Abstract
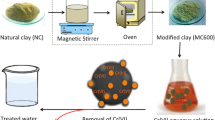
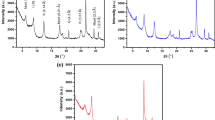
This study investigates a low-cost and economical raw and heat-treated clay sample as a potential adsorbent for a higher uptake of Cu(II), Pb(II) and Cd(II) ions from aqueous solutions. The characterization of the obtained raw and modified clay materials, X-ray fluorescence spectroscopy, thermogravimetric analysis, X-ray diffraction, Fourier transform infrared spectroscopy and scanning electron microscopy were carried out. Compared with the raw clay sample, the adsorption ability of metal ions with the heat-treated clay adsorbent was significantly improved. The effects of calcination temperature, initial metal concentration, solution temperature and contact time on adsorption capacity were investigated. Adsorption data were tested with Langmuir and Freundlich models, and the Langmuir model showed a relatively better fit. The adsorption capacities for Cu (II), Pb (II) and Cd (II) ions were found to be 156.25 mg/g, 172.40 mg/g and 9.15 mg/g, respectively. The adsorption data for heavy metal ions confirmed the pseudo-second-order kinetic model. Heat-treated clay samples showed remarkable adsorption efficiency for heavy metal removal from aqueous systems; therefore, it can be considered as a competent and potential adsorbent for heavy metal removal.
Graphical abstract












Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. Supplementary information is available at Environmental Science and Pollution Research’s website.
References
Abdellaoui Y, Olguín MT, Abatal M et al (2019) Comparison of the divalent heavy metals (Pb, Cu and Cd) adsorption behavior by montmorillonite-KSF and their calcium- and sodium-forms. Superlattices Microstruct. 127:165–175. https://doi.org/10.1016/j.spmi.2017.11.061
Abollino O, Giacomino A, Malandrino M, Mentasti E (2008) Interaction of metal ions with montmorillonite and vermiculite. Appl Clay Sci 38(3–4):227–236. https://doi.org/10.1016/j.clay.2007.04.002
Ahmed Z, Wu P, Jiang L et al (2020) Enhanced simultaneous adsorption of Cd(II) and Pb(II) on octylamine functionalized vermiculite. Colloids Surf A Physicochem Eng Asp 604:125285. https://doi.org/10.1016/j.colsurfa.2020.125285
Auta M, Hameed BH (2014) Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem Eng J 237:352–361. https://doi.org/10.1016/J.CEJ.2013.09.066
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U(VI) ion onto thermally activated bentonite. J Hazard Mater 172(2–3):667–674. https://doi.org/10.1016/j.jhazmat.2009.07.049
Bergaya F, Jaber M, Lambert JF (2011) Clays and clay minerals. In: Galimberti M (ed) Rubber-clay nanocomposites: science, technology, and applications. John Wiley & Sons, Hoboken
Bojemueller E, Nennemann A, Lagaly G (2001) Enhanced pesticide adsorption by thermally modified bentonites. Appl Clay Sci 18(5–6):277–284. https://doi.org/10.1016/S0169-1317(01)00027-8
Brigatti MF, Laurora A, Malferrari D et al (2005) Adsorption of [Al(Urea)6]3+ and [Cr(Urea)6]3+ complexes in the vermiculite interlayer. Appl Clay Sci 30(1):21–32. https://doi.org/10.1016/j.clay.2005.03.001
Cao C-Y, Liang C-H, Yin Y, Du L-Y (2017) Thermal activation of serpentine for adsorption of cadmium. J Hazard Mater 329:222–229. https://doi.org/10.1016/j.jhazmat.2017.01.042
Chai JB, Au PI, Mubarak NM et al (2020) Adsorption of heavy metal from industrial wastewater onto low-cost Malaysian kaolin clay–based adsorbent. Environ Sci Pollut Res 27(12):13949–13962. https://doi.org/10.1007/s11356-020-07755-y
Chen W-J, Hsiao L-C, Chen KK-Y (2008) Metal desorption from copper(II)/nickel(II)-spiked kaolin as a soil component using plant-derived saponin biosurfactant. Process Biochem 43:488–498. https://doi.org/10.1016/j.procbio.2007.11.017
Chen L, Wu P, Chen M et al (2018) Preparation and characterization of the eco-friendly chitosan/vermiculite biocomposite with excellent removal capacity for cadmium and lead. Appl Clay Sci 159:74–82. https://doi.org/10.1016/j.clay.2017.12.050
de Souza FM, dos Santos OAA, Vieira MGA (2019) Adsorption of herbicide 2,4-D from aqueous solution using organo-modified bentonite clay. Environ Sci Pollut Res. 26(18):18329–18342. https://doi.org/10.1007/s11356-019-05196-w
Dev VV, Baburaj G, Antony S et al (2020) Zwitterion-chitosan bed for the simultaneous immobilization of Zn(II), Cd(II), Pb(II) and Cu(II) from multi-metal aqueous systems. J Clean Prod 255:120309. https://doi.org/10.1016/j.jclepro.2020.120309
do Nascimento FH, de Souza Costa DM, Masini JC (2016) Evaluation of thiol-modified vermiculite for removal of Hg(II) from aqueous solutions. Appl Clay Sci 124:227–235. https://doi.org/10.1016/j.clay.2016.02.017
Durand JF (2012) The impact of gold mining on the Witwatersrand on the rivers and karst system of Gauteng and North West Province, South Africa. J Afr Earth Sci 68:24–43
El Ouardi M, Laabd M, Abou Oualid H et al (2019) Efficient removal of p-nitrophenol from water using montmorillonite clay: insights into the adsorption mechanism, process optimization, and regeneration. Environ Sci Pollut Res. 26(19):19615–19631. https://doi.org/10.1007/s11356-019-05219-6
Foroutan R, Mohammadi R, Adeleye AS et al (2019) Efficient arsenic(V) removal from contaminated water using natural clay and clay composite adsorbents. Environ Sci Pollut Res 26(29):29748–29762. https://doi.org/10.1007/s11356-019-06070-5
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57(385471):1100–1107. https://doi.org/10.4236/jep.2017.84030
Gil A, Amiri MJ, Abedi-Koupai J, Eslamian S (2018) Adsorption/reduction of Hg(II) and Pb(II) from aqueous solutions by using bone ash/nZVI composite: effects of aging time, Fe loading quantity and co-existing ions. Environ Sci Pollut Res 25(3):2814–2829. https://doi.org/10.1007/s11356-017-0508-y
González-Pradas E, Socías-Viciana M, Ureña-Amate MD et al (2005) Adsorption of chloridazon from aqueous solution on heat and acid treated sepiolites. Water Res 39(9):1849–1857. https://doi.org/10.1016/j.watres.2005.03.001
Gupta VK and Suhas (2009) Application of low-cost adsorbents for dye removal—a review. J Environ Manag 90:2313–2342. https://doi.org/10.1016/j.jenvman.2008.11.017
Gurgel LVA, Gil LF (2009) Adsorption of Cu(II), Cd(II) and Pb(II) from aqueous single metal solutions by succinylated twice-mercerized sugarcane bagasse functionalized with triethylenetetramine. Water Res 43:4479–4488. https://doi.org/10.1016/j.watres.2009.07.017
Hamdi Karaoǧlu M, Doǧan M, Alkan M (2009) Removal of cationic dyes by kaolinite. Microporous Mesoporous Mater 122(1–3):20–27. https://doi.org/10.1016/j.micromeso.2009.02.013
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70(2):115–124. https://doi.org/10.1016/S1385-8947(98)00076-X
Hu C, Zhu P, Cai M et al (2017) Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: kinetic, thermodynamic and equilibrium studies. Appl Clay Sci 143:320–326. https://doi.org/10.1016/j.clay.2017.04.005
Huang R, Lin Q, Zhong Q et al (2020) Removal of Cd(II) and Pb(II) from aqueous solution by modified attapulgite clay. Arab J Chem 13(4):4994–5008. https://doi.org/10.1016/j.arabjc.2020.01.022
Irawan C, Nata IF, Lee CK (2019) Removal of Pb(II) and As(V) using magnetic nanoparticles coated montmorillonite via one-pot solvothermal reaction as adsorbent. J Environ Chem Eng 7(2):103000. https://doi.org/10.1016/j.jece.2019.103000
Joseph IV, Tosheva L, Doyle AM (2020) Simultaneous removal of Cd(II), Co(II), Cu(II), Pb(II), and Zn(II) ions from aqueous solutions via adsorption on FAU-type zeolites prepared from coal fly ash. J Environ Chem Eng 8(4):103895. https://doi.org/10.1016/j.jece.2020.103895
Kaya M, Dilekoğlu MF, Şahin Ö, Saka C (2016) Plasma treated sepiolite: a new adsorbent for removal of malachite green from contaminated water. Plasma Chem Plasma Process 36(6):1417–1430. https://doi.org/10.1007/s11090-016-9745-y
Kentsa E, Abi CF, Ngomo HM et al (2020) Characterization of Akilbenza clay from Cameroon and its performance for the removal of copper(II) ions from aqueous solution. Environ Sci Pollut Res 27(29):36487–36497. https://doi.org/10.1007/s11356-020-09502-9
Kumrić KR, Crossed D, Signukić ADSB, Trtić-Petrović TM et al (2013) Simultaneous removal of divalent heavy metals from aqueous solutions using raw and mechanochemically treated interstratified montmorillonite/kaolinite clay. Ind Eng Chem Res 52(23):7930–7939. https://doi.org/10.1021/ie400257k
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Li G, Zhang J, Liu J et al (2020) Adsorption characteristics of white pottery clay towards Pb(II), Cu(II), and Cd(II). Arab J Geosci 13(13):1–15. https://doi.org/10.1007/s12517-020-05507-3
Manning BA, Goldberg S (1996) Modeling arsenate competitive adsorption on kaolinite, montmorillonite and illite. Clays Clay Miner 44(5):609–623. https://doi.org/10.1346/CCMN.1996.0440504
Maziarz P, Matusik J (2016) The effect of acid activation and calcination of halloysite on the efficiency and selectivity of Pb(II), Cd(II), Zn(II) and As(V) uptake. Clay Miner 51(3):385–394. https://doi.org/10.1180/claymin.2016.051.3.06
Moraes DS, Rodrigues EMS, Lamarão CN et al (2019) New sodium activated vermiculite process. Testing on Cu2+ removal from tailing dam waters. J Hazard Mater 366:34–38. https://doi.org/10.1016/j.jhazmat.2018.11.086
Pawar RR, Bajaj HC, Lee SM (2016) Activated bentonite as a low-cost adsorbent for the removal of Cu(II) and Pb(II) from aqueous solutions: Batch and column studies. J Ind Eng Chem. 34:213–223. https://doi.org/10.1016/j.jiec.2015.11.014
Poursani AS, Nilchi A, Hassani A et al (2016) The synthesis of nano TiO2; and its use for removal of lead ions from aqueous solution. J Water Resour Prot 8(04):438. https://doi.org/10.4236/jwarp.2016.84037
Qu R, Wang C, Ji C et al (2005) Preparation, characterization, and metal binding behavior of novel chelating resins containing sulfur and polyamine. J Appl Polym Sci 95(6):1558–1565. https://doi.org/10.1002/app.21370
Rao RAK, Kashifuddin M (2016) Adsorption studies of Cd(II) on ball clay: comparison with other natural clays. Arab J Chem 9:S1233–S1241. https://doi.org/10.1016/j.arabjc.2012.01.010
Şahin Ö, Kaya M, Saka C (2015) Plasma-surface modification on bentonite clay to improve the performance of adsorption of methylene blue. Appl Clay Sci 116:46–53. https://doi.org/10.1016/j.clay.2015.08.015
Saka C, Şahin Ö, Demir H, Kahyaoǧlu M (2011) Removal of lead(II) from aqueous solutions using pre-boiled and formaldehyde-treated onion skins as a new adsorbent. Sep Sci Technol 46(3):507–517. https://doi.org/10.1080/01496395.2010.517595
Saka C, Şahin O, Küçük MM (2012) Applications on agricultural and forest waste adsorbents for the removal of lead (II) from contaminated waters. Int J Environ Sci Technol 9(2):379–394. https://doi.org/10.1007/s13762-012-0041-y
Shahraki BK, Mehrabi B, Gholizadeh K, Mohammadinasab M (2011) Thermal behavior of calcite as an expansive agent. J Min Metall Sect B Metall 47(1):89–97. https://doi.org/10.2298/JMMB1101089S
Shang Z, Zhang LW, Zhao X et al (2019) Removal of Pb(II), Cd(II) and Hg(II) from aqueous solution by mercapto-modified coal gangue. J Environ Manag. https://doi.org/10.1016/j.jenvman.2018.10.072
Taghi Ganji M, Khosravi M, Rakhshaee R (2005) Biosorption of Pb, Cd, Cu and Zn from the wastewater by treated Azolla filiculoides with H2O2/MgCl2. Int J Environ Sci Technol 1(4):265–271. https://doi.org/10.1007/bf03325841
Taha AA, Shreadah MA, Heiba HF, Ahmed AM (2017) Validity of Egyptian Na-montmorillonite for adsorption of Pb2+, Cd2+ and Ni2+ under acidic conditions: characterization, isotherm, kinetics, thermodynamics and application study. Asia-Pac J Chem Eng 12(2):292–306. https://doi.org/10.1002/apj.2072
Tu YJ, You CF, Chang CK (2012) Kinetics and thermodynamics of adsorption for Cd on green manufactured nano-particles. J Hazard Mater 235:116–122. https://doi.org/10.1016/j.jhazmat.2012.07.030
Uddin MK (2017) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462
Wang W, Chen H, Wang A (2007) Adsorption characteristics of Cd(II) from aqueous solution onto activated palygorskite. Sep Purif Technol 55(2):157–164. https://doi.org/10.1016/j.seppur.2006.11.015
Wang C, Li J, Wang X et al (2019) Preparation of a hierarchical pore zeolite with high-temperature calcination and acid-base leaching. Clays Clay Miner 67(4):265–274. https://doi.org/10.1007/s42860-019-00025-0
Woumfo D, Kamga R, Figueras F, Njopwouo D (2007) Acid activation and bleaching capacity of some Cameroonian smectite soil clays. Appl Clay Sci 37(1–2):149–156. https://doi.org/10.1016/j.clay.2006.12.008
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. https://doi.org/10.5402/2011/402647
Xu H, Wu Y, Wang J et al (2013) Simultaneous preconcentration of cadmium and lead in water samples with silica gel and determination by flame atomic absorption spectrometry. J Environ Sci (China) 25:S45–S49. https://doi.org/10.1016/S1001-0742(14)60624-0
Yavuz T, Saka C (2013) Surface modification with cold plasma application on kaolin and its effects on the adsorption of methylene blue. Appl Clay Sci 85:96–102. https://doi.org/10.1016/j.clay.2013.09.011
Ye H, Chen F, Sheng Y et al (2006) Adsorption of phosphate from aqueous solution onto modified palygorskites. Sep Purif Technol 50(3):283–290. https://doi.org/10.1016/j.seppur.2005.12.004
Yu S, Zhai L, Zhong S et al (2016) Synthesis and structural characterization of magnetite/sepiolite composite and its sorptive properties for Co(II) and Cd(II). J Taiwan Inst Chem Eng 59:221–228. https://doi.org/10.1016/j.jtice.2015.07.025
Zhong QQ, Yue QY, Li Q et al (2014) Removal of Cu(II) and Cr(VI) from wastewater by an amphoteric sorbent based on cellulose-rich biomass. Carbohydr Polym 111:788–796. https://doi.org/10.1016/j.carbpol.2014.05.043
Acknowledgements
Siirt University Scientific Research Unit supported this study (2014-SİÜFEB-YL2).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
İT and CS contributed to the study conception and design. İT and MŞB performed the experiments. İT, ÖY and CS performed the data analysis. CS wrote the first draft of the manuscript. İT, ÖY and CS reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant competing financial interests or personal relationships to disclose.
Additional information
Editorial responsibility: Babatunde Femi Bakare.
Rights and permissions
About this article
Cite this article
Teğin, İ., Batur, M.Ş., Yavuz, Ö. et al. Removal of Cu (II), Pb (II) and Cd (II) metal ions with modified clay composite: kinetics, isotherms and thermodynamics studies. Int. J. Environ. Sci. Technol. 20, 1341–1356 (2023). https://doi.org/10.1007/s13762-022-04028-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04028-8