Abstract
Algal biomass has been gaining attention over the last decades as it is versatile and can be used in different industries, such as wastewater treatment and bioenergy industries. Microalgae are mixotrophic microorganisms that have potential to utilize nitrogen and phosphate (nutrients) and remove organic matters from wastewater streams. Phycoremediation is an intriguing and cost-efficient technique to simultaneously remove heavy metals from wastewater while removing nutrients and organic matters. The cultivated and produced algal biomass can be a promising candidate and a sustainable feedstock to produce biofuels (e.g., biodiesel, bio-alcohol, and bio-oil) and value-added products such as biochar, glycerol, functional food, and pigments. The algae suspended cultivation systems, WSP and HRAP, are efficient methods for the wastewater treatment in shallow ponds with no mechanical aeration and less required energy consumption, but when a short HRT and minimum evaporation losses are key points in the algal cultivation the PBRs are recommended. It was reported that biosorption and bioaccumulation are the two promising techniques of phycoremediation. Studies showed that among the current processes of algal biomass conversion to biofuels, transesterification of algal lipids and pyrolysis of algal biomass were found to be the most efficient techniques. This review paper investigates the applications of algal biomass in the phycoremediation of wastewater, productions of bioenergy and value-added products by reviewing articles mainly published over the last five years.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing industrialization, urbanization, and farming has significantly increased the amount of wastewater. Disposal of wastewater without prior treatment leads to various problems like algal bloom and consequently, eutrophication which results in loss of biodiversity. Wastewater contains high concentration of chemical oxygen demand (COD), biological oxygen demand (BOD), dissolved and suspended inorganic compounds such as nitrates and phosphates. Industrial wastewater comprises impurities like heavy metals, such as cadmium, nickel, and copper, which further accumulate in human body through food chain resulting in serious health issues (Pavithra et al. 2020).
Phycoremediation is the use of macro- or microalgae for the reduction or biotransformation of pollutants, including nutrients and harmful chemicals from wastewater (Kumar et al. 2018). Biosorption, known as one of the promising phycoremediation techniques, is a bioremediation process that the inactive or dead biological materials take up organic and inorganic components (Rizvi et al. 2020). It is an energy independent technique using various adsorbents such as bacteria, fungi, algae, and plant residue to adsorb heavy metals on their active sites or cell surface (Kushwaha et al. 2020). Vijayaraghavan et al. (2019) did a critical review on biosorption, and reported that seaweeds, well-known algae, has a promising biosorbent capacity to remove a variety of heavy metals contaminants. In contrary to the biosorption, bioaccumulation is a metabolic pathway process where the heavy metal ions would pass through the cell membranes of living microalgae and enter the cytoplasm Danouche et al. (2021). The use of macroalgae in the wastewater treatment process was first introduced by Oswald in 1960s (Salama et al. 2019), and has been known as a sustainable and cost-effective method in wastewater treatment for decades (Bhargava et al. 2020). Bauddh and Korstad (2022) reviewed the heavy metals contaminants and its accumulation mechanisms and the phycoremediation potential of algae to bioremediate the heavy metals in aqueous systems. Leong et al. (2021) reviewed various algal-based treatment technologies including high-rate algal ponds (HRAPs), HRAP-absorption column (HRAP-AC), hybrid algal biofilm-enhanced raceway pond (HABERP) and algal turf scrubber (ATS) in the phycoremediation and illustrated the recent advances in the current technologies.
In recent years, bioenergy as a robust alternative of fossil fuels has drawn more attention. Bioenergy is a type of renewable energy derived from biomass (e.g., agricultural by-products, organic waste, sludge, forest residues and waste, etc.) in form of bioethanol, biogas, biodiesel, biohydrogen, electricity generation which currently accounts about 10% of global energy supply (Duarah et al. 2022). Presently, the world relies on fossil fuels, particularly coal-burning as a major source of energy. However, coal-burning is highly toxic to the environment as it releases a significant amount of greenhouse gases (GHGs) emissions. It is reported that due to industrialization and urbanization, the global energy demand is expected to rise by 9% between the years 2019–2030 (International Energy Agency [IEA], 2020). Hamed et al. (2020) reported that among the bioenergy feedstocks, algae biomass has been reported as one of the most sustainable and efficient alternatives. This is obviously due to the rapid growth rate and high content levels of lipids, proteins, minerals, and carbohydrates in the algal biomass compared to other feedstocks. Production of algae-driven biofuels also results in production of other valuable by-products such as biochar, glycerol, functional food, and pigments, making algae an economically efficient feedstock as well. Kandasamy et al. (2021) reviewed the production of bio-oil from thermochemical processes such as gasification, lipids transesterification, and pyrolysis and indicated that hydrothermal liquefaction (HTL) is the best technology for the algal biomass conversion. In a review study, Ganesan et al. (2020) summarized different technologies using algae to produce biofuels such as bio-alcohol, biodiesel, and bio-oil. They also reviewed the algae harvesting using closed, open and hybrid systems. This review paper focuses on the algae-based studies published recently on wastewater phycoremediation techniques, algal biofuels, and value-added products from algae. The main objective of this study is to provide a timely and comprehensive review on the algae culturing and applications in phytoremediation, biofuel and by-products (value-added products) productions with main focus on the studies published within the last five years in the field.
Algae application in wastewater treatment
To meet the demand in wastewater treatment industry, production of microalgae is an essential factor which needs to be investigated. As shown in Fig. 1, using wastewater in the process of algae cultivation provides an opportunity to simultaneously treat wastewater, which could also reduce the cultivation costs. The cultivation methods of algae from wastewater sources are further discussed in the following sections.
Algae cultivation using wastewater sources
Cultivation system for algae is classified into two main categories; suspended and immobilized. Suspended cultivation system consists of two methods: open pond and photobioreactors. The main characteristics of a cultivation system are optimal light source, effective mass transfer, easy to operate, low contamination rate, economical and high efficiency based on land area.
Open ponds
Open pond systems are preferred at industrial scale due to their low cost and ease of operation. These systems are classified into natural and artificial systems. Natural systems include lakes, lagoons and ponds for algae cultivation. Artificial system includes a large variety of systems like man-made ponds, tanks, and containers (Molazadeh et al. 2019). The preferred systems for the wastewater treatment purpose in these cultivation systems are waste stabilization pond (WSP) and High-Rate Algal Pond (HRAP).
Waste stabilization ponds (WSPs) are large and shallow, and mostly used for the wastewater treatment system in tropical countries (Kumar et al. 2018). They are preferred for the treatment of domestic wastewater streams, municipal wastewater streams, and industrial wastewater streams (food, dairy industry) with high nutrient concentration. The main advantages of WSPs, over traditional (natural) systems, are high removal efficiency of BOD, suspended and dissolved solids, nutrients, and pathogens (Bansah and Suglo 2016). Also, they are cost effective in areas where land is inexpensive and available and are simple to operate and maintain and do not require external electric energy for the treatment process. Organic matter in wastewater streams is oxidized in an algal—bacterial symbiosis system in WSPs where the algal biomass growth results in consuming CO2 and sunlight and subsequently increasing oxygen concentration through the photosynthesis. Producing more oxygen by algae would enable bacteria to breakdown more amount of organic matter and results in accomplishing wastewater treatment in the WSPs (Mahapatra et al. 2022). They also reported that Chlorella, Euglena, Scenedermus, and Microcystis are the predominant algae species found in the WSP systems. The drawbacks of WSP are their long hydraulic retention time ranging from 20 to 30 days, high water losses due to evaporation, and high dependency of such open systems process to the environmental factors like temperature, light intensity and risk of contamination (Nwankwo et al. 2019). Over time, the sludge generation in ponds increases leading to lowering the mean residence retention time or contact time that results in decrease of contaminant removal and therefore significantly affect the pond microbial dynamics results in decreasing efficiency (Coggins et al. 2019). Osman et al. (2021) used a WSP system, Lemna gibba and duckweed pond, to investigate the nutrients and organic removals from industrial wastewater. They reported COD and NH4-N removal percentages of 23% and 75%, respectively, from the industrial wastewater in a 6-day HRT. In a study conducted by Guedes-Alonso et al. (2020), urban wastewater was treated in a 340 m3 constructed wetland system where the BOD, COD, and TSS removal percentages found to be 92%, 62%, and 92%, respectively. The nutrients removal percentages for nitrate, phosphate, and sulfate in this system have reached 26%, 9.5%, and 85%, respectively. Other open pond system is high-rate algal pond system (HRAP), also referred as raceway pond, has three main operations: removal of solids from wastewater, aerobic biological treatment of wastewater and harvesting of algae. In the HRAP system, wastewater treatment is performed by algae-bacteria consortia where algae provide oxygen to the bacteria for degradation of organic matter, reducing BOD and removal of solids, which results in facilitating aerobic treatment of wastewater, and in return bacteria provide CO2 utilized by algae during the photosynthesis (Garfi et al. 2017). Due to having a paddlewheel in the HRAP compartment which provides an optimum horizontal water velocity (about 0.3 m/s), mixing in the HRAP helps collect the algae species in colonies which reduces the sedimentation time required for harvesting of algae. Low depth of HRAP provides high light penetration leading to increased photosynthetic ability and high biomass production (Sutherland et al. 2017; Kim et al. 2018). Craggs et al. (2012) reported that with CO2 addition, algal productivity increased from an annual average of 2.5 g /m2 day to 12–20 g /m2 day. In an investigation, Chambonniere et al. (2021) reported that the presence of high cell density of algal biomass would enhance the sunlight attenuation in the HRAP systems. They also evaluated the mechanism of pathogen removal through the HRAPs and noticed that high pH and algae metabolites enhance the pathogen removal. The CO2 addition promotes algal growth and nutrient removal, reduces pH of wastewater, and maintains C/N ratio. The HRAP systems have lower energy consumption compared to the conventional sludge treatment due to the elimination of mechanical aeration (provides oxygen for bacterial growth) as that criterion is fulfilled by algae. HRAP requires low capital investment and less maintenance compared to the conventional treatment system like activated sludge treatment that requires large amount of energy for sludge removal (1 kWh for conventional compared to 0.2 kWh for HRAP) (Amenorfenyo et al. 2019; Molazadeh et al. 2019). Similar to the WSP, application of HRAP has disadvantages such as high land requirement, risk of contamination and evaporation losses (Arashiro et al. 2018).
Photobioreactors
Another cultivation method in suspended systems are photobioreactors (PBRs). One of the main parameters in PBRs design is to ensure proper light intensity and maximizing the light penetration (by high surface area to volume ratio) as it is essential for the algae growth (Chang et al. 2017). They indicated that the PBRs are applicable at large scale with enhanced temperature control as they provide higher biomass productivity and contamination removal, and lower evaporation loss compared to the open system like WSP and HRAP. The main power requirements in photobioreactor are related to the aeration and cooling systems (about 40% of the total energy requirement) for maintaining the temperature (Clippinger and Davis 2019). Goswami et al. (2019) investigated the treatment of paper industry wastewater using a microalgae-bacteria consortium of Chlorella sp., Klebsiella pneumoniae and Acinetobacter calcoaceticus in a batch mode of PBR with the biomass productivity of 3.17 g/L. They reported the wastewater removal efficiencies of 99.95% and 95.16% for total nitrogen and COD, respectively. In a study conducted by Shetty et al. (2019), a different microalgae-bacteria consortium of Chlorella vulgaris and sludge native bacteria was used to simultaneously treat wastewater and generate biohydrogen. In this study, a batch mode PBR was conducted in 40 ml vials and performed at 24 °C for 72 h under continuous illumination, and the results showed about 75% of TP and TN removals, and a H2 production of 35–40%. Huang et al. (2017) indicated that degassing is essential in photobioreactors as oxygen produced by photosynthesis accumulates leading to a toxic level. They reported that dissolved oxygen oxidizes several enzymes and affect electron transmission resulting in reduced photosynthetic ability of algae. Structural integrity of reactor is at risk in case dissolved oxygen passes the threshold.
Immobilized cell
Immobilization is a technique where the independent movement of algal cells are restricted by artificial or natural means in the aqueous phase. Gross et al. (2015) reported the cell concentration of suspended culture with algal cell concentration of about 0.5 gL−1 (about 0.05% dry basis) and 2–6 gL−1 (0.2–0.6% dry basis) for open pond and photobioreactors, respectively. The low cell concentration requires further separation mechanism for algae harvesting including sedimentation followed by centrifugation that increases the cell concentration to 20% dry basis. To increase the algal cell concentration, biofilm-based systems are used in which algae is grown on a surface (Gross et al. 2015). Selecting a suitable carrier is critical in immobilization process and the surface material ranges from natural materials like polysaccharides to plastics such as high-density polyethylene. Simplified separation process, higher cell density, higher productivity, better cell stability, and biomass recirculation are some of the advantages of algal immobilization cells over the suspended method (Emami Moghaddam et.al. 2018). Boubabidi et al. (2019) reported that the cell immobilization is affected by several factors including physiological state of cells, surface properties and adsorbents, environmental conditions, medium composition, and the pH. Dinesh Kumar et al. (2016) investigated the nutrients removal from a shrimp farm wastewater using biofilter filled with a marine microalgae Picochlorum maculatum. The P. maculatum cells have initially been immobilized in round alginate blocks. The results showed a significant NO2 and NH3 removal percentages of 89.6% and 98.5%, respectively. The 57% and 46.4% of phosphate and nitrate removals were also obtained in this study.
Phycoremediation of wastewater
Application of microalgae in wastewater treatment has been an area of interest in research for many years. Phycoremediation is the use of macro- or microalgae for the reduction or biotransformation of pollutants, including nutrients and harmful chemicals from wastewater (Kumar et al. 2018). Molinuevo-Salces et al. (2019) reported that energy consumption for activated sludge process was approximately 500 Wh per m3 compared to 1.5–8 Wh per m3 for HRAP. Microorganisms employed in phycoremediation do not produce harmful by-products and increase the dissolved oxygen level (Brar et al. 2017). The biofilm-based reactor used in wastewater treatment processes are categorized into rotating algal biofilm reactor, horizontal biofilm reactor and moving bed biofilm reactor (MBBR) (Wang et al. 2016). Miranda et al. (2017) reported a biofilm consisting of two microalgae used in the treatment of wastewater resembling the composition of textile industry effluent with selenium uptake of 38%, followed by the NH4, NO3, and PO4 removals of 24%, 26%, and 17%, respectively. Zkeri et al. (2021) conducted a study on MBBR used in the treatment of dairy wastewater and they reported a complete removal of COD, and 65% and 31% removal rate in ammonium and phosphate, respectively. Other technologies in the field of algal biofilms are algal turf scrubbers and algal wheel. Algae turf scrubbers consist of plastic mesh where the algae would grow on the mesh surface and wastewater is pumped over the algae resulting in removal of contaminants from the wastewater (Ray et al. 2015). The technology is effective in removing nutrients such as nitrogen and phosphorus and assimilates CO2 and is applicable in treatment of low metal concentration in industrial wastewaters (Wollmann et al. 2019).
The major advantage of employing biofilm technology is the lower harvesting cost as algae is grown on a material which can be scrapped for algae biomass removal. Biofilm reactors are closed systems which result in reducing the evaporation loss and risk of contamination (Dwivedi and Dwivedi, 2022). However, compared to the open ponds (WSP and HRAP) which require a minimal capital and operating costs due to a lower energy requirement for mixing, design and operation of photobioreactors (PBRs) require a very high capital cost (Narala et al. 2016).
Biomass harvested can also be used as bio-fertilizer and as a raw material to produce bioethanol, methane and biodiesel. The use of biomass in the production of these value-added products enhances the economic viability of phycoremediation (Gani et al. 2015; Khiewwijit et al. 2018). Use of microalgae is an environment-friendly option which reduces greenhouse gases (GHGs) emissions due to the utilization of CO2 during photosynthesis (Bansal et al. 2018; Sunday et al. 2018). Phycoremediation technique, like HRAP, reduces greenhouse gas emissions by 100–200 tons of CO2 per million liters of treated wastewater compared to the conventional methods like oxidation pond (Amenorfenyo et al. 2019; Molazadeh et al. 2019). Sludge generation is also less compared to the traditional physiochemical methods which results in lowering the operating cost (Dixit and Singh 2015). Koul et al. (2022) reported a study conducted in South India that showed negligible sludge generation when algae are utilized in bioremediation of industrial wastewater.
Algae applications and heavy metals removal
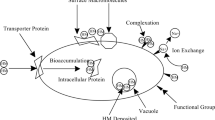
Heavy metals are classified as elements that have high atomic weight and high density. They include both metals and metalloids which are toxic to human health and environment (Tchounwou et al. 2012). The mechanism of utilizing heavy metal by algae is dependent on the cell-wall constituents. Algal cell wall contains polymers (pectin, hemicellulose, cellulose, lignin) and functional groups such as hydroxyl, carboxyl, and amino groups. Algae removes the heavy metals by two distinguished mechanisms; biosorption by non-living microalgae and bioaccumulation by living cells (Chabukdhara et al. 2017).
Biosorption is an extracellular mechanism performed by several techniques like physical adsorption or ion exchange. In physical adsorption the metal ions are attached to the algal cell walls through the weak attraction forces like Van Der Waals forces (Ahmad et al. 2019). In the ion-exchange process, the metal ions are trapped in the cellular structure and adsorbed into the metal binding site on algae. On the algal surface there is a negatively charged functional group which absorbs the positively charged metal ions and consequently results in reducing the heavy metals content in wastewater. Salama et al. (2019) reported that algae have 15.3 to 84.6% higher biosorption capacity as compared to other microorganisms such as bacteria and fungi. Gu and Lan (2021) have investigated the biosorption and effects of heavy metals ions Pb(II), Hg(II), Zn(II), Cd(II), and Cu(II) on the green alga Neochloris oleoabundans. They found that the adsorption capacity of N. oleoabundans biomass is directly proportional to the electronegativity and inversely proportional to the size of the metal ions. Among the metal ions, Pb(II) showed a significant inhibitive effect on the adsorption of other metals in the media. In another study, the biosorption potential of heavy metal ions Cd2+, Co2+, and Zn2+ on Ulva flexuosa biomass has been investigated and the effects of essential parameters including the optimum pH, initial biosorbent dosage, contact time, and agitation were evaluated (Lekshmi et al. 2022). They found that the optimum biosorption is obtained at pH of 4 and with 0.4%, 0.6%, and 0.4% of algal biomass dosages for Cd2+, Co2+, and Zn2+, respectively. After 30 min contact time, the heavy metal removal percentages of cadmium, cobalt and zinc were reached 94.8%, 87.5%, and 90.8%, respectively.
In bioaccumulation the heavy metal ions are transported across the cell membranes and accumulated within the cells which results in reducing the heavy metal content in the wastewater. Bioaccumulation is an active process and heavy metal remediation requires metabolic activity in a living cell (Zainith et al. 2021). Henriques et al. (2017) reported 65%, 95%, and 76% removals of Pb, Hg, and Cd, respectively, during bioaccumulation mechanism for heavy metal uptake. Cader et al. (2019) have investigated the bioaccumulation capacity of heavy metals Cd, Cu, Zn, and Pb on marine algae biomass. They reported that the Bioaccumulation Factors in water (BCFwater) is higher in algae for zinc and copper and the seaweed samples bioaccumulations of heavy metals were reported in the order of Zn > Cu > Pb > Cd. They also found that the highest bioaccumulation level is achieved by green Ulva lactuca algae for Zn followed by the red algae Ceramium rubrum and Cystosera barbata. In another study, Uddin and Lall (2019) have investigated the bioaccumulation potential of Botryococus brurauni for the heavy metal removals of Pb, Cu, and Cd ions from wastewater and aqueous solution. The results showed that the chlorophyll content and consequently the bioaccumulation potential of algal biomass have been significantly suppressed and highly reduced by the concentrations of cadmium followed by lead and copper in the wastewaters.
Algae application in nutrient removal
Wastewater contains carbon, nitrogen, and phosphorus in many forms and the excess of these nutrients results in eutrophication. Algae requires these nutrients for its growth where the process includes several mechanisms for assimilation of such nutrients. For assimilation of different carbon components by algae several mechanisms are followed. Carbonates are utilized by direct uptake or by its conversion to carbon dioxide by carbon anhydrase activity. Carbon dioxide is utilized by algae by direct absorption or through photosynthesis (Mandotra et al.2019; Cai et al. 2013). Nitrogen in wastewater is present as nitrate, nitrite, nitric acid, ammonia, and nitrogen gas. Nitrate is assimilated by microalgae after it is converted to ammonium (Mandotra et al. 2019) and this is due to the fact that ammonium requires the least amount of energy for its assimilation compared to nitrate and nitrite. Nitrate is converted to nitrite by Nitrate reductase (NR), then nitrite is further reduced to ammonium by nitrite reductase (NiR) and at the end ammonium is assimilated by algae as glutamine-by-glutamine synthetase (GS) (Jia and Yuan 2016; Bolay et al. 2018). However, the efficiency of industrialized algae-based treatment systems may hamper due to providing a simultaneous high nutrient removal rate and a stabilized algal biomass consortium. Therefore, to overcome such a drawback, it is necessary to apply a mixed consortia of microalgae-bacteria to remove nutrients from wastewater streams (Mohsenpour et al. 2021). In a study, Sepehri et al. (2020) demonstrated that a consortium consisting of C. vulgaris and nitrifying bacteria would enhance the NH4-N removal rate to a level of 133 mg/L. This improvement has also been shown by Bankston et al. (2020) when they observed that ammonium removal was almost doubled when using a consortium of microalgae—nitrifying bacteria compared to the sole nitrifying system. Phosphorus exists in form of phosphates in industrial wastewaters. Like nitrogen, phosphorus removal is affected by pH and temperature of wastewater (Mandotra et al. 2019). Table 1 shows the contaminant removal efficiency for various species of algae in different industrial wastewater streams. Similarly, Rezvani and Sarrafzadeh (2020) observed that microalgae-bacteria consortium would improve the phosphorus removal efficiency from sole culture of microalgae (4.5 mg/L) or bacteria (2.6 mg/L) to 6.34 mg/L from a mixed consortium. Wang et al. (2020) also reported removal rates of 374 mg/L and 15 mg/L of Ammonium and phosphate, respectively, from a piggery wastewater using Desmodesmus sp. and nitrifying bacteria. These values represent 52% and 100% of efficiency removal levels of NH4+ and PO43−, respectively, in the wastewater stream.
Factors affecting phycoremediation of wastewater
There are critical parameters such as pH, temperature, nutrients, retention time, and light intensity that significantly affect the contaminant removal rate during phycoremediation. Each parameter has its own optimum range which can affect the photosynthetic and consequently the algae contaminant removal efficiency in wastewater treatment process.
pH is one of the main parameters that can significantly affect the growth of algae in wastewater. pH increases throughout the wastewater treatment process due to photosynthesis of algae which results in removal of CO2, hydrogen, and bicarbonate ions where carbon source is limited (Sunday et al. 2018). The optimum algal growth of various species is varied at different pH ranges. Daliry et al. (2017) reported that Chlorella Vulgaris can grow in a broad range of pH, but C. vulgaris shows high growth performance at pH range of 9–10. They also indicated that increasing the pH will increase the salinity of the media and results in inhibiting the algal cell growth. This could be one of the reasons that most of the algal species grow well in the pH range of 6–8. However, Qiu et al. (2017) reported that Chlorella Sorokiniana shows maximum growth at a pH of 6. Thus, optimal pH conditions are varied and highly dependent on the algae species employed in phycoremediation.
Temperature is another effective parameter which can effectively be controlled in closed systems, but it varies in open systems based on the climatic conditions. The optimal temperature range depends on the algal strain and the wastewater stream but generally it is found to be between 20 °C and 30 °C (Singh and Singh 2015). Zainith et al. (2021) reported that the algal growth increases with temperature rise to a certain level. They indicated that at high temperatures, the photosynthesis ability of algae cells is reduced significantly due to inactivation of the essential proteins and results in decreasing the accumulation rate of contaminants on algal cell wall. Similarly, the temperature below 16 °C slows lowering the algal growth and affects the carbon assimilation efficiency of algal strain which results in decrease in pH, and damages the algal cell. Singh and Singh (2015) reported 25 °C as an optimal temperature for Chlorella Pyrenoidosa. While Filippino et al. (2015) reported higher contaminant removal efficiency at 15 °C as compared to 25 °C for Chlorella vulgaris. Thus, optimal temperature highly depends on algal strain.
Nutrients are another essential factor for the algal growth. Algae requires both macronutrients (nitrogen, carbon and phosphorus) and micronutrients (heavy metals) for its growth. When wastewater is used as an algae growth medium, it usually contains essential nutrients. However, in case of any deficiency, further nutrient like CO2 is provided in the form of flue gas (Khan et al. 2018). Kumar et al. (2018) reported that injection of flue gas during phycoremediation had a positive effect on the algae growth.
In a study, Arashiro et al. (2018) indicated that retention time is another important factor which varies depending on the technology used in wastewater treatment and typically ranges from 3 to 36 days. They reported that in photobioreactor, it ranges from 3 to 14 days while in HRAP it is reported about 14 to 21 days and even longer in case of WSPs. Also, they elaborated that retention time depends on the climatic condition. In winter with lower temperature and less light intensity, for the same contaminant removal, higher retention time is required compared to summer.
Light intensity affects the algal activities since light is utilized by algae in photosynthesis and is also essential for their growth and consequently the contaminants removal efficiency. Zenith et al. (2020) investigated the effects of temperature and light on algal growth and reported that algae have higher contaminant removal efficiency and higher biomass productivity during the day or in summer compared to the low temperature and light intensity conditions. Closed systems with artificial illumination prospective would utilize both the artificial lighting and sunlight resulting in an efficient growth and contaminants removal rate. Although light intensity is essential, excessive light inhibits the growth of algae as there is a risk of damage to algal cell at high light intensity (Bwapwa et al. 2017). Anto et al. (2020) reported that algae perform photosynthesis in the active radiation range of 400 to 700 nm.
Algal biorefinery to bioenergy and value-added products
The process of obtaining bioenergy and other value-added products through algal biomass transformation is known as ‘algal biorefinery’ (Chew et al. 2018). There are several biorefinery processes and techniques that are commonly used. The general mechanism of the conversion is presented in Fig. 2 (Bhatia et al. 2020). Algae are recovered from various streams in industrial wastewater (textile, municipal, dairy, etc.), and cultivated in a photobioreactor, using a light source and CO2. The cultivated algal biomass is, then, converted into bioenergy and value-added products through different processes. Malik et al. (2022) have recently reported a new biorefinery route from cultivating microalga Chlamydomonas sp. using urban wastewater as the available low-cost media to converting the algal biomass to value-added products such as 1.83 mg/g of carotenoid and 480 mg/g of lipids. Extracted lipids have been transesterified to produce biodiesel. In this study 131.6 U/mL of α-Amylase and 375–384 mg/g of mycoprotein were also produced using 75–100 g/L of residual algal biomass through the downstream fermentation process.
Algal biorefinery mechanism (Bhatia et al. 2020)
It has been reported that green algae and cyanobacteria are the promising alternative sources of hydrogen (Anto et al. 2020). Fakhimi et al. (2020) investigated the effect of coculturing microalgae Chlamydomonas reinhardtii and bacteria to enhance the hydrogen production. They reported that the coculturing would increase the amount of starch content in the cells, results in reducing the oxygen evolution and consequently higher H2 production. Algal biomass also has been reported as an alternative source of electron donor in microbial fuel cells (MFCs) called Algal Microbial Fuel Cell (AMFC) where photosynthetic organisms would produce electricity (Nagendranatha Reddy et al. 2019).
Algal lipid extraction as pretreatment
Like any other plants, the algae cells consist of carbohydrates, proteins, and lipids. In biorefinery, lipids are the major constituent for biofuels, followed by carbohydrates. Therefore, lipids and carbohydrates are often extracted during pretreatment process to reduce process cost and energy. The post-extraction residual algal biomass is then converted into other value-added products (Chia et al. 2017). Lipid extraction techniques are performed using four approaches: mechanical, biochemical, electromagnetic, and biological. All these approaches and techniques involve damaging the cell walls of algal biomass and collecting the lipid content. The first mechanical technique is the Expeller Press technique, where microalgal biomass is mechanically crushed using an expeller (Demirbas, 2009). Topare et al. (2011) has been successfully extracted 75 mol% of lipids from dry filamentous algae in an experiment. The second mechanical technique is bead beating, where the biomass slurry is spun using high-speed spinning mills and beads to damage the cell walls (Kumar et al. 2015). The highest lipid recovery from agitated bead beating to date is 33 mol% of lipids per 100 g of algae Nannochloropsis cells (Mishra et al. 2017; Gouveia et al. 2012). The next approach is the biochemical approach, where different types of solvents are used to selectively extract lipids from the endogenous cells. The most foundational solvent is chloroform and methanol at a 2:1 ratio, which is known as the ‘Folch’ solvent. The chloroform disrupts hydrophobic interactions between nonpolar and neutral lipids in algal biomass, while methanol penetrates and reduces the lipid chains (Mubarak et al. 2015). Aside from Bligh and Dyer’s solvent, alkali and acid solvents, such as NaOH and H2SO4, are also commonly used in microalgal lipid extraction and bioethanol production (D’Hondt et al. 2017). Sulfuric acid has found to be highly efficient, releasing 96% of glucose in microalga C. vulgaris (Ho et al. 2013). The acid and alkali are usually diluted to avoid the formation of inhibitors and corrosions. The last lipid extraction approach is the biological approach. An indirect biological technique is enzymatic hydrolysis. In this technique, an enzyme is used to lower the activation energy of hydrolysis, providing moderate process conditions (Gong and Bassi 2016). A new direct technique is algicide via microorganisms. Bacteria have been found to secrete a signaling compound called ‘auto-inducers’ for other bacteria from the same population to detect and induce algicidal enzymes, leading to algalysis (Demuez et al. 2015). Chen et al. (2013) found that 21.5 mol% of lipids was extracted from microalgae Chlorella vulgaris using algicidal micro-organism Flammeovirga yaeyamensis. Despite its potential, the technique requires specific conditions and is not suitable for large-scale process.
Direct combustion of algal biomass
Direct combustion is the simplest biorefinery process of algal biomass. In direct combustion, the whole algal biomass is treated as a solid fuel. Microalgal biomass such as H. pluvialis is selected for its lipid content. During the process, the microalgal solid fuel is burned at 800–1000 °C in the presence of excessive air (X. Lee et al. 2020; X. Lee, 2018; Zhao et al. 2016), yielding biodiesel and residual biomass. Conventionally, researchers prefer to employ other biorefinery processes such as liquid-targeted liquefaction for biodiesel production as they believe that biodiesel is solely derived from lipid content of algal biomass (Choi et al. 2019); however, Giostri et al. (2016) argued that lipid extraction techniques ignore the full energy potential of algal biomass. Supporting the argument, Choi et al. (2019) have conducted an experiment to measure the biodiesel potential (BP) in 10 microalgal samples, using solely the convertible lipid fraction in the biomass. The results showed that compared to the lower heating value (LHV) of the entire biomasses, the BPs gained from the convertible lipid fraction of the microalgal samples only measured up to 50.27% of the LHVs. Choi et al. (2019) have concluded that using the entire biomass is up to 8.15 times more energetically efficient than using only the lipid fraction. Moreover, they have deduced that in direct combustion, every component in the biomass, including lipid derivatives, proteins, and carbohydrates are utilized as an energy source. They have also noted that the LHVs depend on the lipid content of the algal biomass, contrary to the popular belief that the biomass density determines the LHVs. Overall, the advantages of the microalgal solid fuel direct combustion process are its simple nature, high energy yield, and energy efficiency. For instance, direct combustion does not involve any carbon-omitting downstream processes, and the algal solid fuel is compatible with conventional coal boilers, requiring no specialized modifications or equipment. Similarly, after pretreatment, the algal solid fuels are grindable, easily chopped and utilized, requiring no extra equipment as well. However, like any process, direct combustion has its limitations. Although the process has lower CO2 emissions compared to the traditional fossil fuel burning, a white fume is reportedly released when the biomass has a moisture content of more than 50 wt% (X. Lee et al. 2020). Consequently, energy-intensive dehydration processes are required as a pretreatment for this biorefinery process in order to prevent white fume emission and to ensure the efficiency of the process. Finally, since algal solid fuels offer lower calorific densities than the fossil fuels, they are not qualified as a standalone fuel; therefore, co-firing of coal-biomass, with a small fraction of biomass, is employed industrially (Giostri et al. 2016).
Transesterification of algal lipids
Transesterification of lipids is the process of using alcohol, with aid of a catalyst, to convert triglycerides to biodiesel and glycerol. Akubude et al. (2019) claimed that transesterification of lipids is the most commercially applied algal biomass biorefinery process for biodiesel production. Sivaramakrishnan and Incharoensakdi (2017) have conducted a successful experiment on lipid transesterification, using microalga Botryococcus sp., dimethyl carbonate (methanol derivative), catalytic lipase, with and without ultrasonic pretreatment. They have concluded that dimethyl carbonate is more costly than methanol, but it assists the glycerol conversion into glycerol carbonate. This aid is important as high glycerol content reduces the quality of biodiesel. The experiment has shown that applying ultrasonic pretreatment is extremely beneficial to the process, as the reaction time reduces by nine-fold. Without the ultrasonic pretreatment, about 78% wt esters/wt oil yield was achieved in 36 h, which is time extensive. With an ultrasonic pretreatment, the process was able to achieve 88% wt esters/ wt oil yield in merely 4 h, reducing the reaction time by nine-fold. Notedly, these results were accomplished under optimum process conditions. The optimum process temperature was found to be 50 °C. The optimum alcohol to oil algal biomass ratio, was found to be 7.5:1 v/v without the pretreatment and 5:1 v/v with the pretreatment (Musa, 2016). Overall, direct transesterification is an effective process despite the reaction time. Surprisingly, the energy consumption for direct transesterification and ultrasonic-assisted transesterification are not much different. Sivaramakrishnan and Incharoensakdi (2017) reported that direct transesterification consumes 126.7 kWh energy, while the ultrasonic-assisted process consumes 127.0 kWh. A drawback of this process is its requirement for dry algal biomass. It is crucial that the biomass has a low moisture content to avoid soap formation. During hydrolysis, fatty acids break free; excessive number of fatty acids lead to soap formation (Musa, 2016). The optimum moisture content for the biomass was found to be 1% v/v, which is extremely low; thus, drying process are employed prior to transesterification, drastically increasing the production cost and energy.
Pyrolysis of algal biomass
Pyrolysis is a thermal decomposition in the absence of oxygen and is recognized as the most efficient thermal algal biomass conversion process (Lee et al. 2020). The process is recognized via its ability to produce products in all three phases: solid, liquid, and gas. The most valuable products are biofuels, which are the liquid bio-oil and non-condensable syngas, and the by-product of solid biochar. Compared to the other thermal conversion processes like direct combustion and gasification, pyrolysis operates at a milder condition and produces higher quality products. The process temperature is lower compared to the other processes, and the produced syngas has high net caloric value. The bio-oil also produces a high heating value that is comparable to the fossil fuels. It has been reported that among the algae biomass species, the highest bio-oil yields obtained from Chlorella at 350 °C and Nannochloropsis sp. at 280 °C with the values of 38.1 wt% and 36.5 wt%, respectively. These values are in a limited range of 28.6–33.0 wt% for other species including Spirulina, Cyanophyta and Euglena (Song et al. 2019). The downside of pyrolysis is that the process loses its efficiency without pretreatment. In addition, the process is most effective when the biomass particle size is small, where for the slow pyrolysis the particle size of > 2 mm and the slow heating rates of < 10 °C are used (Kocer et al. 2018).
The two categories for pyrolysis are conventional pyrolysis and modified pyrolysis. Conventional pyrolysis is the direct pyrolysis which does not employ any assistance from other techniques or materials. The common types of conventional pyrolysis in algal biorefinery are slow and fast pyrolysis. Slow pyrolysis is conducted at a moderate (relatively low) temperature and long vapor residence time, favoring biochar production. Grierson et al. (2011) have conducted an experiment on microalgae, Tetraselmis chui and found that high HHV of bio-oil with high contents of carbon showed both energy potential and high valuable biochar content. Fast pyrolysis is conducted at a high temperature and high heating rate, favoring bio-oil production. Ly et al. (2015) performed fast paralysis on macroalgae Saccharina japonica and found that the biomass-to-bio-oil conversion at optimum conditions is roughly 45 wt%. They have discovered that the biochar and syngas yields increase with temperature rise, while the bio-oil conversion decreases. The advantages of conventional pyrolysis are flexibility, high product quality, low-sulfur product, and large-scale efficiency. The disadvantage of conventional pyrolysis is its high starting cost. There are three primary types of modified pyrolysis: hydro-pyrolysis, catalytic pyrolysis, and microwave-induced pyrolysis. Lee et al. (2020) claimed that hydro-pyrolysis of algal biomass can produce up to 50 wt% of bio-oil. However, finding a suitable catalyst remains a challenge for this type of pyrolysis. The technique is also considered under-studied and requires further investigations. Catalytic pyrolysis also employs a catalyst, which can be acid, base, metal, zeolite, or carbon. The reaction is performed in two stages: primary (in situ) and secondary (ex situ). Catalytic pyrolysis allows for a lower energy requirement as the activation energy and process temperature are lower. It also allows for an easier solid and impurity separation while offering a higher product selectivity. An experiment conducted by Wang et al. (2018) showed that Enteromorpha clathrata produces 37.30–37.86 wt% of bio-oil, 42.86–44.44 wt% of biochar, and 17.70–19.84 wt% of syngas using MgCe/ZSM-5 catalyst. However, like many other catalytic processes, the expensive cost of catalysts remains a drawback of this process. Lastly, microwave-induced pyrolysis uses electromagnetic radiation technique to induce heating (Wang et al. 2015). This type of heating ensures instant penetration, uniform heating, enhanced mass transport, and relatively low activation energy. This pyrolysis technique was developed from the catalytic fast pyrolysis technique (Zhang et al. 2017). Like catalytic pyrolysis, microwave-induced pyrolysis is also performed in two stages: dipolar polarization and ionic conduction. Wang et al. (2015) reported that 27.7 wt% yield of bio-oil was converted from microalga Chlorella vulgaris with microwave-induced pyrolysis at a range of 390 to 700 W power output. Although the experimental result is promising, large-scale refinery and finding a cost effective and suitable microwave receptor remain a challenge for this technique.
Gasification of algal biomass
Gasification of algal biomass converts the biomass into syngas and biochar as a value-added by-product. A common pretreatment technique for this process is torrefaction. Torrefaction uses the temperature of 200–300 °C to burn the algal biomass in the absence of oxygen so that it loses the moisture content and rigid structure and increases its biochar yield. The highest biochar yield (to date), found to be 93.9 wt%, was achieved through dry torrefaction at 200 °C using microalga Chlamydomonas sp. JSC4 (Gan et al. 2018; Chen et al. 2016). However, torrefaction requires energy and time to reach its optimum effectiveness, taking up to 60 min residence time (Gan et al. 2018). Raheem et al. (2018) speculated that microalgae Chlorella vulgaris would be a viable raw material for syngas production. They performed catalytic gasification on the algal biomass and found that process produced three products: tar, syngas, and char. The experiment was conducted using a temperature range of 700–900 °C, reaction time of 15–40 min, and catalyst loading of 5–20 wt%. The results showed that around 64–83 wt% of the product was syngas, and 10–22 wt% was biochar. The rest of the product remained as tar, which amounts to 6–13 wt%. It was also found that the produced syngas consists of 27–46 mol% hydrogen gas (H2). Similarly, a hydrogen-targeted catalytic gasification process was performed with the algae Cladophora glomerata L. by Ebadi et al. (2017) at 700 °C. The results showed that syngas made up about 82–99.7 wt% of the product composition where hydrogen gas (H2) amounts to approximately 38–44 mol% of the syngas. Although the experiment conducted by Ebadi et al. (2017) produced relatively lower hydrogen gas and char, it produced significantly lower molar percentage of tar (0.1–6.5 mol%). Although gasification is a common alternative for thermal conversion of algal biomass, the tar formation and gas cleaning remained as the major obstacles for process commercialization as the syngas tends to be easily contaminated with ammonia and acids (Raheem et al. 2018).
Hydrothermal liquefaction (HTL) of algal biomass
Hydrothermal liquefaction of algae is conversion of algal biomass into biocrude at 280–370 °C and 10–25 MPa in 5–120 min reaction time and in the presence of water, with or without a catalyst (Xu et al. 2018). Biocrude is an intermediate product for biodiesel production. Though not necessary, the use of catalyst is encouraged as it enhances the biocrude yield and quality. In fact, using a catalyst has been reported to increase the maximum biocrude yield to 64% (Xu et al. 2018). Sodium carbonate (Na2CO3), which is a water-soluble alkali salt, is the most common catalyst used in HTL. Jena et al. (2012) was able to derive biocrude yield ranging from 39.9 to 51.6 wt% of biocrude from alga Chlorella pyrenoidosa using sodium carbonate at 350 °C and 60 min residence time. However, as Xu et al. (2018) elaborated, the alkali salts pose a limitation on the process, resulting in solid residue formation and leading to a separation issue. Energy conservation and nutrients recovery are primary advantages of this process. They also claimed that the HTL is an economically effective method, but the primary drawback of the process is the quality of the produced biocrude. The biocrude from HTL tends to contain high moisture content, high viscosity, and high heteroatom content, which are not desirable. In fact, the presence of the heteroatoms such as nitrogen, sulfur, and oxygen disqualify biocrude from being considered as biofuel. The nutrients in algal biomass residue from HTL process can also be recovered and reused for algal growth as well. Wastewater from this process is a course of nutrients which makes it a reliable candidate for the biofuel production (Leng et al. 2018).
Anaerobic digestion of algal biomass
Anaerobic digestion is a biological biogas synthesis process. It involves using microorganisms to degrade organic fractions of the substrate in the absence of O2 (Fernandez et al. 2018). The biogas from anaerobic digestion is consisted of 50–70% methane, 30–45% CO2, < 2% hydrogen, and < 3.5% hydrogen sulfide by volume (Milledge et al. 2019). Anaerobic digestion is an efficient process, generating about 20 m3 of methane per tonne of algae (Milledge et al. 2019). The process can provide a high yield of biomethane because it utilizes a large portion of organic carbons in algal biomass. Anaerobic digestion differs from other processes in the way that it favors macroalgae. Macroalgal biomass contains relatively low lipid content, which is more desirable as lipids often lead to process blockages. Allen et al. (2015) conducted an experiment on macroalga S. latissimi and found that the algae yielded 365 GJ energy per hectare per year. The common issues relating the anaerobic digestion of algal biomass are the energy-intensive drying pretreatment and the high C:N ratio. They also indicated that the acceptable range of C:N ratio for algal biomass is 6:1–20:1; however, the anaerobic digestion algal C:N ratio is 30:1. Because of the high carbon content, solvent extraction is commonly necessary as a pretreatment to reduce the carbon content in the biomass.
In an experiment conducted by Yang et al. (2018), the toxic compounds were extracted and removed using C. vulgaris biomass post-hydrothermal liquefaction wastewater. The C. vulgaris was selected due to the high stability of the strain to use organic carbons as a carbon source for their growth. The HTL process was performed at 270 °C (± 10 °C); the retention time was 1 h, and the solid content was 13 wt%. The wastewater was refrigerated at 4 °C prior to the treatment. Then, the digestion was performed, using swine manure as a source of microbial consortium. The researchers added 10 g/L of wastewater per 20 mL of swine sludge and submerged the sample in a water bath at 37 °C for 50 days. The experiment found that the organic carbons were completely converted to biomethane, and the nitrogen and phosphorus contents were successfully recovered to be used for further algal growth and save cultivation cost. Furthermore, it was also found that the COD, ammonia, and N-heterocyclic, which is an important algal growth inhibitor, were completely digested after the process (Yang et al. 2018).
Microbial fermentation
Microbial fermentation of algal biomass is a process that converts carbohydrates into sugar and eventually bioethanol. The fermentation is commonly performed in a fermenter, which is a type of bioreactor. Sritrakul et al. (2017) indicated that solvent pretreatment is typically necessary in this process; the solvent can dilute acids such as sulfuric, hydrochloric, phosphoric, and nitric acid, as acidic solvent helps in breaking down the algal cell walls. They also reported that the diluted sulfuric acid would promote the hydrolysis yield. The microalga C. vulgaris has been studied as the most potential species converting sugar into bioethanol (V. Kumar et al. 2016). Phwan et al. (2019) has performed a yeast fermentation on C. vulgaris to measure its bioethanol yield. Yeast was chosen as the consortium due to its ability to resist and convert inhibitors and maintain energy-redox balances (Westman et al. 2017). The algal biomass undergoes an enzymic saccharification prior to the fermentation. The saccharification was performed in two steps: liquefaction using the enzyme alpha amylase at the temperature of 90 °C and pH level of 5.5, and saccharification using the enzyme amyloglucosidase at the temperature of 70 °C. The yeast used in this experiment was the S. cerevisiae which had been cultivated in agar solution. After the saccharification, the biomass and yeast are fed into a shaking incubator of 150 rpm for 84 h. The fermentation is isothermal, and the process temperature was maintained at 32 °C. After 84 h, the sugar concentration was measured to be 5.63 g/L, providing a bioethanol yield of only 0.07 g/g algae. Result was unsatisfactory; thus, they performed a few more trials using acid solvent pretreatment. They have found that the algal sample pretreated with H2SO4 provided the highest sugar reduction. The sugar concentration was found to be 6.95 g/L, providing a bioethanol yield of 0.28 g/g dry microalgae. Microbial fermentation can also synthesize biofuel and biohydrogen through different techniques, including direct photolysis, indirect photolysis, and dark fermentation. Direct photolysis uses light and water to ferment algal biomass into H2 and CO2, while indirect photolysis uses light sources in place of the light (Sharma and Arya 2017). These two techniques are not preferred due to their low hydrogen yield, and CO2 synthesis. On the contrary, dark fermentation has been found as a promising technique since it produces many valuable products like biohydrogen, acetic acid, lactic acid, and pyruvate. Dark fermentation is performed in the absence of light source, water, and oxygen. The xylose stored in algal biomass is converted into the valuable by-product pyruvate. For all three techniques, hydrogenase is the key enzyme that catalyzes biohydrogen formation (Xia et al. 2015).
Industrial applications of algal bioenergy and value-added products
Products from algal biomass biorefinery are categorized into biofuels and value-added products. Currently, algal biodiesel is being studied in the energy industry as a potential fossil fuel replacement and it is primarily used in the transportation industry as it is compatible with diesel engines (Rahpeyma and Raheb 2019).
The biofuel is produced from the lipid content of algal biomass via algal biorefinery processes such as direct combustion, transesterification, and pyrolysis. Algal biomass lipid extraction is an excellent pretreatment technique that significantly increases biodiesel yield. Biodiesel derived from algal biomass also meets both the European standard (EN14214) and American standard (ASTM D6751) for diesel quality. The comparison of algal biodiesel against both standards are shown in Table 2, (Akubude et al. 2019). Technically, the algal biofuel is 10–20 times more viscous than conventional diesel, hindering its burning potential; thus, biofuel blending is recommended (Kumar and Thakur 2018). Nevertheless, the use of algal biodiesel and algal biodiesel blends still shows a great potential in replacing fossil fuel, hence reducing net CO2 emissions.
Other than chemical and industrial uses, algal biomass also produce daily-use products such as functional food, pharmaceutical compounds, and pigments as well. Functional food, algal biomass can be ingested directly. Macroalgae, commonly known as ‘seaweed’ is consumed worldwide, especially in Asia. Similarly, microalga Spirulina has been converted into nutraceutical tablets and ingested as a supplement as well. Seaweed and Spirulina have been studied to contain important vitamins, carbohydrates, and proteins (Suganya et al. 2015; Singh et al. 2008). Recently, Zheng et al. (2020) claimed that marine algae have anti-cancer, antioxidant, anti-diabetic, immunity-boosting properties. Algae are also excellent probiotics for digestive health. For these properties, algae and algal extracts are marketed as supplements in the pharmaceutical industry. Pigments such as yellow-orange-red and green colors can also be extracted from algae, depending on the species and strains. The yellow-orange-red pigment is scientifically known as carotenoids, while the green is known as chlorophyll (Nwoba, et al. 2020).
Conclusion
Algal biomass has proven to hold an important dual role in wastewater bioremediation and the production of bioenergy and value-added by-products. Not only is the biomass sustainable and efficient, but it is also versatile and cost effective in both bioremediation and biofuel production processes. Industrialization and urbanization have resulted in an increasing amount of wastewater and global energy demand. Algae have proven to be effective and cost efficient in wastewater treatment in removing harmful impurities, such as heavy metals and nutrients. Algal biomass is relatively inexpensive to cultivate. Some cultivation methods also involve the usage of CO2 as algae food, helping to eliminate CO2 content in industrial areas. However, it is important to note that the effectiveness of algal remediation is dependent on retention time, light intensity, temperature, and pH level. The algal biomass can also be converted into important biofuels, such as biodiesel and other value-added products. The most efficient and commonly used techniques are transesterification of algal lipids and pyrolysis of algal biomass. Biodiesel produced from algal biorefinery meet both the American (ASTM D6751) and European (EN14214) standards for diesel quality as indicated in Table 2. Algal bioethanol also plays an important role in replacing diesel in the transportation industry. Commercially, diesel blends with bioethanol are known as ‘E10’ and ‘E20,’ with 10% and 20% of bioethanol percentage, respectively. Through the overall review from this article, future directions should be toward investigation and review of co-mingled and integrated technologies and processes applied to produce bioenergy and value-added products from algal biomass. Further focus could be given to the recent publications on the comparison between the sole algal biomass and its coculturing with other consortia.
References
Ahmad S, Pandey A, Pathak VV, Tyagi VV, Kothari R (2019) Phycoremediation: Algae as eco-friendly tools for the removal of heavy metals from wastewaters. Bioremediation Ind Waste Environ Saf. https://doi.org/10.1007/978-981-13-3426-9_3
Ajayan K, Selvaraju M, Unnikannan P, Sruthi P (2015) Phycoremediation of tannery wastewater using microalgae scenedesmus species. Int J Phytorem 17(10):907–916
Akubude VC, Nwaigwe KN, Dintwa E (2019) Production of biodiesel from microalgae via nanocatalyzed transesterification process: a review. Materials Science for Energy Technologies 2(2):216–225
Allen E, Wall DM, Herrmann C, Xia A, Murphy JD (2015) What is the gross energy yield of third generation gaseous biofuel sourced from seaweed? Energy 81:352–360
Amenorfenyo DK, Huang X, Zhang Y, Zeng Q, Zhang N, Ren J, Huang Q (2019) Microalgae brewery wastewater treatment: potentials, benefits and the challenges. Int J Environ Res Public Health 16(11):1910
Anto S, Mukherjee SS, Muthappa R, Mathimani T, Deviram G, Kumar SS, Verma TN, Pugazhendhi A (2020) Algae as green energy reserve: technological outlook on biofuel production. Chemosphere 242:120579
Aragaw TA, Asmare AM (2018) Phycoremediation of textile wastewater using indigenous microalgae. Water Pract Technol 13(2):274–284
Arashiro LT, Montero N, Ferrer I, Acien FG, Gomez C, Garfi M (2018) Life cycle assessment of high rate algal ponds for wastewater treatment and resource recovery. Sci Total Environ 622–623(2018):1118–1130
Ballen-Segura M, Rodriguez L, Parra D, Vega A, Perez KM (2016) Using Scenedesmus sp. for the phycoremediation of tannery wastewater. Tecciencia 12(21):69–75
Bankston E, Wang Q, Higgins BT (2020) Algae support populations of heterotrophic, nitrifying, and phosphate-accumulating bacteria in the treatment of poultry litter anaerobic digestate. Chem Eng J 398:125550
Bansah KJ, Suglo RS (2016) Sewage treatment by waste stabilization pond systems. J Energy Nat Resour Manag 7(1):7–14
Bansal A, Shinde O, Sarkar S (2018) Industrial wastewater treatment using phycoremediation technologies and co-production of value-added products. J Bioremediation Biodegradation. https://doi.org/10.4172/2155-6199.1000428
Bauddh K, Korstad J (2022) Phycoremediation: use of algae to sequester heavy metals. Hydrobiology 1(3):288–303
Bhatia SK, Mehariya S, Bhatia RK, Kumar M, Arivalagan P, Awasthi MK, Atabani AE, Kumar G, Kim W, Seo SO, Yang YH (2020) Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci Total Environ 751:141599
Bolay P, Muro-Pastor MI, Florencio FJ, Klahn S (2018) The distinctive regulation of cyanobacterial glutamine synthetase. Life 8(4):52
Bouabidi ZB, El-Naas MH, Zhang Z (2019) Immobilization of microbial cells for the biotreatment of wastewater: a review. Environ Chem Lett 17(1):241–257
Brar A, Kumar M, Vivekanand V, Pareek N (2017) Photoautotrophic microorganisms and bioremediation of industrial effluents: current status and future prospects. 3 Biotech. https://doi.org/10.1007/s13205-017-0600-5
Bwapwa J, Jaiyeola A, Chetty R (2017) Bioremediation of acid mine drainage using algae strains: a review. S Afr J Chem Eng 24(2017):62–70
Cadar E, Sirbu R, Pirjol BSN, Ionescu AM, Pirjol TN (2019) Heavy metals bioaccumulation capacity on marine algae biomass from Romanian Black Sea Coast. Rev Chım (bucharest) 70(8):3065–3072
Cai T, Park SY, Li Y (2013) Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sustain Energy Rev 19(2013):360–369
Chabukdhara M, Gupta SK, Gogoi M (2017) Phycoremediation of heavy metals coupled with generation of bioenergy. Algal biofuels recent advances and future prospects, Springer, Cham, pp 163–188
Chambonniere P, Bronlund J, Guieysse B (2021) Pathogen removal in high-rate algae pond: state of the art and opportunities. J Appl Phycol 33:1501–1511. https://doi.org/10.1007/s10811-020-02354-3
Chang JS, Show PL, Ling TC, Chen CY, Ho SH, Tan CH, Nagarajan D, Phong WN (2017) Photobioreactors. Current Developments in Biotechnology and Bioengineering, Elesvier, pp 313–352
Chen CY, Bai MD, Chang JS (2013) Improving microalgal oil collecting efficiency by pretreating the microalgal cell wall with destructive bacteria. Biochem Eng J 81:170–176
Chen YC, Chen WH, Lin BJ, Chang JS, Ong HC (2016) Impact of torrefaction on the composition, structure and reactivity of a microalga residue. Appl Energy 181(2016):110–119
Chia SR, Ong HC, Chew KW, Show PL, Phang SM, Ling TC, Chang JS (2017) Sustainable approaches for algae utilisation in bioenergy production. Renew Energy 129:838–852
Choi HI, Lee JS, Choi JW, Shin YS, Sung YJ, Hong ME, Sim SJ (2019) Performance and potential appraisal of various microalgae as direct combustion fuel. Bioresour Technol 273:341–349
Clippinger J, Davis R (2019) Techno-economic analysis for the production of algal biomass via closed photobioreactors: future cost potential evaluated across a range of cultivation system designs. National Renewable Energy Laboratory, United states
Coggins LX, Crosbie ND, Ghadouani A (2019) The small, the big, and the beautiful: emerging challenges and opportunities for waste stabilization ponds in Australia. Wiley Interdiscip Rev Water 2019:1–8
Craggs R, Sutherland D, Campbell H (2012) Hectare scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J Appl Phycol 24:329–337
D’Hondt E, Martín-Juárez J, Bolado S, Kasperoviciene J, Koreiviene J, Sulcius S, Bastiaens L (2017) Cell disruption technologies. Microalgae Based Biofuels and Bioproducts. Elesevier, Cham, pp 133–154
Daliry S, Hallajisani A, Mohammadi Roshandeh J, Nouri H, Golzary A (2017) Investigation of optimal condition for chlorella vulgaris microalgae growth. Glob J Environ Sci Manage 3(2):217–230
Danouche M, El Ghachtouli N, El Arroussi H (2021) Phycoremediation mechanisms of heavy metals using living green microalgae: physicochemical and molecular approaches for enhancing selectivity and removal capacity. Heliyon 7(7):e07609
Demirbas A (2009) Effects of temperature and particle size on bio-char yield from pyrolysis of agricultural residues. J Anal Appl Pyrol 72(2):243–248
Demuez M, Gonzalez-Fernandez C, Ballesteros M (2015) Algicidal microorganisms and secreted algicides: new tools to induce microalgal cell disruption. Biotechnol Adv 33(8):1615–1625
Dinesh Kumar S, Santhanam P, Park MS, Kim M-K (2016) Development and application of a novel immobilized marine microalgae biofilter system for the treatment of shrimp culture effluent. J Water Process Eng 13:137–142
Dixit S, Singh DP (2015) Phycoremediation: future perspective of green technology. Algae and Environmental Sustainability, Springer, New Delhi, pp 9–21
Duarah P, Haldar D, Patel AK, Dong CD, Singhania RR, Purkait MK (2022) A review on global perspectives of sustainable development in bioenergy generation. Bioresour Technol 348:126791
Dwivedi S, Dwivedi N (2022) Microalgae: a potential tool for wastewater treatment and its biotechnological applications. An Integration of Phycoremediation Processes in Wastewater Treatment. Elsevier, Cham, pp 121–134
Ebadi AG, Hisoriev H, Zarnegar M, Ahmadi H (2017) Hydrogen and syngas production by catalytic gasification of algal biomass (Cladophora glomerata L.) using alkali and alkaline-earth metals compounds. Environ Technol 40(9):1178–1184
Emami Moghaddam SA, Harun R, Mokhtar MN, Zakaria R (2018) Potential of zeolite and algae in biomass immobilization. BioMed research international, Hindawi
Fakhimi N, Gonzalez-Ballester D, Fernández E, Galván A, Dubini A (2020) Algae-bacteria consortia as a strategy to enhance H2 production. Cells 9(6):1353
Fernandez S, Srinivas K, Schmidt AJ, Swita MS, Ahring BK (2018) Anaerobic digestion of organic fraction from hydrothermal liquefied algae wastewater byproduct. Bioresour Technol 247:250–258
Filippino KC, Mulholland MR, Bott CB (2015) Phycoremediation strategies for rapid tertiary nutrient removal in a waste stream. Algal Res 11:125–133
Gan YY, Ong HC, Show PL, Ling TC, Chen WH, Yu KL, Abdullah R (2018) Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energy Conserv Manag 165(2018):152–162
Ganesan R, Manigandan S, Samuel MS, Shanmuganathan R, Brindhadevi K, Chi NTL, Pugazhendhi A (2020) A review on prospective production of biofuel from microalgae. Biotechnol Rep 27:e00509
Gani P, Sunar NM, Peralta HM, Latiff AA, Parjo UM, Razak AR (2015) Phycoremediation of wastewater and potential hydrocarbon from microalgae: a review. Adv Environ Biol 9(20):1–8
Garfi M, Flores L, Ferrer I (2017) Life cycle assessment of wastewater treatment systems for small communities: activated sludge, constructed wetlands and high rate algal ponds. J Clean Prod 161(2017):211–219
Giostri A, Binotti M, Macchi E (2016) Microalgae cofiring in coal power plants: innovative system layout and energy analysis. Renew Energy 95:449–464
Gong M, Bassi A (2016) Carotenoids from microalgae: a review of recent developments. Biotechnol Adv 34(8):1396–1412
Goswami G, Makut BB, Das D (2019) Sustainable production of bio-crude oil via hydrothermal liquefaction of symbiotically grown biomass of microalgae-bacteria coupled with effective wastewater treatment. Sci Rep 9:15016. https://doi.org/10.1038/s41598-019-51315-5
Gouveia, L., Batista, P.A., Marques, AP, Moura, P. (2012). Exploring Scenedesmus obliqus and Nannochloropsis sp. Potential as a sustainable raw material for biofuels and high added value compounds, In Livro de actas do Congresso Iberoamericano Sobre Biorrefinerias, 701–707.
Grierson S, Strezov V, Shah P (2011) Properties of oil and char derived from slow pyrolysis of Tetraselmis chui. Bioresour Technol 102(17):8232–8240. https://doi.org/10.1016/j.biortech.2011.06.010
Gross M, Jarboe D, Wen Z, W. (2015) Biofilm-based algal cultivation system. Appl Microbiol Biotechnol 99(2015):5781–5789
Gu S, Lan CQ (2021) Biosorption of heavy metal ions by green alga Neochloris oleoabundans: Effects of metal ion properties and cell wall structure. J Hazard Mater 418:126336
Guedes-Alonso R, Montesdeoca-Esponda S, Herrera-Melián JA, Rodríguez-Rodríguez R, Ojeda-González Z, Landívar-Andrade V, Santana-Rodríguez JJ (2020) Pharmaceutical and personal care product residues in a macrophyte pond-constructed wetland treating wastewater from a university campus: presence, removal and ecological risk assessment. Sci Total Environ 703:135596
Hamed SM, Kapoore RV, Raut MP, Vaidyanathan S, Wright PC (2020) Influence of nutrient status on the biohydrogen and lipid productivity in Parachlorella kessleri: a biorefinery approach. Appl Microbiol Biotechnol 104(23):10293–10305
Heriques B, Rocha L, Lopes CB, Duarte AC, Vale C, Pardal MA, Pereira E (2017) A macrolgae based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J Environ Manage 191:275–289
Ho SH, Huang SW, Chen CY, Hasunuma T, Kondo A, Chang JS (2013) Bioethanol production using carbohydrate-rich microalgae biomass as feedstock. Bioresour Technol 135:191–198
Huang Q, Jiang F, Wang L, Yang C (2017) Design of photobioreactors for mass cultivation of photosynthetic organisms. Engineering 3(2017):318–329
International Energy Agency [IEA]. (2020). World Energy Outlook 2020 [Annual Report]. IEA. https://www.iea.org/reports/world-energy-outlook-2020
Jena U, Das KC, Kastner JR (2012) Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl Energy 98(2012):368–375
Jia H, Yuan Q (2016) Removal of nitrogen from wastewater using microalgae and microalgae-bacteria consortia. Cogent Environ Sci 2(2016):1–15
Kandasamy S, Narayanan M, He Z, Liu G, Ramakrishnan M, Thangavel P, Carvalho IS (2021) Current strategies and prospects in algae for remediation and biofuels: an overview. Biocatal Agric Biotechnol 35:102045
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed and other products. Microb Cell Fact 2018:1–21
Khiewwijit R, Rijnaarts H, Temmink H, Keesman K (2018) Glocal assessment of integrated wastewater treatment and recovery concepts using partial nitration/Anammox and microalgae for environmental impacts. Sci Total Environ 628–629(2018):74–84
Kim B-K, Choi J-E, Cho K, Kang Z, Ramanan R, Moon D-G, Kim H-S (2018) Influence of water depth on microalgal production, biomass harvest and energy consumption in high rate algal pond using municipal wastewater. J Microb Biotechnol 28(4):630–637
Kocer AT, Inan B, Ozcimen D (2018) A comparison of bioethanol and biochar production from various algal biomass samples and sweet sorghum energy crop. Environ Res Technol 1:43–50
Koul B, Sharma K, Shah MP (2022) Phycoremediation: a sustainable alternative in wastewater management regime. Environ Technol Innov 25:102040
Kumar M, Thakur IS (2018) Municipal secondary sludge as carbon source for production and characterization of biodiesel from oleaginous bacteria. Bioresource Technol Reports 4:108–113. https://doi.org/10.1016/j.biteb.2018.09.011
Kumar KS, Dahms HU, Won EJ, Lee JS, Shin KH (2015) Microalgae— A promising tool for heavy metal remediation. Ecotoxicol Environ Saf 113(2015):329–352
Kumar VB, Pulidindi IN, Kinel-Tahan Y, Yehoshua Y, Gedanken A (2016) Evaluation of the potential of chlorella vulgaris for bioethanol production. Energy Fuels 30(4):3161–3166. https://doi.org/10.1021/acs.energyfuels.6b00253
Kumar S, Mal G, Sharma HR (2018) Waste stabilization ponds: a technical option for liquid waste management in rural areas in Haryana under Swachh Bharat Mission-Gramin. Environ Int J Sci Technol 13(2018):177–187
Kushwaha A, Rani R, Patra JK (2020) Adsorption kinetics and molecular interactions of lead [Pb (II)] with natural clay and humic acid. Int J Environ Sci Technol 17(3):1325–1336
Lee XJ (2018) Evaluation of cost effective adsorbent and biochar from Malaysia oil palm wastes: synthesis, characterization and optimization studies. University of Nottingham, Thesis
Lee XJ, Ong HC, Gan YY, Chen W-H, Mahlia TMI (2020) State of art review on conventional and advanced pyrolysis of macroalgae and microalgae for biochar, bio-oil and bio-syngas production. Springer, Energy Conversion and Management, p 210
Lekshmi R, Rejiniemon TS, Sathya R, Kuppusamy P, Al-Mekhlafi FA, Wadaan MA, Rajendran P (2022) Adsorption of heavy metals from the aqueous solution using activated biomass from Ulva flexuosa. Chemosphere 306:135479
Leng L, Li J, Wen Z, Zhou W (2018) Use of microalgae to recycle nutrients in aqueous phase derived from hydrothermal liquefaction process. Biores Technol 256:529–542
Leong YK, Huang CY, Chang JS (2021) Pollution prevention and waste phycoremediation by algal-based wastewater treatment technologies: the applications of high-rate algal ponds (HRAPs) and algal turf scrubber (ATS). J Environ Manage 296:113193
Ly HV, Kim SS, Woo HC, Choi JH, Suh DJ, Kim J (2015) Fast pyrolysis of microalga Saccharina japonica in a bubbling fluidized-bed reactor for bio-oil production. Energy 93(2):1436–1446
Mahapatra S, Samal K, Dash RR (2022) Waste Stabilization Pond (WSP) for wastewater treatment: a review on factors, modelling and cost analysis. J Environ Manage 308:114668
Malik S, Shahid A, Betenbaugh MJ, Liu CG, Mehmood MA (2022) A novel wastewater-derived cascading algal biorefinery route for complete valorization of the biomass to biodiesel and value-added bioproducts. Energy Convers Manage 256:115360
Mandotra SK, Lolu AJ, Kumar S, Ramteke PW, Ahluwalia AS (2019) Integrated approach for bioremediation and biofuel production using algae. A Trajectory Towards a Sustainable Environment, Restoration of Wetland Ecosystem, pp 145–160
Milledge J, Nielsen B, Maneein S, Harvey P (2019) A brief review of anaerobic digestion of algae for bioenergy. Energies. https://doi.org/10.3390/en12061166
Miranda AF, Ramkumar N, Andriotis C, Holtkemeier T, Yasmin A, Rochfort S, Wlodwic D, Morrison P, Roddick F, Spangenberg G, Lal B, Subudhi S, Mouradov A (2017) Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels 10:120
Mishra V, Dubey A, Prajapti SK (2017) Algal biomass pretreatment for improved biofuel production. Springer, Algal Biofuels, pp 259–280
Mohsenpour SF, Hennige S, Willoughby N, Adeloye A, Gutierrez T (2021) Integrating micro-algae into wastewater treatment: A review. Sci Total Environ 752:142168
Molazadeh M, Ahmadzadeh H, Pourisnfar HR, Lyon S, Rampelotto PH (2019) The use of microalgae for coupling wastewater treatment with CO2 Bio fixation. Front Bioeng Biotechnol 7(2019):1–12
Molinuevo-Salces B, Riaño B, Hernández D, Cruz García-González M (2019) Microalgae and wastewater treatment: advantages and disadvantages. Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. Springer, Singapore, pp 505–533
Mubarak M, Shaija A, Suchithra TV (2015) A review on the extraction of lipid from microalgae for biodiesel production. Algal Res 7:117–123
Musa IA (2016) The effects of alcohol to oil molar ratios and the type of alcohol on biodiesel production using transesterification process. Egypt J Pet 25(1):21–31
Nagendranatha Reddy C, Nguyen HTH, Noori MT, Min B (2019) Potential applications of algae in the cathode of microbial fuel cells for enhanced electricity generation with simultaneous nutrient removal and algae biorefinery: current status and future perspectives. Bioresour Technol 292:12201
Narala RR, Garg S, Sharma KK, Thomas-Hall SR, Deme M, Li Y, Schenk PM (2016) Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front Energy Res 4:29
Nwankwo IH, Nwaiwu NE, Nwabanne JT (2019) The dynamics of solids removal in waste stabilization ponds. J Eng Res Rep 7(4):1–10
Nwoba EG, Ogbonna CN, Ishika T, Vadiveloo A (2020) Microalgal pigments: a source of natural food colors. Microalgae biotechnology for food, health and high value products. Springer, Singapore, pp 81–123
Osama R, Awad HM, Zha S, Meng F, Tawfik A (2021) Greenhouse gases emissions from duckweed pond system treating polyester resin wastewater containing 1, 4-dioxane and heavy metals. Ecotoxicol Environ Saf 207:111253
Pavithra KG, Kumar PS, Jaikumar V, Vardhan KH, Sundar Rajan P (2020) Microalgae for biofuel production and removal of heavy metals: a review. Environ Chem Lett 18(2020):1905–1923
Phwan CK, Chew KW, Sebayang AH, Ong HC, Ling TC, Malek MA, Show PL (2019) Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae. Biotechnol Biofuels 12:191
Qiu R, Gao S, Lopez PA, Ogden KL (2017) Effects of pH on cell growth, lipid production and CO2 addition of microalgae Chlorella sorokiniana. Algal Res 28:192–199
Raheem A, Ji G, Memon A, Sivasangar S, Wang W, Zhao M, Taufiq-Yap YH (2018) Catalytic gasification of algal biomass for hydrogen-rich gas production: parameter optimization via central composite design. Energy Convers Manage 158:235–245
Rahpeyma SS, Raheb J (2019) Microalgae biodiesel as a valuable alternative to fossil fuels. BioEnergy Res 12(4):958–965
Ranjith Kumar R, Hanumantha Rao P, Arumugam M (2015) Lipid extraction methods from microalgae: a comprehensive review. Front Energy Res 2:61
Ray NE, Terlizzi DE, Kangas PC (2015) Nitrogen and phosphorus removal by the algal turf scrubber at an oyster aquaculture facility. Ecol Eng 131:27–32
Rezvani F, Sarrafzadeh MH (2020) Autotrophic granulation of hydrogen consumer denitrifiers and microalgae for nitrate removal from drinking water resources at different hydraulic retention times. J Environ Manage 268:110674
Rizvi S, Goswami L, Gupta SK (2020) A holistic approach for Melanoidin removal via Fe-impregnated activated carbon prepared from Mangifera indica leaves biomass. Biores Technol Rep 12:100591
Salama EL, Roh HS, Dev S, Khan MA, Shanab RA, Chang SW, Jeon BH (2019) Algae as a green technology for heavy metal removal from various wastewater. World J Microbiol Biotechnol 35(2019):1–19
Sasi PK, Viswanathan A, Mechery J, Thomas D, Jacob JP, Paulose SV (2020) Phycoremediation of paper and pulp mill effluent using Planktochlorella nurekis and Chlamydomonas reinhardtii: a comparative study. J Environ Treat Tech 8(2):809–817
Sepehri A, Sarrafzadeh MH, Avateffazeli M (2020) Interaction between Chlorella vulgaris and nitrifying-enriched activated sludge in the treatment of wastewater with low C/N ratio. J Clean Prod 247:119164
Sharma A, Arya SK (2017) Hydrogen from algal biomass: a review of production process. Biotechnol Rep 15:63–69. https://doi.org/10.1016/j.btre.2017.06.001
Shetty P, Boboescu IZ, Pap B, Wirth R, Kovács KL, Bíró T, Maróti G (2019) Exploitation of algal-bacterial consortia in combined biohydrogen generation and wastewater treatment. Front Energy Res 7:52
Singh SP, Singh P (2015) Effect of temperature and light on growth of algal species: a review. Renew Sustain Energy Rev 50(2015):431–444
Singh S, Kate BN, Banerjee UC (2008) Bioactive compounds from cyanobacteria and microalgae: an overview. Crit Rev Biotechnol 25(3):73–95
Sivaramakrishnan R, Incharoensakdi A (2017) Production of methyl ester from two microalgae by two-step transesterification and direct transesterification. Environ Sci Pollut Res Int 24(5):4950–4963. https://doi.org/10.1007/s11356-016-8217-5
Soeprobowati TR, Hariyati R (2017) The phycoremediation of textile wastewater discharge by chlorella pyrenoidosa H. Chick Arthrospira platensis Gomont and Chaetoceros calcitrans Paulson H Takano. Aquac Aquar Conserv Legis 10(3):640–651
Song W, Wang S, Xu D, Guo Y, Yang C, Zhang J, Li Y (2019) Comprehensive potential evaluation of the bio-oil production and nutrient recycling from seven algae through hydrothermal liquefaction. Korean J Chem Eng 36(10):1604–1618
Sritrakul N, Nitisinprasert S, Keawsompong S (2017) Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric Nat Resour 51(6):512–519
Suganya T, Varman M, Masjuki HH, Renganathan S (2015) Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: a biorefinery approach. Renew Sustain Energy Rev 55:909–941
Sunday ER, Uyi OJ, Caleb OO (2018) Phycoremediation: an eco-solution to environmental protection and sustainable remediation. J Chem Environ Biol Eng 2(1):5–10
Sutherland DL, Turnbull MH, Craggs RJ (2017) Environmental drivers that influence microalgal species in full-scale wastewater treatment high rate algal ponds. Water Res 124(2017):504–512
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metals toxicity and the environment. Experientia Suppl 101(2012):133–164
Topare NS, Raut SJ, Renge VC, Khedkar SV, Chavan YP, Bhagat SL (2011) Extraction of oil from algae by solvent extraction and oil expeller method. Int J Chem Sci 9(4):1746–1750
Uddin A, Lall AM (2019) Phycoremediation of heavy metals by Botryococus brurauni from wastewater. Biosci, Biotechnol Res Asia 16(1):129–133
Vijayaraghavan K, Reddy DHK, Yun YS (2019) Improving the quality of runoff from green roofs through synergistic biosorption and phytoremediation techniques: a review. Sustain Cities Soc 46:101381
Wang N, Tahmasebi A, Yu J, Xu J, Huang F, Mamaeva A (2015) A Comparative study of microwave-induced pyrolysis of lignocellulosic and algal biomass. Bioresour Technol 190:89–96
Wang Y, Ho SH, Cheng CL, Guo WQ, Nagarajan D, Ren NQ, Lee DJ, Chang JS (2016) Perspectives on the feasibility of using microalgae for industrial wastewater treatment. Biosour Technol 222(2016):485–497
Wang S, Cao B, Liu X, Xu L, Hu Y, Afonaa-Mensah S, Abomohra AE, He Z, Wang Q, Xu S (2018) A comparative study on the quality of bio-oil derived from green macroalga Enteromorpha clathrata over metal modified ZSM-5 catalysts. Bioresour Technol 256:446–455
Wang M, Zhang SC, Tang Q, Shi LD, Tao XM, Tian GM (2020) Organic degrading bacteria and nitrifying bacteria stimulate the nutrient removal and biomass accumulation in microalgae-based system from piggery digestate. Sci Total Environ 707:134442
Westman JO, Wang R, Novy V, Franzen CJ (2017) Sustaining fermentation in high- gravity ethanol production by feeding yeast to a temperature-profiled multifeed simultaneous saccharification and co-fermentation of wheat straw. Biotechnol Biofuels 10:213. https://doi.org/10.1186/s13068-017-0893-y
Wollmann F, Dietze S, Ackermann JU, Bley T, Walther T, Steingroewer J, Krujatz F (2019) Microalgae wastewater treatment: Biological and technological approaches. Eng Life Sci 19(2019):860–871
Xia A, Cheng J, Song W, Su H, Ding L, Lin R, Cen K (2015) Fermentative hydrogen production using algal biomass as feedstock. Renew Sustain Energy Rev 51:209–230
Xu D, Lin G, Guo S, Wang S, Guo Y, Jing Z (2018) Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: a critical review. Renew Sustain Energy Rev 97:103–118
Yang L, Si B, Tan X, Chu H, Zhou X, Zhang Y, Zhao F (2018) Integrated anaerobic digestion and algae cultivation for energy recovery and nutrient supply from post-hydrothermal liquefaction wastewater. Bioresour Technol 266:349–356
Zainith S, Saxena G, Kishor R, Bhargava RN (2021) Application of microalgae in industrial effluent treatment contaminants removal and biodiesel production: opportunities challenges, and future Bioremediation for environmental sustainability approaches to tackle pollution for cleaner and greener society. Elesevier, Singapore, pp 481–517
Zhang J, Rong J, Wang Q (2017) Mixotrophic cultivation, a preferable microalgae cultivation mode for biomass/bioenergy production, and bioremediation, advances and prospect. Int J Hydrogen Energy 42(12):8505–8517
Zhao B, Su Y, Liu D, Zhang H, Liu W, Cui G (2016) SO2/NOx emissions and ash formation from algae biomass combustion: Process characteristics and mechanisms. Energy 113:821–830
Zheng LX, Chen XQ, Cheong KL (2020) Current trends in marine algae polysaccharides: the digestive tract, microbial catabolism, and prebiotic potential. Int J Biol Macromol 151:344–354. https://doi.org/10.1016/j.ijbiomac.2020.02.168
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Algal biomass dual roles in phycoremediation of wastewater and production of bioenergy and value-added products.
Author information
Authors and Affiliations
Contributions
NL contributed to investigation, methodology, visualization, writing—original draft. YG contributed to investigation, methodology, visualization, writing—original draft. GA contributed to validation, writing—review and editing. VR contributed to conceptualization, supervisor, validation, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Editorial responsibility: Jing Chen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Razaviarani, V., Arab, G., Lerdwanawattana, N. et al. Algal biomass dual roles in phycoremediation of wastewater and production of bioenergy and value-added products. Int. J. Environ. Sci. Technol. 20, 8199–8216 (2023). https://doi.org/10.1007/s13762-022-04696-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04696-6