where Qi is the Quality rating of ith parameter for a total of n water quality parameters Vactual (mg/l) is the actual value of the water quality parameter obtained from analysis.
Videal (mg/l) is the Ideal value of that water quality parameter can be obtained from the standard Tables.
The relative (unit) weight (Wi) was calculated by a value inversely proportional to the recommended standard (Si) for the corresponding parameter using Eq. (4):
where I is given by I = 1/∑1/Si and Wi is the Relative (unit) weight for nth parameter Si is the standard permissible value for nth parameter I is proportionality constant. The Relative (unit) weight (Wi) to different water Quality parameters is inversely proportional to the recommended standards for the corresponding parameters. Finally, the overall WQI was calculated by combining the quality rating with the unit weight linearly by using Eq. (5):
where, Qi is the Quality rating and Wi is the Relative (unit) weight
The water quality was categorized according to Table 4.
Results and discussion
Water use license and removal efficiency
The obtained water quality results from clarifier effluent and permeate are demonstrated graphically (Figs. 4, 5, 6, 7, 8, 9, 10, 11) and presented in Table 5.
The water quality trends for physicochemical and biological characteristics were presented against the Water Use License (WUL). Analyses for secondary clarifier effluent showed that ammonia, conductivity and E.coli did not fully comply with the WUL during the study. Non-compliance for ammonium nitrogen (NH4+) and electrical conductivity (EC) was due to failure of BNR which could be due to critical equipment failures linked to nitrification in the BNR. E.coli non-compliance was attributed to no disinfection (Chlorination) done in the clarifiers effluent. Water quality parameters that did not comply with the WUL for permeate were NH4+, pH, EC and E.coli.
NH4+ concentration ranged between 4 and 7.5 mg/L which was due to membrane fouling resulting in flux decline, and it was addressed by membrane back flushing and air scoring. Similar results were reported by Mahmoud & Soumaya (2020) who demonstrated that membrane fouling contributes to high NH4+ concentration in the permeate and can be reduced by back flushing and air scoring. A decrease in pH to 6.4, due to alkalinity consumption could be explained by nitrification taking place in the pilot plant. Hwang et al., (2000), Hou et al., (2014) and Belmonte et al., (2017) reported similar results that a reduction in the alkalinity concentration increased the nitrite accumulating efficiency in the process and this can attribute to pH value decreasing inside the reactor which stimulated the inhibitory effect of free nitrous acid on nitrite oxidizing bacteria. EC ranged between 80 and 86 mS/m due to poor nitrification and also poor alkalinity consumption resulting in conductivity increasing, this was caused by microfiber filter inability to retain dissolved ions. Similar results were reported by Levin (2007) who demonstrated that conductivity increases if the transformation of ammonium to nitrate produces hydrogen ions and no alkalinity consumption. E.coli ranged between 500 counts/L and 12,000 counts/L which could be linked to biofilm augmentation on the micro filter. Nonwoven membrane microfiltration results showed excellent removal efficiency for E.coli which was 90% without disinfection. Therefore, for these bacteria nonwoven membrane demonstrated a sufficient barrier. These results could be explained by microbial attachment and formation of biofilm on the membrane surface. The flow through the microfilters enhanced contact of the attached microbes and possible food sources in the wastewater. The microfilters also received aeration. This may have encouraging growth of facultative microbes and higher predator species as mentioned by Aybar et al., (2019). Koltuniewicz & Drioli (2008) and Bodzek et al., (2019) reported that E.coli from municipal wastewater after primary and secondary treatment using microfiltration membranes showed significant removal, and high removal efficiency of E.coli was explained by filtration layer formation on the surface of the membrane, which performed as a secondary film layer. The formation of biofilm on the membrane surface may also lead to biofouling; however, Wang & Waite (2009) reported that biofouling layer can work as an additional layer in the submerged MBR improving the water quality if it is well maintained. In addition, Dreszer et al., (2014) also reported that bacteria cell in biofilm formation does not reduce the performance of the membrane, however extracellular polymeric substance (EPS) plays an important role in the performance decline of membrane. Aybar et al., (2019) also reported that re-aeration of final effluent affects bacterial activities of E.coli as these conditions become unfavorable for their enzymes.
Removal efficiency for COD was obtained to be 25%. The results indicated that there was further oxidation of organic matter that was taking place in the membrane pilot plant due to sufficient oxygen supply for the facultative microbes that have attached on the membrane surface. The aeration mechanism of this patented microfilter also enhances smaller air bubbles to be in close vicinity of the biofilm layers formed on the filtration surface, augmenting nitrification. According to the study done by Mahmoud & Soumaya (2020), it was reported that oxygen eliminates the organic contaminants, and it is required for the bacteria to allow biodegradation to take place. Obtained NH4+ removal efficiency was 22%, these results indicated that there was sufficient oxygen and further nitrification was taking place due to continuous aeration of the pilot. Metcalf & Eddy (2002) reported that for nitrification to take place effectively the process needs to be operated at an optimum DO level. NO3− removal efficiency obtained was 12.6%, the results showed that further denitrification took place in the pilot due to further oxidation of organic matter created by the recycle stream from filtrate unit back to the pilot tank. Makaya et al., (2007) reported that when the oxygen demand is high, nitrification decreases and nitrates removal efficiency increases. PO4 and SS obtained removal efficiency of 7.8% and 6.3% respectively, much difference was not seen for these parameters due to instrument detector limit of 0.1 for PO4 and 10 for SS. EC obtained lowest removal efficiency of 5.4%, these results indicated that there was no net change ion content and conductivity remained the same. This is comparable with the results obtained by Howard et al., (2004) and Levin (2007) who reported that when the nitrate content increases, the phosphate content decreases resulting in conductivity remaining the same for influent and effluent, showing that there is no net change in the ion content, see Fig. 11. Low concentration of dissolved ion in the secondary clarifier effluent may also attribute low conductivity removal efficiency (Lajci et al. 2017).
Single factor pollution index
The Single Factor Pollution Index was calculated to determine the extent of pollution in the final effluent discharge from BNR clarifier effluent and the permeate as indicated in Tables 6 and 7, respectively.
According to the single factor pollution index BNR clarifier effluent water quality for E.coli indicated serious pollution, the pollution level was above 5 as per standard Table 3. This pollution range indicated that excessive pollution is occurring at the downstream where the effluent is discharged. High E.coli may present health problems in the receiving environment and limit reuse purposes. Okah et al. (2007) and Akpor and Muchie (2011) studied the presence of E. coli in streams in which wastewater is discharged. The results confirmed that E. coli can cause infections such as diarrhea for adults and kidney failure for children. Conductivity showed medium pollution with the rating between 1 and 2. High conductivity indicates that dissolved salts or chemical ions are high and it affects the ecosystem. According to Masters & Ela (2007) and Pal et al. (2015) who studied the presence of conductivity on lakes, dissolved ion concentrations control the survival, reproduction, and growth of aquatic organisms. COD, pH and SS indicated slight pollution with the level rating between 0.5 and 1. The presence of biodegradable organic matters in water is noticed through high COD and DO levels decrease affecting the aquatic life. Van den Brand, et al., (2015) and Edokpayi et al. (2017) studied the presence of COD in streams in which wastewater is discharged. The results indicated that a high COD level decreases the DO level in streams and affects the functioning of the ecosystem. pH level needs to range between (6.5 and 8.5), if the pH level is out of this range, it indicates that there is industrial pollution and aquatic life will be affected. Akpor and Muchie (2011) studied the presence of pH in streams and reported that low pH level kills the aquatic life and cause physical damage and leaving them vulnerable to diseases. SS reduces photosynthesis and physically harms the aquatic life. Akpor and Muchie (2011) also reported that SS reduces penetration of sunlight and aquatic life are physical harmed. NH4+, NO3− and PO4 rated less than 0.4 which indicated non-pollution.
According to the single factor pollution index the permeate water quality indicated that E.coli, EC, pH and SS are slightly polluted, these parameters are rated between the level of 0.5 – 1. NH4+, NO3−, PO4 and COD rated less than 0.4 which indicated non-pollution.
Water quality index
To determine water reuse options, the water Quality Index was calculated for BNR clarifier effluent and permeate. The calculated WQI for BNR clarifier effluent is presented in Table 8 and was found to be 28.03.
According to the standard Table 6 for classification of WQI, the water quality falls within the range of 26–50, therefore it can be classified as good water. Good water refers to the reuse options that include domestic, irrigation and industrial. However, it is limited to water treatment reuse for drinking purposes. The WQI was affected by high conductivity and E.coli counts from final effluent clarifiers, thereby limiting the direct reuse without chlorination for E.coli. In agreement with the results obtained by Mohamad (2019) reported that the presence of E.coli in the BNR effluent discharged to the downstream affected the WQI and Rim-Rukeh & Agbozu (2013) reported that the presence of conductivity can also affect WQI. According to the results presented in Table 9, the WQI for membrane filtration was found to be 21.13, falling within the range of 0–25.
According to the standard rating Table 6, the WQI category presents the excellent water quality which can be reused for drinking, domestic, irrigation and industrial purposes. E.coli counts from 4974.48 to 294.33 counts/L, COD improved from 36.67 to 25.19 mg/L and conductivity improved from 85.28 to 80.04 mS/m. The standard of E.coli according to the WUL at this WCW is 500 counts/L. Similar results were obtained by Wintgens et al., (2005) in Windhoek, Namibia using UF membrane for BNR clarifier effluent and major successes were achieved on the removal of microbial and chemical components without disinfection.
Conclusion
The permeate from these tests showed that NO3−, PO4, COD and SS fully complied with the WUL standard. NH4+, pH, EC and E.coli did not comply fully with the WUL standard during the study. For the permeate sample, the highest removal efficiency obtained in this study using nonwoven membrane filtration was for E.coli, at 90%, followed by COD 25%, NH4N 22%, NO3− 12.6%, PO4 7.8%, SS 6.3% and the lowest was EC 5.2%. Therefore, it was concluded that nonwoven membrane filtration is able to improve the removal efficiency of clarifier effluent. This could be due to microbes attachment on the membrane, evident by the reduction in E.coli, COD and NH4+. The average pollution index for BNR clarifier effluent and permeate was determined to be 1.74 (medium polluted) and 0.52 (slightly polluted), respectively. The use of nonwoven membrane filtration presented small, significant improvements in the extent of pollution in the effluent discharged to the downstream. According to the results of WQI BNR clarifier effluent, the water quality rating is 28.03, it is regarded as good water and can be reused for domestic, Irrigation and Industrial, except drinking purposes. WQI for permeate rated is 21.13. According to the standard rating of WQI the water quality at permeate is regarded as excellent water quality which can be reused for drinking (with additional treatment such as activated carbon and additional disinfection), domestic, irrigation and industrial. As nonwoven membrane filtration decreases microbial contamination, it lowers chlorine demand of wastewater final effluent, and also showed that it can improve nitrification, alkalinity consumption and has strength in further oxidizing organic matters. The low cost and local fabrication of such a polishing treatment support this treatment as a possible alternative.
References
Akpor OB, Muchie M (2011) Environmental and public health implications of wastewater quality. Afr J Biotechnol 10:2379–2387
Anspach FB, Petsch D, Deckwer WD (1999) Model studies on the mechanism of endotoxin adsorption on flat-sheet microfiltration membrane adsorbers. Can J Chem Eng 77:921–930
Aybar M, Perez-Calleja P, Li M, Pavissich JP, Nerenberg R (2019) Predation creates unique void layer in mebrane-aerated biofilms. Water Res 149:232–242
Belmonte M, Hsieh CF, Campos JL, Guerrero L, Mendez R, Mosquera-Corral A, Vidal G (2017) Effect of free ammonia, free nitrous acid and alkalinity on the partial nitrification of pretreated pig slurry, using an alternating oxc/anoxi SBR. BioMed Res Int 2017:1–7
Bodzek M, Konieczny K, Rajca M (2019) Membranes in water and wastewater disinfection—review. Arch Environ Protect 45(1):3–18
Chang WK, Hu AY, Y HR and Tzou WY, (2007) Membrane biorector with nonwoven fabrics as solid-liquid separation media for wastewater treatment. Desalination 202:122–128
Donnenfeld Z (2018) South Africa water crisis is bigger than the Cape. Institute of Security Studies (ISS), Pretoria, 2018
Donnenfeld Z, Crookes C and Hedden S (2018) A delicate balance Water scarcity in South Africa. Water Research Council, Pretoria
Dreszer C et al (2014) In-situ biofilm characterization in membrane systems using optical coherence tomography: formation, structure, detachment and impact of flux change. Water Res 67:243–254
Edokpayi J, Odiyo J, Durowoju O (2017) Impact of wastewater on surface water quality in developing countries: a case study of South Africa. ResearchGate,. https://doi.org/10.5772/66561,401-416
Fujii T, Sugino H, Rouse JD, Funekawa K (2002) Characterization of the microbial community in an anaerobic ammonium-oxidizing biofilm cultured on a nonwoven biomass carrier. J Biosci Bioeng 94:412–418
Hou B, Han H, Jia S, Zhuang H, Zhao Q, Xu P (2014) Effect of alkalinity on nitrite accumulation in Environment of coal chemical industry wastewater using moving bed biofilm reactor. J Environ Sci (china) 26:1014–1022
Howard KWF (2015) Sustainable cities and the groundwater governance challenge. Environ Earth Sci 73:2543–2554
Howard I, Espigares E, Lardelli P, Martin JL, Och EM (2004) Evaluation of microbiologigal and physiochemical inductors for wastewater treatment. Environ Toxicology 19:241–249
Hwang BH, Hwang KY, Choi ES, Choi DK, Jung JY (2000) Enhanced nitrite build-up in proportion to increasing alkalinity / NH4 ratio of influent in biofilim reactor. Biotechnol Lett 22:1287–1290
Kesari KK, Sani R, Jamal QMS, Tripathi P, Lae JA, Jha NK, Siddiqui MH, Kumar P, Tripathi V, Ruokolainer J (2021) Wastewater Treatment and Reuse: a Review of its Applications and Health Implications. Water Air Soil Pollut 232:208
Kiyak Y, Mazé B, Pourdeyhimi B (2019) Microfiber nonwovens as potential membranes. Sep Purif Rev 48:282–297
Koltuniewicz AB, Drioli E (2008) Membranes in clean technologies. Wiley-VchVerlag GmbH, Weinheim
Lajci N et al (2017) Performance Evaluation of Skenderaj Wastewater Treatment Plant, Kosova. J Int Environ Appl Sci 12(1):82–89
Levin E (2007) Conductivity measurements for controlling municipal wastewater treatment. Researchgate 15:51–62
Mahmoud B, Soumaya F (2020) Removal of contaminants and pathogens from secondary wastewater effluents using Hollow fiber microfiltration membrane. Int J Environ Sci Natural Resources 23:78–83
Makaya E, Hoko Z, Parawira W, Svotwa E (2007) An assessment of the effectiveness of biological nutrient removal from wastewater: a case for hatcliffe sewage treatment works in Zimbabwe. Electronic J Environ Agriculture Food Chem 10:2409–2419
Masters GM, Ela WP (2007) Introduction engineering and science, 3rd edn. Pearson, New York
Metcalf and Eddy (2002) Wastewater engineering: treatment and reuse, 4th edn. McGraw Hill, Boston
Mohamad NI (2019) Effluent quality assessment of selected wastewater treatment plant in Jordan for irrigation purposes: water quality index approach. J Ecological Eng 20:206–216
Munz G, Mori G, Salvadori L, Barberio C, Lubello C (2008) Process efficiency and microbial monitoring in MBR (membrane bioreactor) and CASP (conventional activated sludge process) treatment of tannery wastewater. Bioresour Technol 99:8559–8564
Okah AT, Odjadjare EE, Igbinosa EO, Osode AN (2007) Wastewater treatment plants as a source of microbial pathogens in receiving water sheds. African J Biotechnol 6:2932–2944
Ottoson J, Hansen A, Bjorlenuis B, Norder H, Stenstrom TA (2006) Removal of viruses, parasitic ptotozoa and microbial indicators in conventional and membrane processes in a wastewater pilot plant. Water Res 40:1449–1957
Pal M, Samal NR, Roy PK, Roy MB (2015) Electrical conductivity of Lake water as enviromental monitoring—a case of Rudrasagar Lake. J Environ Sci Toxicology Food Technol 3:66–71
Pauwels R, Fru Ngwa F, Deconinck S, Verstraete W (2006) Effluent quality of a conventional activated sludge and a membrane bioreactor system treating hospital wastewater. Environ Technol 27:395–402
Ren X, Shan HK, Jang N, Lee YG, Bae M, Lee J, Cho K, Kim IS (2010) Novel membrane bioreactor (MBR) coupled with a nonwoven fabric filter for household wastewater treatment. Water Res 44:751–760
Rim-Rukeh A, Agbozu IE (2013) Impact of partially treated sewage effluent on the water quality of recipient Epie Creek Niger Delta, Nigeria using Malaysian Water Quality Index (WQI). J Appl Sci Environ Manange 17:5–12
Tchobanoglous GJ, Bourgeous K, McArdle J, Genest P, Tylla M (1998) Ultrafiltration as an advanced tertiary treatment process for municipal wastewater. Desilination 119:315–322
Van den Brand TP, Roest K, Chen G, Brdjanovic D, Van Loosdrecht MCK (2015) Effect of chemical oxygen demand, nutrients and salinity on sulfate—reducing bacteria. Environ Eng Sci 32:1–7
Van Nieuwenhuijzen AF, Evenblij H, Uijterlinde CA, Schulting FL (2008) Review on the state of science on membrane bioreactors for municipal wastewater treatment. Water Sci Technol 27:395–402
Wang XM, Waite TD (2009) Role of Gelling soluble and colloidal microbial products in membrane fouling. Environ Sci Techn 43:9341–9347
Wintgens T, Melin T, Schafer AI, Muston M, Bixio D, Thoeye C (2005) The role of membrane processes in municipal wastewater reclamation and reuse. Desalination 178:1–11
Zhu H, Wen X, Huang X (2012) Characterization of membrane fouling in a microfiltration ceramic membrane system treating secondary effluent. Desalination 284:324–331
Acknowledgements
The authors are grateful to Ekurhuleni Water Care Company Operations Department executive manager Mr Fortune Mabunda and Mr Livhuwani Mathomu manager at Waterval WCW for allowing us to do the study at the plant. Gratitude also to Scientific services executive manager Alison Chapman for allowing analysis of samples.
Funding
Open access funding provided by Vaal University of Technology.
Author information
Authors and Affiliations
Contributions
JK: Supervision, Formal analysis, Investigation, Validation, Visualization, Writing review and editing. MSM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization. Writing original draft. JT: Conceptualization, Funding acquisition, Project administration, Supervision, Formal analysis, Investigation, Validation, Visualization.
Corresponding author
Ethics declarations
Conflict of interest
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. None of the authors of this paper has a financial or personal relationship with other persons or organizations that might inappropriately influence or bias its content.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kabuba, J., Masala, M.S. & Topkin, J. Application of nonwoven microfiltration membrane on activated sludge final effluent: improving wastewater quality for reuse. Int. J. Environ. Sci. Technol. 20, 13277–13288 (2023). https://doi.org/10.1007/s13762-023-04876-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04876-y