Abstract
Water pollution connected with rapid industrial growth is one of the most challenging issues worldwide. The disposal of heavy metals turns out to be complex and expensive, so several researchers have tried to remove these pollutants based on abundantly available, inexpensive materials, such as agsricultural waste to be used as sorbents; however, most of these materials have not achieved sufficient removal rates. Consequently, research has been conducted for economic, environmentally benign, and efficient byproduct materials. Among the most auspicious techniques was the extraction of microcrystalline cellulose, chemically modified by a low-molecular-weight organic acid such as citric acid (McC-CA); such materials are powerful chelators for the removal of heavy metals from water bodies. The Taguchi robust design approach was used in present study to optimize the factors determing the efficieny of heavy metal removal, namely ion concentration, pH-value, adsorbent dosage, and contact time, through an orthogonal array (OA) L16 = 44 in batch absorbtion experiments. The results illustrated the optimum combination for Co (II) and Cs (I) adsorption was pH (5–6), C (1–50 mg L−1), D (3–4 g L−1), T (60–100 min) according to contour plots and verification tests, Where the percent removal reached 74 and 88% for cobalt and cesium respectively when using this optimal combination. Furthermore, when this combination was applied to 60Co and 137Cs the percent removal ranged from 96.01 to 90.28% for 60Co, and 100 to 94.25% for 137Cs. Therefore, it can be inferred that the use of McC-CA constitutes an effective tool to remove cobalt and cesium ions from waterbodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulosic waste has extensive applications in the safe and stable solidification of hazardous waste and applications in construction sector (Saleh and Eskander 2009; Eskander and Saleh 2012; Saleh and Eskander 2012; Saleh et al. 2014). In this context, rice straw is one of the most prevalent agricultural byproducts since it is disposed of by combustion, which creates a hazardous environmental phenomenon, namely the Black Cloud. Egypt is one of the major rice straw producers in the world (Elbasiouny and Elbehiry 2020). Rice straw is a burden and causes harmful effects by environmental pollution, in addition to economic and social risks originating from the spread and increase of diseases resulting from environmental pollution (El safty 2020). The most enticing and promising, eco-friendly, renewable, and sustainable way to reutilize these byproduct material from rice industry is microcrystalline cellulose (McC) extraction from rice straw for the sustainable removal of various aquatic pollutants from wastewater (Liu et al. 2021). McC can be prepared from rice straw, which is regarded as one of the most serious environmental challenges in Egypt, when farmers remove it by incineration, causing above-mentioned environmental problems. McC is a prevalent polymer in nature, which has rife applications in catalysis, ion exchange, and adsorption processes (El-sakhawy and Hassan 2007; Trache et al. 2016; Dawoud et al. 2021). Notwithstanding the efficacy of cellulose in heavy metals adsorption from wastewater, it is even more efficient and reactive if it is activated via a low molecular weight organic acid (Adel and El-shinnawy 2012; Hokkanen et al. 2016). This eutrophication phenomenon utilizing citric acid is regarded to be one of the most influential cellulose activation mechanisms since citric acid interacts with the McC surface groups by formation of chemical bonds; this way, strong claws are generated, which enables modified McC to adsorb heavy metal ions by formation of chelat complexes. In addition, attachment of citric acid increases the number of hydroxyl and carboxyl groups on the surface of McC. This effect can be observed by Fourier transform infrared spectroscopy (FTIR) analysis; here, spectra different to untreated cellulose appear (Nagarajan et al. 2020).
Cobalt and cesium, which are biochemically similar to potassium and calcium, are among the most critical heavy metals in the ecosystem. For example, low dosages of these heavy metals can accumulate in human and animal food chains and cause diseases like different types of cancer (Hassan et al. 2022; El Adham et al. 2022; Chen et al. 2015; Harari et al. 2015). As a result, treating radioactive waste containing these dangerous radioisotopes is a critical step in ensuring the safe handling of such effluents under precise wastewater treatment guidelines. Although solvent extraction is a very successful method for removing or recycling ions in effluents with relatively high ions concentrations, the loss of costly and hazardous additives as a result of dissolving them in water limits the adoption of this technology (Moamen et al. 2020). As a result, at low ion concentrations, this approach is uncompetitive. Adsorption is among the most effective methods in which heavy metals are removed from wastewater, especially at low concentrations. A robust experimental design shall be used for performing the experiments to examine different combinations of variables under study at different levels to disclose the optimum conditions and achieve the best outcomes of using adsorption as an effective method to remove heavy metals from wastewater (Kundu et al. 2015). Genichi Taguchi’s optimization technique has been developed to conceptualize experiments; this technique has turned out as very valuable, especially in product development and industrial engineering, and are based on two central ideas, namely Parameter Design and Tolerance Design. A specific series of experiments needs to be performed with an ‘orthogonal array’ which are matched with all control variables and yet reduced in the number of experimental trials (Yang and Tarng 1998). The robust Taguchi method provides costs, time, and resources used in the experimental study. Signal to noise ratio (S/N) is computed to define the optimum configuration and design accuracy for each combination (George and Tembhurkar 2020). In present study, the preparation of treated microcrystalline cellulose modified with citric acid (McC-CA) from rice straw, and the adsorption variables for heavy metals have been optimized using the L16 Taguchi design for maximum removal of cobalt and cesium from aqueous solution. Besides, the adsorption mechanism was elucidated by FTIR, scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDX). This study presents a sustainable and effective solution to remove heavy metals and radioisotopes from water bodies by upcycling agricultural waste.
Materials and methods
Laboratory experiments were conducted in the Radioisotopes Department and the Soil and Water Research Department of the Nuclear Research Center, Atomic Energy Authority, Egypt.
Preparation of adsorbent material
Rice straw was collected from the farm of Soil and Water Research Department as a zero-cost agricultural waste. The straw was repeatedly washed with tap water to remove dust and soluble impurities; subsequently, it was washed with distilled water and dried for 48 h in shade, and stored for 24 h in an air furnace at 333–343 K.
20 g of dried rice straw simmered with 1 L of 1% H2SO4 for 45 min. (Fig. 1A), then dispersed and cleaned with distilled water until the pH-value was neutral. Obtained material was boiled in 1 L of 1.5 M NaOH and 5% H2O2 for 30 min (Fig. 1B). Afterwards, the supernatant was removed and remaining fibers cleaned with a lot of distilled water. Subsequently, the purified fibers were added to 0.5 L of 0.5% NaClO solution and boiled for 30 min. for bleaching (Fig. 1C), then McC was filtrated and washed with distilled water and dried as shown in Fig. 1 (Taye et al. 2019).
Modification with citric acid (CA)
Bleached McC was mixed with 100 mL of 0.5 M citric acid and stirred for 30 min. The produced fibers were dried at 50 °C for 24 h, followed by raising the temperature to 120 °C for 90 min., to ensure that the thermochemical reaction was completed. After cooling, the McC-CA was washed with double distilled deionized water until the filtrate was not turning turbid when 0.1 M lead (II) nitrate solution was dropped in (Hassan et al. 2019).
Ultimately, it was dried at 50 °C for 24 h and ground, the thus obtained McC-CA was sieved to prepare the adsorbent with a size range of 150 to 370 µm, moisture content 4.62 (%), bulk density of 0.285 (g/mL), apparent density of 0.154 (g/mL), and packed in air-tight glass bottles.
McC-CA characterization
The ground McC-CA was characterized to study the adsorption mechanism for Co(II) and Cs(I) by using FTIR, SEM, and EDX.
Spectroscopic investigation by FTIR before and after CA modification
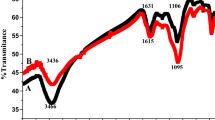
The ground McC-CA was characterized by FTIR spectroscopy using an FTIR—8201 PC, Shimadzu, before and after CA modification as illustrated in Fig. 2a. Where the FTIR spectra of citric acid-modified cellulose may indication the formation of ester bonds. As a result of heating, the anhydride-formation reaction between the citric acid carboxyl groups, and the reaction of this anhydride group with cellulose’s hydroxyl groups took place, which resulted in an esterification reaction; two free carboxyl groups of esterified products remain per citric acid molecule now linked to McC, which serve for chelation; further this chelation is reinforced by replacing the hydrogen on the carboxylic acid groups with sodium.
Consequently, McC-CA was the anchor chelation ligand for Co(II) and Cs(I) from the aqueous solution as shown in Fig. 2b. A large, intensive band around 1732 cm−1 is visible, indicating the carbonyl ester bond. The peaks at around 1048 cm–1 were attributes of the C=O group of diverse hydroxyl expanding and thus can be related to the cellulose structure. The wide absorption peaks which were at around 3394 cm−1 also indicated the presence of carboxylic O–H groups and the large stretching in this band because of citric acid linkage. The strong peak and its expansion in the case of modified McC-CA were also observed at a wavelength of 664 cm−1, indicating the O–C–O in the McC network. The peak was also found at 2905 cm−1 owing to the elongated expansion of C–H in the pyranoid circle (Madivoli et al. 2016; Tan et al. 2016; Ávila Ramírez et al. 2017; Nhung and Thanh 2019).
Evaluation of McC by SEM before and after modification with CA
The Philips XL 30 (SEM), Japan, which works in the form of primary electron beams ranging from 5 to 30 keV, was used to obtain SEM particle imagery before and after CA modification. Like other cellulosic materials, the potent cellular structure offered a framework ranging from nanometers to micrometers. Figure 3 displays the surface morphologies of the samples of McC rice straw before and after treatment with CA. The observed specimens were fibrils, and the modification had no adverse effect on the morphological aspect. Furthermore, all figures showed interwoven and slender- fibers.
Analysis of McC-CA by EDX microscopy before and after Co (II) and Cs (I) adsorption
The chemical composition of McC-CA before and after Co(II) and Cs(I) adsorption by chalation was analyzed by Philips XL 30 Scanning Electron Microscope with an attached EDX Unit. EDX microanalysis shows that McC-CA was an efficient adsorbent for Co(II) and Cs(I) as illustrated in Table 1.
Experimental design
The Taguchi method is an efficient tool for advanced experimental design and is used in various engineering processes. Moreover, it decreases the impact of uncontrollable variables by using an orthogonal array, which reduces the number of experiments (Aguedal et al. 2018).
It’s an environmentally friendly approach for treatment of wastewater polluted by heavy metals and radionuclides; it saves high quantities of hazardous and expensivematerials, time, and labour-related efforts.
In present study, several adsorption experiments were conducted in batch mode to adsorb Co(II) and Cs(I) from aqueous solution, where the removal yield was the main objective response (Eq. 1). Controllable factors and their levels are indicated in Table 2: the initial metal concentration, pH-value, sorbent (McC-CA) dose, and contact time were optimzed. L16 (44) was designed as an orthogonal array structure to assess the optimum operating conditions of the adsorption process.
where Co and Ce were the initial and equilibrium concentrations of the metal ions in solution (mg L-1), respectively (Abdel-sabour et al. 2018).
Adsorption of 60Co and 137Cs radioisotopes
60Co and 137Cs adsorption batches were performed by using 10 mL of radioactive waste solutions at room temperature. The control factors were pH-value 6, agitation time (t) 120 min, and sorbent Dose (D) 4 g.L−1 applied with different initial radiation activities (200.07, 440.13, 650.27 and 912.31 Bq) for 60Co and 240.18, 410.77, 610.82 and 843.72 Bq for 137Cs. The removal by adsorption was tested using the multichannel detector analyzer of sodium iodide (NaI), PCAP, USA, where the removal yield is the main objective response (Eq. 2).
where Ct and Cb are the initial and equilibrium radioactivity of the radionuclides in solution (Bq), respectively (Saleh et al. 2019).
Results and discussion
Taguchi’s L16 experimental study
In the current work, the Taguchi strategy orthogonal array contains several levels for each factor. An L16 (44) is implemented to attain the best possible conditions to improve Co(II) and Cs(I) removal by using the adsorbent McC-CA; designed experimental trials are illustrated in Table 3. Data were analyzed by Minitab Software (Minitab Inc., Version 18, USA) to assess the impact and importance for removal efficiency of each individual factor. The flowchart for the Taguchi process is shown in Fig. 4.
Signal to Noise (S/N) ratio examination
The impact of assessed factors on every process is called “noise”, and the reaction to each operational variable's change is known as “signal”. Different forms of (S/N) process were developed, e.g., “smaller is better”, “larger is better” besides “nominal is better” (Maazinejad et al. 2020). The higher S/N ratio refers to higher quality characteristics. As the target of the current research is to remove Co(II) and Cs(I) at the maximum adsorption rate conceivable, the “larger is better” category was adopted and calculated according to Eq. (3).
where n is the number of all tests and \({{\varvec{y}}}_{{\varvec{i}}}\) is the measured number of all trials ith (Gupta and Lataye 2018).
In this study, reaction to the S/N ratio response accompanied by adsorption yield corresponding to L16 (44) orthogonal array is measured and listed in Table 3. The removal yield for both Co(II) and Cs(I) was assessed according to the research design used for this analysis for every combination of process parameters in the study, the mean values were obtained by each trial. The ideal removal yield of Co(II) and Cs(I) was calculated using the signal to noise (S/N) ratio as indicator. The high removal yield results correspond to a rise in the adsorption efficiency of these metals by McC-CA and a reduction of time, effort, and expenditure. Table 3 represents the results of S/N ratios for measured values of Co(II) and Cs(I) removal by using McC-CA. The results of the effects for each control factor (Ion Metal Concentration (C), Degree of Acidicy-Alkalinity (pH), Adsorbent Dosage (D), and Time (t)) on the percent removal was quantified by S/N ratio responses shown in Table 4. This table, using the Taguchi method, displays the combination of the optimal values of control variables, which optimize the removal yield for Co(II) and Cs(I) by using McC-CA. The means of S/N ratios are plotted against the levels for each control parameter. Figure 5a–d shows the removal yield for Co(II), while the removal yield for Cs(I) is presented in Fig. 5e–h. The higher S/N bold values in Table 4 indicate the ideal level of each control parameter for the removal yield for Co (II) and Cs (I) by using McC-CA. Based on the aforementioned findings, the ideal combination for Co(II) removal by McC-CA were recognized as A1B3C4D4,i.e., Ion concentration (level 1, S/N = 35.58), pH-value (level 3, S/N = 35.91), Sorbent Dose (level 4, S/N = 35.26) and Time (level 4, S/N = 35.35). This means that the maximum Co(II) removal yield by McC-CA has been attained at following conditions: Ion concentration 10 mg L−1, pH-value 6, Sorbent Dose 4 mg L−1, and Contact Time 120 min. Identically, the optimal combination of factors favouring Cs(I) removal were determined as A1B3C4D4, i.e., Ion concentration (level 1, S/N = 36.88), pH-value (level 3, S/N = 37.10), Sorbent Dose (level 4, S/N = 36.54) and Contact Time (level 4, S/N = 36.68), consequently depending on parameter level and S/N ratio.
The maximum Cs (I) percent removal by McC-CA has been attained with Ion concentration 10 mg L−1, pH 6, Sorbent Dose 4 g L−1, and Contact Time 120 min. These variations in the values for removal of Co(II) and Cs(I) can result from a synergistic effect of different alternative control factor combinations and their individual levels.
Accordingly, Co(II), and Cs(I) removal efficiencies are affected by the hydrogen ion concentration, hence, by the pH-value. The results demonstrated that the removal yield increases with increasing pH-value of the solution for both metal ions under investigation. The increase in adsorption is probably due to the decreasing concentration of positively charged hydrogen ions, which leads to less competition between them and the also positively charged Co(II) and Cs(I) ions for the available McC-CA adsorption sites to be occupied (Çelebi et al. 2020). As expected, also the removal yield increases with increasing McC-CA dosage because more binding sites for ions are available at a higher dose of adsorbent (Pavithra et al. 2021). In addition, the removal yield increased at lower ions concentrations and vice versa, because of a higher number of binding sites being available for a lower quantitity of metal ions (Ray et al. 2021).
Evaluation of experimental results
The performance for removal of Co (II) and Cs (I) by McC-CA was determined based on experimental results, and is shown in Table 3. The relationships between control factors and the integrated and combined effect for Control variables on removal yield for Co(II) and Cs(I) by McC-CA are shown graphically using a two-dimensional contour plot. According to the graphic illustration in Fig. 6, the adsorption removal yield of both Co(II) and Cs(I) exhibited an increasing propensity with the decrease in the initial ion concentration and that is contrary to previous expectations, whereas the increase in ion metal concentration raises the quantity of metal adsorbed, however decreasing the removal yield because of a limited number of available active sites on the efficient adsorption surface (Vayenas et al. 2001). On the other hand, the removal yield for both Co(II) and Cs(I) improved with an increase in pH-value as long as the reaction takes place in the acidic range, whereas it is adversely affected when the medium conditions approache the neutral pH-value, which is due to an increase in hydrogen ion concentration at a lower pH level, which is affecting the mobility of the studied metal ions; this effect is less pronounced at pH = 6, which has the highest removal efficieny. Furthermore, the main adsorption mechanism of Co(II) and Cs(I) can be an electrostatic mechanism between the negative charge of some anionic radicals of the metals and the adsorbent surface, which are positively charged. As a result, the removal yield decreased as the reaction approached neutral pH-value (Aguedal et al. 2018).
Sorbent Dose (D) is the next desicive parameter regarding the effect on removal efficiency after the pH-value and Ion metal Concentration (C);. This might be due to the increase in the quality of surface properties of the adsorbent, like surface area and surface electric charge (Razmi and Ghasemi-Fasaei 2018). As a result of the fast kinetics of the adsorption reaction, less influence of contact time can be considered (Azizi et al. 2011). The contour plot displays the influence of the control factors derived from the mathematical evaluation of the Taguchi method on both Co(II) and Cs(I) removal by using McC-CA to assess the results of the experimental study where the optimum combination for Co(II) and Cs(I) adsorption was pH-value of 5–6, C between 1 and 50 mg L−1, D of 3–4 g L−1, and contact time t between 60 and 100 min.
Comparison of calculated and expected results.
Confirmation tests were necessary for the Taguchi optimization approach to validate the optimal combination of variables under study (George and Tembhurkar 2020). For the investigation of optimum removal efficiency for Co(II) and Cs(I), the following Eqs. (4) and (5), respectively, were used:
where A1, B3, C4, D4 present the optimum level mean values of removal yield for Co(II) and Cs(I) by McC-CA according to Table 5. \({T}_{Co(II)}\) and \({T}_{Cs(I)}\) Co (II) and Cs(I) removal yield values were obtained by the experimental analysis based on average valued taken from Table 3 (Gupta and Lataye 2018).
As a result of the calculations of removal yields for Co(II) and Cs(I) by McC-CA according to Fig. 6, the optimum control factors are A1B3C4D4. Therefore, the optimum calculated removal yield is RCo(II)pred. = 77.41%, and RCs(I) pred. = 91.14%, The results from confirmation experiments by using Eqs. (4), (5) for A1B3C4D4 present a for Co(II) and Cs(I) removal efficiency by McC-CA of 78.02% and 91.14%, respectively, which is close to the calculated values. Consequently, the experimental results from the Taguchi approach are contrasted by the predicted values obtained from prediction equations. The estimated and measured values are very similar and within the permissible limitations. The suitability of the Taguchi approach was also confirmed by choosing a random combination, which is A4B2C3D1, and the experimental values were as follows: 45.12% and 52.67% for Co(II) and Cs(I) respectively, while the predicted results were very similar: 44.87% and 51.56%, respectively, thus demonstrating the appropriateness of the Taguchi approach in determining the optimal combination and that its results can be relied upon (Table 6).
60Co and 137Cs adsorption by using McC-CA with the guidance of Taguchi’s L16 experimental study results
Based on the chemical properties of the metals, there is no difference between the stable and the radioactive isotopes (Saleh et al. 2020a,b,c). As a result, the optimum conditions for the controllable parameters pH-value, Sorbent Dose, and Time levels (B3C4D4) were used throughout the experiments dedicated to 60Co and 137Cs removal by McC-CA. As illustrated in Fig. 7, the removal yield was 96.01, 94.27, 92.52, and 90.28% for 60Co, and 100, 98.13, 95.85, and 94.25% for 137Cs, respectively. Since the initial radiation activity is due to an increase in the initial concentration of the elements under study, the trend goes in the same direction, hence, any decrease in the removal efficiency is because of the increase in the initial radiation activity.
Conclusion
According to the findings of this study, Taguchi’s design of experimental technique is one of the smartest ways for optimizing various process parameters. The Taguchi L16 orthogonal array (44) was used to design experiments to determine the optimal combination of four factors (Ion concentration, pH-value, adsorbent dosage, and contact time); every factor has been tested at four levels in batch mode for evaluating cobalt and cesium adsorption on Microcrystalline Cellulose treated by Citric Acid extracted from rice straw (McC-CA). The optimal combination from the significant parameters was pH-value 7, C 10 mg L−1, D 4 g L−1, and t of 120 min. At these conditions, the removal yield amounted to 74.37% and 88.58% for Co(II) and Cs(I), respectively. When the optimal combination was used for 60Co and 137Cs, the removal yield exceeded 95% for 60Co and achieved 100% for 137Cs. According to present study, it has been shown that McC-CA originating extracted rice straw could be a promising alternative adsorbent for Co(II) and Cs(I) mitigation from polluted wastewater provided that the suggested optimal combination is adhered to during the adsorption process.
Abbreviations
- McC-CA:
-
Microcrystalline celluolose modified by citric acid
- OA:
-
Orthogonal array
- Co(II):
-
Cobalt ions
- 60Co:
-
Radioisotope for Cobalt
- 137Cs:
-
Radioisotope for Cesium
- FTIR:
-
Fourier transform infrared spectroscopy
- SEM:
-
Scanning Electron Microscope
- EDX:
-
Energy-Dispersive X-ray Spectroscopy
- S/N:
-
Signal to noise ratio
References
Abdel-sabour MF, Atomic E, Authority E, Dawoud MA (2018) Removal of Cu ( II ) and Pb ( II ) from aqueous solution using treated rice straw. Int J Adv Res 3:1260–1271
Adel AM, El-shinnawy NA (2012) International Journal of Biological Macromolecules Hypolipidemic applications of microcrystalline cellulose composite synthesized from different agricultural residues. Int J Biol Macromol 51:1091–1102. https://doi.org/10.1016/j.ijbiomac.2012.08.003
Aguedal H, Iddou A, Locs J (2018) Optimization of the adsorption process of bezaktiv turquoise blue «VG» textile dye onto diatomite using the Taguchi method. Key Eng Mater 762:81–86. https://doi.org/10.4028/www.scientific.net/KEM.762.81
Ávila Ramírez JA, Fortunati E, Kenny JM et al (2017) Simple citric acid-catalyzed surface esterification of cellulose nanocrystals. Carbohydr Polym 157:1358–1364. https://doi.org/10.1016/j.carbpol.2016.11.008
Azizi SN, Abrishamkar M, Kazemian H (2011) Using of taguchi robust design method to optimize effective parameters of methylene blue adsorption on ZSM-5 zeolite. Asian J Chem 23:100–104
Çelebi H, Gök G, Gök O (2020) Adsorption capability of brewed tea waste in waters containing toxic lead ( II ), cadmium ( II ), nickel ( II ), and zinc ( II ) heavy metal ions. Sci Rep. https://doi.org/10.1038/s41598-020-74553-4
Chen GR, Chang YR, Liu X et al (2015) Prussian blue (PB) granules for cesium (Cs) removal from drinking water. Sep Purif Technol 143:146–151. https://doi.org/10.1016/j.seppur.2015.01.040
Dawoud MMA, Hegazy MM, Helew WK, Saleh HM (2021) Overview of environmental pollution and clean management of heavy metals and radionuclides by using microcrystalline cellulose. J Nucl Ene Sci Power Gener Technol 3:2
El-sakhawy M, Hassan ML (2007) Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carb Polymers 67:1–10. https://doi.org/10.1016/j.carbpol.2006.04.009
El Adham EK, Hassan AI, Dawoud MMA (2022) Evaluating the role of propolis and bee venom on the oxidative stress induced by gamma rays in rats. Sci Rep 12:1–22
El safty A (2020) Environmental and health impact of open burning Rice straw. Egy J of Occ Med 44(3):679–708. https://doi.org/10.21608/ejom.2020.118349
Elbasiouny H, Elbehiry F (2020) Rice production in Egypt: The challenges of climate change and water deficiency. Climate change impacts on agriculture and food security in Egypt. Springer, Cham, pp 295–319
Eskander SB, Saleh HM (2012) Cement mortar-degraded spinney waste composite as a matrix for immobilizing some low and intermediate level radioactive wastes: Consistency under frost attack. J Nucl Mater 420:491–496
George AM, Tembhurkar AR (2020) Taguchi experimental design for adsorptive removal of fluoride from water using novel Ficus Glomerata Bark-developed biosorbent. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-020-02787-w
Gupta TB, Lataye DH (2018) Adsorption of indigo carmine and methylene blue dye: Taguchi’s design of experiment to optimize removal efficiency. Sadhana - Acad Proc Eng Sci 43:1–13. https://doi.org/10.1007/s12046-018-0931-x
Harari F, Bottai M, Casimiro E et al (2015) Exposure to lithium and cesium through drinking water and thyroid function during pregnancy: a prospective cohort study. Thyroid 25:1199–1208. https://doi.org/10.1089/thy.2015.0280
Hassan AI, Bondouk II, Omar K, Esawii HA, Saleh HM (2022) Chemical toxicity assessment and physiological investigation in rats exposed to pyrethroid insecticide type 1 and possible mitigation of propolis. EuroBiotech J. 6:9–26. https://doi.org/10.2478/ebtj-2022-0002
Hassan MM, Tucker N, Joo M, Guen L (2019) Graphical abstract l P re of. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115675
Hokkanen S, Bhatnagar A, Sillanpää M (2016) Treated water SC. Water Res. https://doi.org/10.1016/j.watres.2016.01.008
Kundu A, Sen Gupta B, Hashim MA, Redzwan G (2015) Taguchi optimization approach for production of activated carbon from phosphoric acid impregnated palm kernel shell by microwave heating. J Clean Prod 105:420–427. https://doi.org/10.1016/j.jclepro.2014.06.093
Liu R, Rahman M, Li C, Chai M (2021) Catalytic pyrolysis of microcrystalline cellulose extracted from rice straw for high yield of hydrocarbon over alkali modified ZSM-5. Fuel 285:119038. https://doi.org/10.1016/j.fuel.2020.119038
Maazinejad B, Mohammadnia O, Ali GAM et al (2020) Taguchi L9 (34) orthogonal array study based on methylene blue removal by single-walled carbon nanotubes-amine: Adsorption optimization using the experimental design method, kinetics, equilibrium and thermodynamics. J Mol Liq 298:112001. https://doi.org/10.1016/j.molliq.2019.112001
Madivoli E, Kareru P, Gachanja A et al (2016) Adsorption of selected heavy metals on modified nano cellulose. Int Res J Pure Appl Chem 12:1–9. https://doi.org/10.9734/irjpac/2016/28548
Moamen OAA, Hassan HS, Zaher WF (2020) Sr 2 + ions by a novel scavenger. Ecotoxicol Environ Saf 189:110013. https://doi.org/10.1016/j.ecoenv.2019.110013
Nagarajan KJ, Balaji AN, Rajan STK, Ramanujam NR (2020) Jo ur l P re of. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2020.115997
Nhung HL, Thanh ND (2019) CELLULOSE MODIFIED WITH CITRIC ACID AND ITS ABSORPTION OF Pb2+ and Cd2+ IONS. Sci Forum. https://doi.org/10.3390/ecsoc-13-00231
Pavithra S, Thandapani G, Sugashini S et al (2021) Chemosphere Batch adsorption studies on surface tailored chitosan / orange peel hydrogel composite for the removal of Cr ( VI ) and Cu ( II ) ions from synthetic wastewater. Chemosphere 271:129415. https://doi.org/10.1016/j.chemosphere.2020.129415
Ray S, Mishra AK, Kalamdhad AS (2021) Evaluation of equilibrium, kinetic and hydraulic characteristics of Indian bentonites in presence of heavy metal for landfill application. J Clean Prod 317:128396. https://doi.org/10.1016/j.jclepro.2021.128396
Razmi B, Ghasemi-Fasaei R (2018) Investigation of Taguchi optimization, equilibrium isotherms, and kinetic modeling for phosphorus adsorption onto natural zeolite of clinoptilolite type. Adsorpt Sci Technol 36:1470–1483. https://doi.org/10.1177/0263617418779738
Saleh HM, Eskander SB (2009) Long-term effect on the solidified degraded cellulose-based waste slurry in cement matrix. Acta Montan Slovaca 14:291–297
Saleh HM, Eskander SB (2012) Characterizations of mortar-degraded spinney waste composite nominated as solidifying agent for radwastes due to immersion processes. J Nucl Mater 430:106–113
Saleh HM, Eskander SB, Fahmy HM (2014) Mortar composite based on wet oxidative degraded cellulosic spinney waste fibers. Int J Environ Sci Technol 11:1297–1304
Saleh HM, Mahmoud HH, Aglan RF, Bayoumi TA (2019) Biological treatment of wastewater contaminated with Cu(II), Fe(II) and Mn(II) using Ludwigia stolonifera aquatic plant. Environ Eng Manag J 18:1327–1336
Saleh HM, Moussa HR, El-Saied FA et al (2020a) Mechanical and physicochemical evaluation of solidifed dried submerged plants subjected to extreme climatic conditions to achieve an optimum waste containment. Prog Nucl Energy 122:103285
Saleh HM, Moussa HR, El-Saied FA et al (2020b) Adsorption of cesium and cobalt onto dried Myriophyllum spicatum L. from radio-contaminated water: experimental and theoretical study. Prog Nucl Energy 125:103393
Saleh HM, Moussa HR, Mahmoud HH et al (2020c) Potential of the submerged plant Myriophyllum spicatum for treatment of aquatic environments contaminated with stable or radioactive cobalt and cesium. Prog Nucl Energy 118:103147. https://doi.org/10.1016/j.pnucene.2019.103147
Tan KB, Abdullah AZ, Horri BA, Salamatinia B (2016) Adsorption mechanism of microcrystalline cellulose as green adsorbent for the removal of Cationic methylene blue dye. J Chem Soc Pakistan 38:651–664
Taye M, Chaudhary BU, Kale RD (2019) Extraction and Analysis of Microcrystalline Cellulose from Delignified Serte Leaf Fiber Wastes. J Nat Fibers 00:1–13. https://doi.org/10.1080/15440478.2019.1697992
Trache D, Hussin MH, Hui Chuin CT et al (2016) Microcrystalline cellulose: Isolation, characterization and bio-composites application—a review. Int J Biol Macromol 93:789–804. https://doi.org/10.1016/j.ijbiomac.2016.09.056
Vayenas CG, Bebelis S, Pliangos C et al (2001) Electrochemical activation of catalysis: promotion, electrochemical promotion, and metal-support interactions. Springer Science & Business Media
Yang WH, Tarng YS (1998) Design optimization of cutting parameters for turning operations based on the Taguchi method. J Mater Process Technol 84:122–129. https://doi.org/10.1016/S0924-0136(98)00079-X
Acknowledgment
The authors would like to acknowledge Professor Amal I. Hassan, Egyptian Atomic Energy Authority for her guidance in improving this manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Editorial responsibility: Samareh Mirkia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dawoud, M.M.A., Hegazi, M.M., Saleh, H.M. et al. Removal of stable and radio isotopes from wastewater by using modified microcrystalline cellulose based on Taguchi L16. Int. J. Environ. Sci. Technol. 20, 1289–1300 (2023). https://doi.org/10.1007/s13762-022-04073-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04073-3