Abstract
The new clay modified with triazole and triazolium ligands was prepared in this research. These materials were applied as abundant and eco-friendly adsorbents for removal of heavy metal ions such as Pb(II), Co(II) and Zn(II) ions. The adsorption efficiency of these materials was calculated by relevant equations such as Langmuir and Freundlich as well as kinetic studies with pseudo-first-order and pseudo-second-order models. These adsorbents proved to be very active on heavy metal ion adsorption. The characterization of these new materials was carried out by various techniques such as X-ray diffraction, thermogravimetric analysis, scanning electron microscope (SEM), X-ray photoelectron spectroscopy and energy-dispersive X-ray spectroscopy as well as SEM-map analysis. Eventually, the catalytic activity of the adsorbents which treated with heavy metal ion solutions was studied in the reduction of nitroarenes to its corresponding amines. The prepared adsorbent–catalyst materials indicated efficient catalytic activity in the reduction of nitroarenes to amines in ambient conditions.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals toxicities are one of the most dangerous environmental contaminants which have a relatively high density compared to water (Häni 1990; Ngah and Hanafiah 2008; Fu and Wang 2011). The contamination of water and soil recourses by heavy metals is a serious worldwide problem, and it is challenging to eliminate their long-term effects (Agudosi et al. 2018a, b). The removals of these contaminants metals are crucial because they are persistent, bioaccumulative, and able to disrupt the metabolic functions and vital organs in livelihoods. Another toxic and hazardous category of organic compounds are nitroarenes, which are the common environmental contaminants and considered as major industrial activities, mining and coal combustion (Lin 1987; Tokiwa et al. 1986; Zwirner-Baier and Neumann 1999). Reduction of the nitroarenes is undoubtedly the most important way to decrease the possibility of environmental degradation by aromatic nitro compounds (Riefler and Smets 2000; Sabbioni and Jones 2002). Extensive research has been carried out to introduce materials which can remove and alleviate heavy metal ions from the wastewaters. Some of the recent important methods used for the removal of heavy metal ions are chemical precipitation (Charerntanyarak 1999), membrane filtration (Bessbousse et al. 2008), ion exchange (Da̧browski et al. 2004), electrolytic methods (Nojiri et al. 1980), and solvent extraction (Yun et al. 1993). However, these methods have several disadvantages such as high reagent requirement, unpredictable metal ion removal, generation of toxic sludge, etc. Among the different techniques, the adsorption process is considered as the best one because it is straightforward, economically very significant, practical and versatile, which has become the most preferred method for removal of toxic contaminants from wastewater. Such materials are zeolites, biosorbents, activated carbon, fly ash, mesoporous and clays which have gained more attention than the other adsorbents due to the high surface area as well as being eco-friendly (Zhu et al. 2012; Padilla-Ortega et al. 2013).
Clay is a naturally occurring compound of aluminum silicate ((AlO)2 SiO3) composed of fine-grained minerals which have gained much attention during the recent decades (Alvarez-Ayuso and Garćia-Sánchez 2003; Uddin 2017). They are defined as soils with particles smaller than 2 μm having a layer structure (Velde 1992; Chenu and Plante 2006). Small size and layer structure provide them with a high surface area; hence, it is capable of heavy metal adsorption within its layers (Velde 1992; Bhattacharyya and Gupta 2008). Among different kinds of clays, clinochlore is an important one which belongs to the chlorite group (Welch and Marshall 2001; Kleppe et al. 2003; Gopal et al. 2004). The chemical formula of clinochlore is (Mg5Al)(AlSi3)O10(OH)8, and it crystallizes in monoclinic (Welch and Marshall 2001; Kleppe et al. 2003; Gopal et al. 2004). Due to strong interactions between the elements such as nitrogen, sulfur and phosphor with few heavy metals like Cu, Cd, Pb, Zn, Ni, Co and also in order to increase adsorption efficacy, the clays are modified with sorts of ligand (Celis et al. 2000; Esalah and Husein 2008; Jlassi et al. 2018). Potentially, clays can physisorbed the metals in their structures, but in this case, the adsorption capacity has a limited value which could be increased by modification of clay surface (Altın et al. 1998; Lin and Juang 2002; Bhattacharyya and Gupta 2008).
In this study, we modified the raw clinochlore with triazole and triazolium ligands and its application for adsorption of heavy metal ions such as Co(II), Pb(II) and Zn(II) from industrial wastewater is presented. Moreover, the catalytic activities of the modified clinochlore which are treated with heavy metal ions were proved in the reduction of nitroarenes to amines.
Materials and methods
Experimental
All materials were purchased from Sigma-Aldrich, Acros and Merck Millipore. Reactions were monitored by thin-layer chromatography (TLC) using Merck silica gel 60F254 glass plate with 0.25 mm thickness. Clinochlore was purchased from Daneshmand chemical trading co (Iran). Column chromatography was carried out on silica gel 60 Merck (230–240 mesh) in a 2-cm-diameter column. 1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively, on a Bruker Avance HD. Chemical shifts are given on the δ-scale in ppm, and residual solvent peaks were used as internal standards. X-ray diffraction (XRD) patterns were recorded using Philips X’Pert Pro instrument. The SEM images were captured with JEOL JSM 840. XPS analyses were performed using a K-Alpha spectrometer. Thermogravimetric analysis was conducted from room temperature to 800 °C in an oxygen flow using a NETZSCH STA 409 PC/PG instrument.
Synthesis of (1-benzyl-1H-1,2,3-triazol-4-yl) methanol (Tzl-OH)
In a 10-mL flask, sodium azide (1.5 mmol, 97.5 mg), benzyl bromide (1 mmol, 0.11 mL), methanol (2 mL) and deionized water (0.1 mL) were added and stirred for 30 min at room temperature. Then propargyl alcohol (3 mmol, 0.17 mL) and copper iodide (0.1 mmol, 19 mg) were added to the mixture, and the reaction was stirred at room temperature. After 72 h, the reaction mixture was diluted with deionized water and extracted with ethyl acetate (3 × 5 mL). The combined organic layers were dried over Na2SO4. Afterward, the resulting solution was concentrated with a rotary evaporator and further purification was done by column chromatography. The product was achieved in 90% isolated yield. In order to dry the resultant product, it was placed in 60 °C oven for 24 h.
Synthesis of 1-benzyl-4-(hydroxymethyl)-3-methyl-1H-1,2,3-triazolium (IL-Tzl-OH)
In a 10-mL flask, dry acetonitrile (5 mL) was added to (1-benzyl-1H-1,2,3-triazol-4-yl)methanol (2 mmol, 378 mg) and stirred for 10 min. Then methyl iodide (10 mmol, 0.623 mL) was added to the mixture slowly, and the reaction was stirred for 24 h under reflux condition. After the reaction time, the mixture was washed with ethyl acetate (3 × 5 mL) and the resulting sticky oil was placed in the rotary evaporator to remove the remaining amount of methyl iodide. The product was obtained in 96% isolated yield. The resulting product was placed in an oven of 50 °C under vacuum condition for overnight.
Preparation of Cl@clinochlore (Cl@Clin)
In a 50-mL flask, dry toluene (10 mL) was added to clinochlore (1 g), and it was sonicated for 15 min. Next (3-chloropropyl)triethoxysilane (5 mmol, 1.2 mL) was added to the mixture slowly, and it was reflux for 24 h under argon atmosphere. After 24 h, the whole reaction mixture was poured to a 15-mL falcon and centrifuged at 4000 rpm for 15 min. Then, the supernatant was decanted, and the remaining solid was washed with deionized water (10 mL) and acetone (10 mL). Next, the mixture was centrifuged again and the final sediment was placed in an oven of 60 °C for 24 h.
Preparation of Tzl-OH@Clin and IL-Tzl-OH@Clin
In a 25-mL flask, dry THF (10 mL) was added to Tzl-OH (1 mmol, 189 mg) or IL-Tzl-OH (1 mmol, 331 mg) and stirred for 15 min at room temperature under argon atmosphere. Then, sodium hydride (NaH) (2 mmol, 48 mg) was added to the mixture under argon atmosphere. The mixture was stirred at room temperature for 1 h. Next, Cl@Clin (0.5 g) was added to the mixture, and the reaction was refluxed under argon atmosphere for 24 h. Afterward, the reaction was allowed to reach the room temperature, and the residue solid was separated by centrifugation (15 min at 3500 rpm). The resulting sediment was washed with deionized water (10 mL) and acetone (10 mL), respectively. The obtained material (Tzl-OH@Clin) or (IL-Tzl-OH@Clin) was placed in an oven of 60 °C for 24 h.
Adsorption experiments
In order to perform adsorption experiments, the adsorbent (5 mg) was added to the aqueous solution (20 mL, 20 mg/L) of Co(II), Zn(II) and Pb(II). The mixture was stirred for 3 h to reach equilibrium adsorption capacity. Then, the mixture was centrifuged at 2000 rpm for 15 min, and atomic adsorption analysis (AAS) of the resulting supernatant determined the concentration of heavy metal ions after treatment. All the adsorption experiments were carried out at room temperature (25 °C).
The equilibrium adsorption capacity and equilibrium adsorption capacity at the time (t) were calculated by the following equations.
where C0 is initial heavy metal ion concentration (mg/L), Ce is the concentration of heavy metal ion after equilibrium (mg/L), Ct is the concentration of heavy metal ion at time t (mg/L), V is the solution volume (L), and m is the mass of adsorbent (g).
Effect of initial heavy metals ion concentrations on equilibrium adsorption capacities
In order to investigate the effect of initial heavy metal ion concentration on adsorption capacity, the same process as the above part was done except the initial heavy metal ion concentrations were changed to 10–80 mg/L for Pb(II), Zn(II) and Co(II). In order to prevent heavy metal ion precipitation by OH− and higher efficiency of adsorbents, the pH values were set to 5 for this experiment.
Effect of pH on the equilibrium adsorption capacity
To study the equilibrium adsorption capacity changes in different pH values, the experiments were carried out on the pH range of 2–8. The pH value was fixed to 2 by using the buffer solution of KCl/HCl, sodium acetate/acetic acid for pH range of 3–6 and HEPES-Na (4-(2-hydroxyethyl)-piperazine-1-ethanesulfonic acid, sodium salt) for pH range of 7–8.
Kinetic study
To study kinetic behavior of adsorbent, the equilibrium adsorption capacities of adsorbents (5 mg) in the treatment with solutions (20 mL, 20 mg/L) of Pb(II), Zn(II) and Co(II) were determined in the time intervals of 0–180 min and the pH value was set to 5.
Procedure for adsorption of heavy metal ions in industrial wastewater
In order to study removal efficacy in industrial wastewaters, first, the solution (20 mg/L, 20 mL) of the Pb(II), Co(II) and Zn(II) was made and treated with Tzl-OH@Clin(10, 50 and 100 mg) for 3 h. Then, the whole mixture was centrifuged, and the concentrations of heavy metal ions were determined by atomic absorption of the supernatants.
Determination of Co(II) concentration on the Tzl-OH@Clin structure by AAS
In order to determine the concentration of adsorbed Co(II) in the absorbent structure, the adsorbent treated with Co(II) solution (20 mg) was dissolved in aqua regia (5 mL) and stirred for 12 h. Then, the mixture was poured to the 15-mL falcon, and it was centrifuged at 3000 rpm for 10 min. Next, the supernatant was decanted to the volumetric flask (25 mL) and reached the total volume to 25 mL via deionized water. Afterward, the concentration of the metal was gained by the AAS of the resulting solution.
Procedure for reduction of nitroarenes
To perform nitro reduction reaction, in a 10-mL flask, Tzl-OH@Clin treated with Co(II) solution (15 mg, 1.60 mol%) was added to the sodium borohydride solution [2.7 mM in 1.5 mL mixture of deionized water and THF (9:1)]. Then the nitroarene (1 mmol) was added to the mixture slowly and stirred for different time intervals. The completion of the reaction was monitored by TLC. After the reaction was completed, the mixture was diluted with deionized water (4 mL) and it was extracted with ethyl acetate (3 × 2 mL). Next, the combined organic layer was treated with sodium sulfate and it was filtered and the resulting organic layer was concentrated with a rotary evaporator. The yield was obtained by gas chromatography (GC), and the further purification was done by column chromatography.
Results and discussion
Synthesis of (1-benzyl-1H-1,2,3-triazol-4-yl)methanol (Tzl-OH) and 1-benzyl-4-(hydroxymethyl)-3-methyl-1H-1,2,3-triazolium (IL-Tzl-OH) was proceeded by the initial synthesis of benzyl azide, and its reaction was shown with propargyl alcohol in the presence of copper iodide as the catalyst at room temperature. The corresponding 1,2,3-triazole (Tzl-OH) product was treated with methyl iodide under reflux condition to obtain 1,2,3-triazolium (IL-Tzl-OH) product. Further, to modify raw clinochlore with triazole and triazolium ligand, firstly the chlorinated clinochlore (Cl@Clin) was made by adding 3-chloropropyltriethoxysilane to the fresh clinochlore and then the grafting process for both Tzl-OH and IL-Tzl-OH to Cl@Clin was done by treating ligands with sodium hydride in THF and adding Cl@Clin at the end. Finally, the clinochlore modified with triazole (Tzl-OH@Clin) and triazolium (IL-Tzl-OH@Clin) was obtained (Scheme 1).
The 1H and 13C NMR spectra of both Tzl-OH and IL-Tzl-OH were investigated, and it shows synthesis processes of the corresponding products were successful (Figs. 1 and 2 ESI).
The thermogravimetric analysis (TGA) of Cl@Clin, Tzl-OH@Clin and IL-Tzl-OH@Clin showed a weight loss at 120 °C, which is due to the presence of some water molecules in the structures. Also, related to the Cl@Clin, two increases on the weight loss were observed in 200 °C for Tzl-OH@Clin and IL-Tzl-OH@Clin, which is according to the additional of organic groups contained in grafted ligands to the Cl@Clin. Overall weight loss of IL-Tzl-OH@Clin was about 54.12% and for Tzl-OH@Clin was about 42.13%, which is smaller than IL-Tzl-OH@Clin. This may relate to addition of methyl and some heavy elements such as iodine (I−) to the triazole structure (Fig. 1).
The X-ray photoelectron spectroscopy (XPS) study of the Tzl-OH@Clin in the nitrogen region showed two peaks at the binding energies of 400.18 and 401.88 eV which are related to the triazole nitrogen (Fig. 2a) (Fortgang et al. 2016). The XPS spectra of IL-Tzl-OH@Clin at nitrogen region showed a single peak at the binding energy of 402 eV which is related to triazolium nitrogens (Fig. 2b) (Obadia et al. 2015).
In order to investigate the structure of clinochlore, the SEM images at different magnifications were taken (Fig. 3).
An X-ray powder diffraction (XRD) spectrum of clinochlore was also examined (Fig. 4), and the XRD pattern was similar to previous literature (Hemanthkumar et al. 2009).
Heavy metal ion adsorption
In order to study the adsorption ability of clinochlore, Tzl-OH@Clin and IL-Tzl-OH@Clin, the solution of Zn(II), Co(II) and Pb(II) (20 mg/L) was treated with the specified amount of adsorbents (5 mg) at room temperature. Equilibrium adsorption capacities (qe) were achieved after 3-h stirring of the adsorbents with metal ion solutions. In order to compare the adsorption efficacy of three adsorbents, the qe values of three adsorbents were obtained in different equilibrium concentrations (Ce), and the results are shown in Fig. 5. According to the results, the qe values for Tzl-OH@Clin were higher than raw clinochlore and IL-Tzl-OH@Clin which shows better interactions of triazole nitrogens with heavy metal ions (Fig. 5).
At the same above conditions, the experiments were performed in a different range of pH (2–8). The results indicated that on the pH of 5, the maximum amounts of qe were obtained and at higher pH, the amounts of qe were decreased. It may be due to precipitation of metal ions with hydroxide ions (OH−). Also, at pH 4, the qe values did not have significant changes. On the pH amounts of 3 and 2, the qe values decreased and it is probably caused by disturbance of H+ Ions and its interaction with triazole nitrogens which may prevent metal ions from being adsorbed by Tzl-OH@Clin and IL-Tzl-OH@Clin. No significant changes were observed in raw clinochlore as an adsorbent with pH decreasing (Fig. 6).
The effect of initial heavy metal ion concentration on adsorption capacity was also examined. The adsorption behaviors were studied with Langmuir and Freundlich equations (Eqs. 3 and 4).
where Ce (mg/L) is equilibrium concentration, qe (mg/g) is equilibrium adsorption capacity, KL is Langmuir constant which is related to binding affinity, qmax is the maximum adsorption capacity (mg/g), Kf is the Freundlich constant which is related to the adsorption capacity, and n is related to adsorption intensity.
The two isotherm models were fitted (Fig. 7), and the results are collected in Table 1.
According to results, the qmax values for the adsorption of Co(II) by raw clinochlore, Tzl-OH@Clin and IL-Tzl-OH@Clin were 28.73, 84.74 and 65.78, respectively. In addition, the adsorption capacity of Tzl-OH@Clin for adsorbing Co(II) was higher than that of the other two adsorbents. Also, in the case of adsorbing other metal ions, the results indicated that the best adsorption capacities were achieved by using Tzl-OH@Clin as the adsorbent. However, the adsorption capacity of IL-Tzl-OH@Clin was a bit lower than Tzl-OH@Clin, which may be due to the ionic structure of the adsorbent and establishing a weaker binding with metal ions. Also, the existence of the counter anion around the triazolium structure may cause some congestion for the adsorption process. The KL values of the three adsorbent were ranged from 0.0641 to 0.3715, all of which are positive values and show the adsorption would be desirable. The correlation coefficient (R2) values for the Langmuir model were from 0.9972 to 0.9998, all of which are close to 1, and it made the Langmuir model so favorable. The studies of Freundlich isotherms revealed the Freundlich parameters [the adsorption intensity (n) and adsorption capacity (Kf)]. The n values of the three adsorbents were ranged from 2.39 to 5.42. The more n value is greater than 1 (1/n closer to 0), the more adsorbent shows heterogeneous behaviors (de Sá et al. 2017). In this study, the maximum n values were achieved for IL-Tzl-OH@Clin as adsorbent, and in this regard, the n values for adsorption of Co(II), Pb(II) and Zn(II) were calculated to be 4.31, 4.00 and 5.42, respectively. The Kf values for Tzl-OH@Clin and IL-Tzl-OH@Clin as adsorbents were much greater than raw clinochlore, which results from the interactions between metal ions and ligands. Investigation of the correlation coefficient (R2) values indicated that the minimum value for R2 in Langmuir model was 0.9972 and the maximum value was 0.9998, all of which are very close to 1. In Freundlich model, these values are 0.8883 and 0.9620, respectively. By comparing R2 values for Langmuir and Freundlich model, it can be concluded that the R2 values for Langmuir model are closer to 1 than those for Freundlich model. It means the adsorption model of adsorbents is more corresponding to Langmuir model than to Freundlich model.
Also, the kinetic study of the adsorption process was carried out by using pseudo-first-order model and pseudo-second-order model (Eqs. 5 and 6).
where qe (mg/g) and qt (mg/g) are the adsorption capacity at equilibrium and adsorption capacity at time (t), respectively. In addition, K1 (1/min) and K2 (g/mg min) are the pseudo-first-order and pseudo-second-order constants, respectively.
The two kinetic models were fitted (Fig. 8) and the kinetic parameters calculated (Table 2).
According to the results, the R2 values for the pseudo-first-order ranged from 0.9753 to 0.9938 and for pseudo-second-order it ranged from 0.9994 to 0.9998. The R2 values of pseudo-second-order model closer to 1 than those of pseudo-first-order model, which declares that the adsorption of metal ions by adsorbents is mostly performed through chemical adsorption (Ho and McKay 1998).
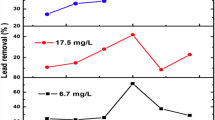
The adsorption capacities at different time intervals were also studied (Fig. 9). The results showed a sharp increase in the initial 25 min. After 25 min, the adsorption capacity did not change significantly.
The application of the adsorbents was also studied in the adsorption of heavy metal ions contained in wastewaters. In this regard, the specific amount of Tzl-OH@Clin (10, 50 and 100 mg) was treated with the wastewater (20 mL) and stirred for 3 h in order to reach the equilibrium adsorption capacity at the room temperature. In the next step, the adsorbent was separated by centrifugation and the AAS of the remaining supernatant showed that the heavy metal ions were mostly removed after treated with adsorbent. The results are presented in Table 3.
In the case of using 10 mg of Tzl-OH@Clin for adsorption of Co(II), Pb(II), and Zn(II), removal efficacy of 91%, 89.50%, and 90.25% was obtained, respectively. When the adsorbent amount was increased to 50 mg, these values were changed to 95%, 95.15% and 95.75%. By increasing adsorbent amount to 100 mg, almost all the metal ions were adsorbed (99.40%, 98.50% and 98.90% for Co(II), Pb(II) and Zn(II), respectively).
To find out the relationship between temperature and adsorption ability, we carried out the adsorption process at 25 °C, 35 °C and 45 °C (Fig. 10).
In this regard, thermodynamic parameters such as standard enthalpy (ΔH°), standard entropy (ΔS°) and Gibbs free energy (ΔG°) were calculated (Table 4). The aforementioned parameters were calculated by Eqs. 7 and 8 as follows:
where R (8.314 J/mol K) is the universal gas constant, T (°K) is the solution temperature, and Kd is the distribution coefficient.
To calculate the free Gibbs energy (ΔG°), Eq. 9 was used:
The results of these calculations are inserted in Table 4. According to the results, ΔH° and ΔS° values are both positive, which means the adsorption processes are likely to be endothermic and carried out by entropy, respectively (Boparai et al. 2011). Furthermore, results of free Gibbs energy calculations showed that the values are all negative, which showed that the adsorption is a spontaneous process and the degree of spontaneity increased with raising temperature (Table 4).
In order to indicate the presence of metal ions in the adsorbent structure, XPS analysis was taken from Tzl-OH@Clin treated with standard metal ion solutions. Results showed two peaks at the binding energy of 780.68 and 796.58 eV, which are related to Co(II) 2p3/2 and Co(II) 2p1/2, respectively. Also, two deconvoluted peaks at 785 and 800 eV are related to Co(III) (Fig. 11a) (Patil et al. 2014). The study of XPS spectra in the Pb region revealed that two peaks at binding energy of 138 and 142.88 eV are related to Pb(II) 4f7/2 and Pb(II) 4f5/2. Moreover, two peaks at 139.48 and 144.38 eV are related to Pb(II) ions of PbO species (Fig. 11b) (Burungale et al. 2016). In the Zn region, two peaks at 1021.98 and 1045.08 eV are related to Zn(II) species (Fig. 11c) (Xu et al. 2013).
The SEM-map and EDX analysis of Tzl-OH@Clin treated with metal ion solutions proved the presence of metal ions species in the adsorbents structures (Figs. 12 and 13).
Comparison of this work with some other relevant literatures showed that the prepared adsorbents exhibit appropriate adsorption capacities, and in some cases it is superior to some other related works. In addition, Tzl-OH@Clin could adsorb three kinds of heavy metal ions with an acceptable adsorption capacity values and also being as an eco-friendly adsorbent would make this clay-based adsorbent to be as a useful material on industries specifically in the way of declining pollutions (Table 5). Moreover, to broad our perspective in the applications of the prepared adsorbents, we investigated the catalytic ability of the aforementioned adsorbents.
In order to find out the catalytic activity of the adsorbents, the specific amount of them (15 mg) was treated with heavy metal ion solutions. After 3 h (equilibrium time), the adsorbents were separated and washed by deionized water and acetone. Then, the concentrations of the metals adsorbed in the structure adsorbents were determined by AAS. Next, reduction of 1-chloro-4-nitrobenzene was selected as a model reaction to study the catalytic activity of the adsorbents with metals in their structures. In the beginning, the reactions were undergone with 15 mg of three adsorbents containing metals ions as the catalyst (0.5–1.6 mol% of metal in its structure) in deionized water as the solvent, sodium borohydride as the reducing agent and at room temperature. The highest yield was obtained using Tzl-OH@Clin treated with Co(II) as the catalyst (Table 6).

In order to reach the optimized condition for the reduction of nitroarenes by Tzl-OH@Clin, we studied the reduction of 1-chloro-4-nitrobenzene at different temperatures (Table 7). Results indicated that 63% and 75% of the corresponding product were achieved when the temperatures were 25 and 35 °C, respectively (entries 1 and 2, Table 7). Moreover, very good and excellent yields were obtained in the cases of 50 and 70 °C (entries 3 and 4, Table 7). However, as the amines are such sensitive agents to oxidization, so the high temperature like 70 °C could make amines to be oxidized by the oxygen contained in the atmosphere. In this regard, we chose 50 °C as the optimum temperature and tried to investigate the solvent effect in the reduction efficiency. In the next study, we determined the effect of different solvents in the reduction progression. Results showed the corresponding product was obtained in poor-to-moderate yields by using organic solvents as the solvent of the reduction (entries 1–7, Table 8). In the case of ethanol and water, 65 and 88% of the product were attained, respectively (entries 8 and 9, Table 8). Next, we investigated the impact of additional THF, EtOH and acetone to the aqueous medium. Results indicated that 95, 92 and 90% of the product were obtained by the addition of THF, EtOH and acetone, respectively (entries 10–12, Table 8). Eventually, 15 mg of the Tzl-OH@Clin treated with Co(II) [contains 1.60 mol% of Co(II)], H2O/THF (9:1) as the solvent and NaBH4 (4 equivalent) at 50 °C were selected as the optimization reaction condition.


By reaching the optimized reaction condition, reductions of other nitroarene derivatives were undergone (Table 9). The results indicated that nitronaphthalene was reduced to its corresponding amine harder than nitrobenzene due to its lower solubility (entries 1–2, Table 9). Also, 4-chloroaniline and 4-bromoaniline were achieved in excellent yields due to the existence of withdrawing groups on the para position of nitro group (entries 3–4, Table 9). Reduction of ortho and para nitro toluene achieved the corresponding products in excellent yields, but regarding the steric effect of ortho position, the ortho toluidine was gained in longer time and lower yield than para toluidine (entries 5–6, Table 9). Presence of the electron-donating groups such as methoxy (–OMe) and hydroxy (–OH) on the nitrobenzene ring could reduce the reaction rate and yields. In the case of 4-nitroanisole and nitrophenols isomers, corresponding products were obtained in excellent (94%) and good yields (81–88%), respectively (entries 7–10, Table 9). The catalyst effectively reduced the nitroarenes, and amine products were achieved in good-to-excellent yields (Table 9).

Furthermore, to investigate the reusability of Tzl-OH@Clin, 1-chloro-4-nitrobenzene was selected as the model reaction. The catalyst was recycled for up to 4 cycles in the reduction. During the first 3 cycles, just a little change was observed (15% decline in the product yield). However, in the run 4th, the yield was dropped to 57%, which may be related to the leaching of the Co species into the reaction medium (Table 10). These results indicated that the catalyst could play a dominant role in the future industries by being as a sustainable and reusable adsorbent/catalyst type.

Conclusion
The modification of the clinochlore was carried out by attachment of triazole and triazolium ligands to the clinochlore surface, and the new prepared materials (Tzl-OH@Clin and IL-Tzl-OH@Clin) showed excellent capability in the adsorption of toxic heavy metal ions such as Co(II), Pb(II) and Zn(II). Treated Tzl-OH@Clin with the solutions of heavy metal ions, particularly Co, exhibited good catalytic activity in the reduction of nitroarenes. In the end, these materials indicated good adsorption efficacy in the heavy metal ion adsorption of industrial wastewaters and also could be as an effective catalyst after treated with heavy metal ion solutions.
References
Agudosi E, Salleh M, Abdullah E, Mujawar M, Khalid M, Azni A (2018a) Characterization of crystallized struvite on wastewater treatment equipment: prospects for crystal fertilizer production. Desalin Water Treat 113:205–212
Agudosi ES, Abdullah EC, Mubarak N, Khalid M, Pudza MY, Agudosi NP, Abutu ED (2018b) Pilot study of in-line continuous flocculation water treatment plant. J Environ Chem Eng 6(6):7185–7191
Altın O, Özbelge HÖ, Doğu T (1998) Use of general purpose adsorption isotherms for heavy metal–clay mineral interactions. J Colloid Interface Sci 198(1):130–140
Alvarez-Ayuso E, Garćia-Sánchez A (2003) Removal of heavy metals from waste waters by natural and Na-exchanged bentonites. Clays Clay Miner 51(5):475–480
Anirudhan T, Suchithra P (2010) Heavy metals uptake from aqueous solutions and industrial wastewaters by humic acid-immobilized polymer/bentonite composite: kinetics and equilibrium modeling. Chem Eng J 156(1):146–156
Anirudhan T, Jalajamony S, Sreekumari S (2012) Adsorption of heavy metal ions from aqueous solutions by amine and carboxylate functionalised bentonites. Appl Clay Sci 65:67–71
Bentouami A, Ouali M (2006) Cadmium removal from aqueous solutions by hydroxy-8 quinoleine intercalated bentonite. J Colloid Interface Sci 293(2):270–277
Bessbousse H, Rhlalou T, Verchère J-F, Lebrun L (2008) Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J Membr Sci 307(2):249–259
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140(2):114–131
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186(1):458–465
Burungale VV, Devan RS, Pawar SA, Harale NS, Patil VL, Rao V, Ma Y-R, Eun J, Kim JH, Patil PS (2016) Chemically synthesized PbS nano particulate thin films for a rapid NO2 gas sensor. Mater Sci Pol 34(1):204–211
Celis R, Hermosin MC, Cornejo J (2000) Heavy metal adsorption by functionalized clays. Environ Sci Technol 34(21):4593–4599
Charerntanyarak L (1999) Heavy metals removal by chemical coagulation and precipitation. Water Sci Technol 39(10–11):135
Chenu C, Plante A (2006) Clay-sized organo-mineral complexes in a cultivation chronosequence: revisiting the concept of the ‘primary organo-mineral complex’. Eur J Soil Sci 57(4):596–607
Da̧browski A, Hubicki Z, Podkościelny P, Robens E (2004) Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 56(2):91–106
de Sá A, Abreu AS, Moura I, Machado AV (2017) Polymeric materials for metal sorption from hydric resources. In: Water purification. Elsevier, pp 289–322
Eren E, Afsin B, Onal Y (2009) Removal of lead ions by acid activated and manganese oxide-coated bentonite. J Hazard Mater 161(2–3):677–685
Esalah J, Husein MM (2008) Removal of heavy metals from aqueous solutions by precipitation–filtration using novel organo-phosphorus ligands. Sep Sci Technol 43(13):3461–3475
Fortgang P, Tite T, Barnier V, Zehani N, Maddi C, Lagarde F, Loir A-S, Jaffrezic-Renault N, Donnet C, Garrelie F (2016) Robust electrografting on self-organized 3D graphene electrodes. ACS Appl Mater Interfaces 8(2):1424–1433
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Gopal N, Narasimhulu K, Rao JL (2004) Optical absorption, EPR, infrared and Raman spectral studies of clinochlore mineral. J Phys Chem Solids 65(11):1887–1893
Häni H (1990) The analysis of inorganic and organic pollutants in soil with special regard to their bioavailability. Int J Environ Anal Chem 39(2):197–208
Hemanthkumar GN, Parthasarathy G, Chakradhar RPS, Omkaram I, Rao JL, Ratnakaram YC (2009) Electron paramagnetic resonance studies on clinochlore from Longitudinal Valley area, northeastern Taiwan. Phys Chem Miner 36:447–453
Ho Y, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Jlassi K, Abidi R, Benna M, Chehimi MM, Kasak P, Krupa I (2018) Bentonite-decorated calix [4] arene: a new, promising hybrid material for heavy-metal removal. Appl Clay Sci 161:15–22
Kleppe AK, Jephcoat AP, Welch MD (2003) The effect of pressure upon hydrogen bonding in chlorite: a Raman spectroscopic study of clinochlore to 26.5 GPa. Am Miner 88(4):567–573
Kumrić KR, Đukić AB, Trtić-Petrović TM, Vukelić NS, Stojanović Z, Novaković JDG, Matović LL (2013) Simultaneous removal of divalent heavy metals from aqueous solutions using raw and mechanochemically treated interstratified montmorillonite/kaolinite clay. Ind Eng Chem Res 52(23):7930–7939
Le VT, Tran TKN, Tran DL, Le HS, Doan VD, Bui QD, Nguyen HT (2018) One-pot synthesis of a novel magnetic activated carbon/clay composite for removal of heavy metals from aqueous solution. J Dispers Sci Technol. https://doi.org/10.1080/01932691.2018.1541414
Lin GHY (1987) Toxicity of nitroaromatic compounds. J Appl Toxicol 7(2):151
Lin S-H, Juang R-S (2002) Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J Hazard Mater 92(3):315–326
Ltaief OO, Siffert S, Fourmentin S, Benzina M (2015) Synthesis of faujasite type zeolite from low grade Tunisian clay for the removal of heavy metals from aqueous waste by batch process: kinetic and equilibrium study. C R Chim 18(10):1123–1133
Ngah WW, Hanafiah M (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99(10):3935–3948
Nojiri N, Tanaka N, Sato K, Sakai Y (1980) Electrolytic ferrite formation system for heavy metal removal. J Water Pollut Control Fed 52:1898–1906
Obadia MM, Mudraboyina BP, Serghei A, Montarnal D, Drockenmuller E (2015) Reprocessing and recycling of highly cross-linked ion-conducting networks through transalkylation exchanges of C–N bonds. J Am Chem Soc 137(18):6078–6083
Padilla-Ortega E, Leyva-Ramos R, Flores-Cano J (2013) Binary adsorption of heavy metals from aqueous solution onto natural clays. Chem Eng J 225:535–546
Patil U, Lee SC, Sohn J, Kulkarni S, Gurav K, Kim J, Kim JH, Lee S, Jun SC (2014) Enhanced symmetric supercapacitive performance of Co(OH)2 nanorods decorated conducting porous graphene foam electrodes. Electrochim Acta 129:334–342
Riefler RG, Smets BF (2000) Enzymatic reduction of 2,4,6-trinitrotoluene and related nitroarenes: kinetics linked to one-electron redox potentials. Environ Sci Technol 34(18):3900–3906
Sabbioni G, Jones CR (2002) Biomonitoring of arylamines and nitroarenes. Biomarkers 7(5):347–421
Tokiwa H, Ohnishi Y, Rosenkranz HS (1986) Mutagenicity and carcinogenicity of nitroarenes and their sources in the environment. CRC Crit Rev Toxicol 17(1):23–58
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Velde B (1992) Introduction to clay minerals: chemistry, origins, uses and environmental significance. Chapman and Hall Ltd, London
Welch MD, Marshall WG (2001) High-pressure behavior of clinochlore. Am Miner 86(11–12):1380–1386
Xu D, Fan D, Shen W (2013) Catalyst-free direct vapor-phase growth of Zn1−xCuxO micro-cross structures and their optical properties. Nanoscale Res Lett 8(1):46
Yan L, Li S, Yu H, Shan R, Du B, Liu T (2016) Facile solvothermal synthesis of Fe3O4/bentonite for efficient removal of heavy metals from aqueous solution. Powder Technol 301:632–640
Yun CH, Prasad R, Guha AK, Sirkar KK (1993) Hollow fiber solvent extraction removal of toxic heavy metals from aqueous waste streams. Ind Eng Chem Res 32(6):1186–1195
Zhu Y, Hu J, Wang J (2012) Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J Hazard Mater 221:155–161
Zwirner-Baier I, Neumann H-G (1999) Polycyclic nitroarenes (nitro-PAHs) as biomarkers of exposure to diesel exhaust. Mutat Res Genet Toxicol Environ Mutagen 441(1):135–144
Acknowledgements
The authors are grateful to Jaber Ibn Hayyan Laboratory Foundation of NSTRI and Iran National Science Foundation (INSF-Grant No. 96001155) for supporting this research work. We are also grateful to Dr. Amir Hossien Ghorashi for his valuable comments and thoroughly editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Fatih Şen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kakaei, S., Khameneh, E.S., Hosseini, M.H. et al. A modified ionic liquid clay to remove heavy metals from water: investigating its catalytic activity. Int. J. Environ. Sci. Technol. 17, 2043–2058 (2020). https://doi.org/10.1007/s13762-019-02527-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02527-9