Abstract
In present work, the hydrodynamic studies and determination of oxygen mass transfer coefficients (KLa) were conducted in a multistage flexible fibre biofilm reactor developed for treating milk processing wastewater. In this regard, the residence time distributions in the MS-FFBR under various operating conditions as water flow rates, airflow rates, and hydraulic retention times were analysed using tracer experiments. The results revealed that the reactor’s hydraulic regime is similar to a continuous stirred tank reactor. Furthermore, the multistage flexible fibre biofilm reactor exhibited a lower oxygen mass transfer coefficient (KLa of 11.955 1/h at AFR/WFR of 47) than that reported for continuous stirred tank reactor in the literature at similar WFRs and AFRs (KLa of 15 1/h at AFR/WFR of 40). From the results, dissolved oxygen transfer was hindered to some extent owing to the presence of the fibre packing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The management and treatment of industrial wastewaters containing high organic contents is a major environmental challenge. For example, milk processing wastewater (MPW) contains high amounts of organic matter, nutrients, solids, and detergents originated from sanitization and cleaning processes (Liu and Haynes 2010; Andrade et al. 2014; Rahimi et al. 2016). The release of such wastewaters without proper treatment into the receiving water sources causes deteriorative effects like eutrophication phenomenon, oxygen depletion, and ammonia toxicity (Buabeng-Baidoo et al. 2017). Therefore, the treatment of MPW is of great significance to reduce its adverse effects on human life and the environment. Various biological treatment technologies that were recently employed for the treatment of such a high strength wastewater (Rezaee et al. 2015; Asadi et al. 2016; Rahimi et al. 2016; Abdulgader et al. 2019, 2020a; b). Some of the high rate biological treatment technologies applied to treat the high strength wastewaters include up-flow anaerobic, anoxic and oxic (A2O) bioreactor; up-flow aerobic–anoxic flocculated sludge bioreactor (UAASB); up-flow aerobic/anoxic sludge bed (UAASB) bioreactor; and hybrid airlift bioreactor (HALBR) (Asadi et al. 2012; Amini et al. 2013; Abyar et al. 2018; Mirghorayshi et al. 2021).
Overall, aerobic biological treatment technology could be made more cost-effective by reducing the energy consumption in aeration processes (Longo et al. 2016). In this aspect, the oxygen mass transfer from the gas phase into the liquid phase as well as the flow hydrodynamics plays significant roles in the efficient performance of aerobic biological wastewater treatment systems (Chen et al. 2009). Due to the importance of these factors, much effort has been dedicated to the study of hydrodynamic and the determination of oxygen mass transfer coefficients in various biological systems (Chen et al. 2009; Luo et al. 2011; Lei and Ni 2014; Dias et al. 2018).
Biofilm reactors or attached growth systems are known as cost-effective systems and hence are increasingly used in industrial wastewater treatment processes. For instance, submerged aerated filters, moving bed biofilm reactors, integrated fixed-film activated sludge and flexible fibre biofilm reactors are some of the biofilm reactors utilized in wastewater treatment (Rusten et al. 1992; Malovanyy et al. 2015; Abdulgader et al. 2020a). Biofilm reactors support and allow microorganisms to grow on carrier media, thereby, promote high biomass retention, prevent washout, and enhance the development of slow-growing bacteria. Furthermore, attached growth systems maximize the loading capacity and treatment efficiency when compared to the activated sludge process (ASP) at decreased footprints (McQuarrie and Boltz 2011).
Compared to other biofilm reactors, flexible fibre biofilm reactor (FFBR) has further advantages such as lower capital costs, higher resistance towards organic loading shocks and biofilm clogging, and easier control of effluent quality (Chen et al. 2009). Therefore, the FFBR has been employed extensively to treat high strength food processing wastewaters (Yu et al. 2003; Abdulgader et al. 2009, 2020a, b). Recently, we investigated the functionality of the FFBR in various configurations as single-stage flexible fibre biofilm reactor (SS-FFBR), sequencing batch flexible fibre biofilm reactor (SB-FFBR), multistage flexible fibre biofilm reactor (MS-FFBR) for treating the milk processing wastewater under different operating and process conditions (Abdulgader et al. 2019, 2020a, 2021; b).
The evaluation of hydrodynamics and determination of oxygen mass transfer coefficient in a single-stage FFBR has been carried out (Chen et al. 2009). In this research, hydrodynamic behaviour was studied to determine oxygen mass transfer coefficient in a multistage flexible fibre biofilm reactor (MS-FFBR). The effects of three operating parameters, hydraulic retention time (HRT), airflow rate (AFR, QG) and water flow rate (WFR, QL) were studied to investigate the hydrodynamics and oxygen transfer efficiency. Tracer experiments were also carried out to determine mean residence time distribution in the MS-FFBR. This study was conducted in January 2010 at Griffith University, Australia.
Materials and methods
Bioreactor description
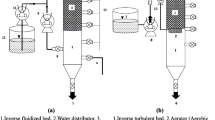
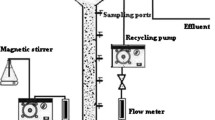
In this research, a laboratory-scale multistage flexible fibre biofilm reactor was made of acrylic (Perspex) with thickness and dimensions of 6 mm and 500 * 125 * 650 mm, respectively, for the treatment of the actual milk processing wastewater. The characteristics of the wastewater used are given in our previously published paper (Abdulgader et al. 2020a). The reactor was divided into four compartments (stages) with equal sizes by means of baffles. The total and working volumes of the reactor were 40.6 and 32 L, respectively. The oxygen was introduced into the reactor using two identical air diffusers for the supply of required dissolved oxygen (DO) levels and adequate mixing. The airflow rate (AFR) was monitored in situ with airflow meters. The reactor was followed by a cylindrical-shaped settling tank in order to settle suspended solids (SS) washed out from the reactor and get a clear effluent. The total volume of the settling tank was 12.5 L. A schematic diagram of the experimental apparatus is delineated in Fig. 1. A seven-flexible fibre bundle was placed in all four compartments of the reactor. Details regarding the characteristics of the flexible fibre used in this research can be found elsewhere (Abdulgader et al. 2019).
Residence time distribution
The residence time distribution was determined using tracer tests. The tracer test is commonly conducted to investigate the hydraulic performance of bioreactors. This test is usually done by pulse input or C-curve and F-curve inputs.
In the C-curve test, to evaluate and understand the hydraulic characteristics of multiple reactors in series, a tracer was instantaneously introduced in the first stage of the reactor. The experiment was conducted at hydraulic retention times (HRTs) of 2, 4 and 8 h, corresponding to water flow rates (WFRs, QL) of 271, 135, and 68 mL/min, respectively. In addition, the airflow rate (AFR, QG) was controlled at 564, 270, and 135 mL/min at HRTs of 2, 4, and 8 h, respectively. Based on predetermined values of AFRs and HRTs, the ratio of the airflow rate to water flow rate (AFR/WFR) was kept 2 in all experiments. A solution of food dyes was pulsed into the influent stream as a tracer. For a completely mixed reactor in series, the theoretical mean residence time was calculated using the following equation (Levenspiel 1999; Metcalf 2003):
where \(E(t)\) is the residence time distribution in one reactor, \(\theta_{i}\)= \(\frac{t}{{\overline{N} t_{i} }}\) is mean residence time in the N reactor, \(\tau \) is theoretical hydraulic residence time, and N is the number of reactors.
In the F-curve test, a dye solution as tracer with initial concentration of C0 was continuously injected to the reactor. This test was conducted at constant AFR of 807 mL/min and HRTs of 4 and 8 h corresponding to the AFR/WFR ratios of 6 and 11.8, respectively. The tracer response curve is determined as C/C0. It should be noted that the theoretical mean residence time can be obtained from the F-curve by integrating Eq. 1.
The reactor was free of any suspended materials such as microorganisms during tracer experiments. The dye concentration of effluent samples was measured by using an absorbance of UV–Vis spectrophotometer at a wavelength of 629 µm (Shimadzu Corporation, UV 1601).
Determination of oxygen mass transfer coefficient
To estimate the capability of dissolved oxygen mass transfer into the bulk water phase in the MS-FFBR, the oxygen mass transfer coefficient (KLa) was measured by using clean tap water. The oxygen transfer rate was determined by direct measurement of the rate of the increase in the dissolved oxygen (DO) concentration in the reactor. Then, the DO concentration was decreased by passing pure nitrogen gas into a mixer equipped wastewater tank to nearly zero. The reactor was aerated through the air sparger mounted at the bottom of the reactor. Simultaneously, water was introduced into the reactor by a peristaltic pump at water flow rate (WFR, QL) of 1.0178 L/h and HRT of 8 h. The experiments were conducted at airflow rates (AFRs, QG) of 15, 48.2, 94.5, 142.4, 190, and 239.8 L/h, which corresponded to the AFR/WFR ratios of 15, 47, 93, 140, 187, and 235, respectively. The dissolved oxygen (DO) concentration in the reactor was recorded with a DO meter at intervals of every 20 s. The oxygen mass transfer coefficient was quantified by the equation below (Amaral et al. 2008).
where KLa is the overall mass transfer coefficient (1/h), Cs is dissolved oxygen saturation concentration at the test temperature (20 °C) and pressure (1.01325 × 105 Pa) (mg/L), C is DO concentration at time t (mg/L), t0 is initial time (h), t is time (h), and QL and V are the water flow rate (m3/h) and the volume of the reactor (m3), respectively.
This experiment was conducted at a water flow rate (WFR, QL) of 1.0178 L/h and a reactor volume of 8 L. Hence, the value of QL/V was 0.127, which was much less than KLa. Therefore, Eq. 2 can be simplified to the following equation:
Plot of t−t0 versus ln (Cs−C0)/(Cs−C) was drawn for the FFBR to obtain an overall oxygen mass transfer coefficient, KLa, at different ratios of AFR/WFR. To determine the relationship between AFR/WFR ratio and the value of KLa, a correction coefficient was considered.
Results and discussion
Distribution of hydraulic retention time in the MS-FFBR
In the C-curve method, a tracer was instantaneously added into the first stage of the reactor and the tracer concentration in the effluent was measured as a function of time. All the experimental results and theoretical data obtained from C-curve test are shown in Tables S1-3 (Supporting information). Figure 2 illustrates the experimental and theoretical results for the MS-FFBR (the whole bioreactor) as a function of time at HRT of 2, 4, and 8 h, respectively. The data showed almost complete tracer recovery at all conditions studied. The experimental data obtained at various HRTs are in close agreement with those achieved for completely mixed flow reactors. In addition, the data of the distribution of hydraulic retention time in the MS-FFBR were similar at various HRTs. Hence, HRT was not affected by the flexible fibre packing in the MS-FFBR, and in practice, the concerned reactor can be considered as a continuous stirred tank reactor (CSTR). The results of this work are in agreement with those reported by Yu et al. (2003), while Dias et al. (2018) in their study concluded the presence of packing media indicated a positive impact on the reactor effective volume.
The experimental data or calculated theoretical data achieved from F-curve are listed in Tables S4-6 (Supporting information). Moreover, Fig. 3 displays F-curve experimental and computed data at HRTs of 2, 4 and 8 h for the whole reactor. As can be seen from Fig. 3, the actual and theoretical data reported at HRT of 2 h are in close agreement. In contrast, the results at HRTs of 4 and 8 h exhibited some deviations. This was attributed to the insufficient sparging energy resulting from the airflow rate applied, so that the portions of the reactor contents may be unmixed with the incoming water with dead zones being developed inside the reactor (Levenspiel 1999; Metcalf 2003). Therefore, the HRT slightly affected the residence time distribution of the bioreactor. However, the flexible fibre packing media located in the centre of the individual reactor stages had no impact on the regime and the reactor can be consider to act as a completely mixed reactor. The results of this study are in agreement with literature data (Plascencia-Jatomea et al. 2015). The calibration curve for the tracer experiments is shown in Figure S1 (Supporting information).
Oxygen mass transfer coefficient
The oxygen mass transfer coefficient (KLa) is one of the most important parameters in aerobic bioreactors. It depends on various factors such as geometrical and operational characteristics of the reactor, media composition, and microorganisms present (Amaral et al. 2008). The data obtained from this experiment and the calculated data for the estimated mass transfer coefficients are given in Tables S6-11 (Supporting information). Figure 4 shows the regression plots used to determine dissolved oxygen mass transfer coefficients.
The KLa values of MS-FFBR at various AFR/WFR ratios are given and graphically presented in Table 1 and Fig. 5, respectively. The results clearly show that the KLa notably increased with increasing the AFR/WFR ratio. The correlation equation acquired for the MS-FFBR is as follows:
The values of oxygen mass transfer coefficients obtained from the MS-FFBR (KLa of 11.955 1/h at AFR/WFR of 47) were lower than those reported by Chen et al. (KLa of 15 1/h at AFR/WFR of 40) (Chen et al. 2009). On the other hand, these values were higher than those achieved by Rodgers et al. in a vertically moving biofilm system used for industrial wastewater treatment (Rodgers et al. 2004). In a study conducted by Wutz et al. (2016), KLa of around 4.3 1/h was reported at AFR of 20 L/h in stirred tank reactors (Wutz et al. 2016). In another research, the KLa value was obtained to be 20 1/h at 70 rpm in a spin filter bioreactor (Niño-López and Gelves-Zambrano 2015). Salehpour et al. (2019) achieved the maximum KLa of 0.031 1/s in an airlift reactor (ALR) with a net draft tube (NDT).
In the MS-FFBR, the oxygen mass transfer coefficient seemed to be less sensitive to variations in the AFR/WFR ratio. Hence, the existence of flexible fibre packing may slow down the oxygen mass transfer rate. This may be attributed to the interference caused by the flexible fibre on the distribution of the air bubbles within the individual compartments of the reactor. It can be said that the capacities of the oxygen mass transfer in the MS-FFBR are similar to those previously reported for a single-stage FFBR (Chen et al. 2009). Other studies showed a positive effect of media on oxygen mass transfer (Dias et al. 2018). However, KLa values reported by Salehpour et al. and Yazdian et al. are much higher than those of values reported in this study, which may be due to differences in the configuration of reactors (Yazdian et al. 2010; Salehpour et al. 2019).
Conclusion
The residence time distribution of MS-FFBR developed to treat milk processing wastewater (MPW) has been evaluated at various HRTs using tracer experiments. The results of experiments revealed that the residence time distributions in the MS-FFBR were very close to the theoretical values of the C-curve and also F-curve especially at 2 h HRT. Therefore, it can be concluded that the flow regime is not affected by the flexible fibre as packing media, and the reactor can be described as a CSTR. In this study, KLa values were lower (KLa of 11.955 at AFR/WFR of 47) in comparison with those obtained from the literature (KLa of 15 at AFR/WFR of 40). As a conclusion, the presence of packing media may reduce the oxygen mass transfer coefficient due to their interference on the distribution of the air bubbles.
Availability of data and materials
Not applicable.
References
Abdulgader M, Yu QJ, Zinatizadeh A, Williams P (2009) Biological treatment of milk processing wastewater in a sequencing batch flexible fibre biofilm reactor. Asia Pacif J Chem Eng 4:698–703. https://doi.org/10.1002/apj.320
Abdulgader M, Yu J, Zinatizadeh AA et al (2019) Process analysis and optimization of single stage flexible fibre biofilm reactor treating milk processing industrial wastewater using response surface methodology (RSM). Chem Eng Res Des 149:169–181. https://doi.org/10.1016/j.cherd.2019.07.011
Abdulgader M, Yu QJ, Zinatizadeh AA et al (2020a) Application of response surface methodology (RSM) for process analysis and optimization of milk processing wastewater treatment using multistage flexible fiber biofilm reactor. J Environ Chem Eng 8:103797. https://doi.org/10.1016/j.jece.2020.103797
Abdulgader M, Yu QJ, Zinatizadeh AA et al (2020b) Performance and kinetics analysis of an aerobic sequencing batch flexible fibre biofilm reactor for milk processing wastewater treatment. J Environ Manag. https://doi.org/10.1016/j.jenvman.2019.109793
Abdulgader M, Yu QJ, Zinatizadeh AA et al (2021) Treatment capacity of a novel flexible fibre biofilm bioreactor treating high-strength milk processing wastewater. Environ Technol (united Kingdom). https://doi.org/10.1080/09593330.2021.1992509
Abyar H, Younesi H, Bahramifar N, Zinatizadeh AA (2018) Biological CNP removal from meat-processing wastewater in an innovative high rate up-flow A2O bioreactor. Chemosphere. https://doi.org/10.1016/j.chemosphere.2018.09.047
Amaral PFF, Freire MG, Rocha-Leão MHM et al (2008) Optimization of oxygen mass transfer in a multiphase bioreactor with perfluorodecalin as a second liquid phase. Biotechnol Bioeng 99:588–598. https://doi.org/10.1002/bit.21640
Amini M, Younesi H, Zinatizadeh Lorestani AA, Najafpour G (2013) Determination of optimum conditions for dairy wastewater treatment in UAASB reactor for removal of nutrients. Bioresour Technol 145:71–79. https://doi.org/10.1016/j.biortech.2013.01.111
Andrade LH, Mendes FDS, Espindola JC, Amaral MCS (2014) Nanofiltration as tertiary treatment for the reuse of dairy wastewater treated by membrane bioreactor. Sep Purif Technol 126:21–29. https://doi.org/10.1016/j.seppur.2014.01.056
Asadi A, Zinatizadeh AAL, Sumathi S (2012) Simultaneous removal of carbon and nutrients from an industrial estate wastewater in a single up-flow aerobic/anoxic sludge bed (UAASB) bioreactor. Water Res 46:4587–4598
Asadi A, Zinatizadeh AA, Van Loosdrecht M (2016) High rate simultaneous nutrients removal in a single air lift bioreactor with continuous feed and intermittent discharge regime: process optimization and effect of feed characteristics. Chem Eng J 301:200–209. https://doi.org/10.1016/j.cej.2016.04.144
Buabeng-Baidoo E, Mafukidze N, Pal J et al (2017) Study of water reuse opportunities in a large-scale milk processing plant through process integration. Chem Eng Res Des 121:81–91. https://doi.org/10.1016/j.cherd.2017.02.031
Chen Y, Yu J, Xu H, Chen Y (2009) Oxygen transfer and hydrodynamics in a flexible fibre biofilm reactor for wastewater treatment. Chin J Chem Eng 17:879–882. https://doi.org/10.1016/S1004-9541(08)60291-8
Dias J, Bellingham M, Hassan J et al (2018) Impact of carrier media on oxygen transfer and wastewater hydrodynamics on a moving attached growth system. Chem Eng J 351:399–408. https://doi.org/10.1016/j.cej.2018.06.028
Lei L, Ni J (2014) Three-dimensional three-phase model for simulation of hydrodynamics, oxygen mass transfer, carbon oxidation, nitrification and denitrification in an oxidation ditch. Water Res 53:200–214. https://doi.org/10.1016/j.watres.2014.01.021
Levenspiel O (1999) Chemical reaction engineering. Ind Eng Chem Res 38:4140–4143
Liu YY, Haynes RJ (2010) Long-term irrigation with dairy factory wastewater influences soil quality. World Acad Sci Eng Technol 46:576–580
Longo S, d’Antoni BM, Bongards M et al (2016) Monitoring and diagnosis of energy consumption in wastewater treatment plants. A state of the art and proposals for improvement. Appl Energy 179:1251–1268
Luo L, Liu F, Xu Y, Yuan J (2011) Hydrodynamics and mass transfer characteristics in an internal loop airlift reactor with different spargers. Chem Eng J 175:494–504. https://doi.org/10.1016/j.cej.2011.09.078
Malovanyy A, Trela J, Plaza E (2015) Mainstream wastewater treatment in integrated fixed film activated sludge (IFAS) reactor by partial nitritation/anammox process. Bioresour Technol 198:478–487. https://doi.org/10.1016/j.biortech.2015.08.123
McQuarrie JP, Boltz JP (2011) Moving bed biofilm reactor technology: process applications, design, and performance. Water Environ Res 83:560–575. https://doi.org/10.2175/106143010x12851009156286
Metcalf E (2003) Wastewater engineering: treatment, disposal, 4th edn. McGraw-Hill, Singapore
Mirghorayshi M, Zinatizadeh AA, van Loosdrecht M (2021) Simultaneous biodegradability enhancement and high-efficient nitrogen removal in an innovative single stage anaerobic/anoxic/aerobic hybrid airlift bioreactor (HALBR) for composting leachate treatment: process modeling and optimization. Chem Eng J 407:127019. https://doi.org/10.1016/j.cej.2020.127019
Niño-López LC, Gelves-Zambrano GR (2015) Simulating gas-liquid mass transfer in a spin filter bioreactor. Rev Fac Ing 1:163–174. https://doi.org/10.17533/udea.redin.n75a16
Plascencia-Jatomea R, Almazán-Ruiz FJ, Gómez J et al (2015) Hydrodynamic study of a novel membrane aerated biofilm reactor (MABR): tracer experiments and CFD simulation. Chem Eng Sci 138:324–332. https://doi.org/10.1016/j.ces.2015.08.004
Rahimi Z, Zinatizadeh AA, Zinadini S (2016) Milk processing wastewater treatment in a bioreactor followed by an antifouling O-carboxymethyl chitosan modified Fe3O4/PVDF ultrafiltration membrane. J Ind Eng Chem 38:103–112. https://doi.org/10.1016/j.jiec.2016.04.011
Rezaee S, Zinatizadeh AAL, Asadi A (2015) High rate CNP removal from a milk processing wastewater in a single ultrasound augmented up-flow anaerobic/aerobic/anoxic bioreactor. Ultrason Sonochem 23:289–301. https://doi.org/10.1016/j.ultsonch.2014.10.018
Rodgers M, Zhan XM, Casey A (2004) Oxygen transfer and industrial wastewater treatment efficiency of a vertically moving biofilm system. Water Air Soil Pollut 151:165–178. https://doi.org/10.1023/B:WATE.0000009913.90786.47
Rusten B, Odegaard H, Lundar A (1992) Treatment of dairy wastewater in a novel moving bed biofilm reactor. Water Sci Technol 26(3–4):703–711. https://doi.org/10.2166/wst.1992.0451
Salehpour R, Jalilnejad E, Nalband M, Ghasemzadeh K (2019) Hydrodynamic behavior of an airlift reactor with net draft tube with different configurations: numerical evaluation using CFD technique. Particuology. https://doi.org/10.1016/j.partic.2019.09.005
Wutz J, Lapin A, Siebler F et al (2016) Predictability of kLa in stirred tank reactors under multiple operating conditions using an Euler–Lagrange approach. Eng Life Sci 16:633–642. https://doi.org/10.1002/elsc.201500135
Yazdian F, Hajiabbas MP, Shojaosadati SA et al (2010) Study of hydrodynamics, mass transfer, energy consumption, and biomass production from natural gas in a forced-liquid vertical tubular loop bioreactor. Biochem Eng J 49:192–200. https://doi.org/10.1016/j.bej.2009.12.013
Yu QJ, Xu H, Yao D, Williams P (2003) Development of a two-stage flexible fibre biofilm reactor for treatment of food processing wastewater. Water Sci Technol 47:189–194
Acknowledgements
The authors are grateful to Griffith University for their endless support of this research. A big thank you also goes to the National Food Milk Ltd plant manager for his great help and excellent cooperation.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Not applicable.
Author information
Authors and Affiliations
Contributions
MA: Conceptualization, Investigation, Methodology, Writing—original draft, Data curation, Formal analysis. QJY: Supervision, Conceptualization, Resources, Methodology, Funding acquisition, Writing—review and editing. AAZ: Supervision, Conceptualization, Methodology, Writing—review and editing. PW: Supervision, Writing—review and editing. ZR Software, Validation, Writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Editorial responsibility: Samareh Mirkia.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdulgader, M., Yu, Q.J., Zinatizadeh, A.A. et al. Oxygen transfer and hydrodynamic evaluation in a multistage flexible fibre biofilm reactor. Int. J. Environ. Sci. Technol. 20, 12417–12426 (2023). https://doi.org/10.1007/s13762-023-04835-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-023-04835-7