Abstract
Organophosphate pesticides are extravagantly used in worldwide context to get rid of insects. Due to their relevance and edges in agriculture, organophosphate pesticide can effortlessly stretch to the aquatic ecological units and, correspondingly, cause threats to aquatic animals, like fish. Profenofos could be a diligent and cytotoxic organophosphate pesticide. Animals become liable to profenofos by means of water and food. Present experiment determines toxicological impacts of profenofos through the in vivo presentation of silver barb (Barbonymus gonionotus) to 1/10th and 1/2 of the median lethal concentration (LC50) of profenofos as sublethal exposures for variable periods (7, 15, and 30 days). Succeeding recuperation arrays were additionally appraised yielding the exposed fish in tap water (without profenofos) for the identical time as they had been treated with profenofos. The study findings showed that, with the advancement of time and concentrations, treated fish demonstrated significantly (p < 0.05) bigger intensity of nuclear anomalies (nuclear aberrations of erythrocyte-NAE), for instance, micronucleus, swollen nucleus, terminal nucleus, extended nucleus, and karyopyknosis and, furthermore, cell variations from the standard structure of erythrocyte (cellular abnormalities of erythrocyte—CAE), for example, demembranated, crescentic, spindle-shaped, almond-formed, and twin-molded cells. Recuperation data showed that B. Gonionotus recovered spontaneously and the abnormal erythrocytic parameters were normalized with a concentration and duration-dependent fashion. Thus, supported these knowledge, we tend to convert that erythrocytic variations (NAE and CAE) from the standard structure could fill in as an evaluation of the hazard caused by organophosphate pesticides on non-target living beings, notably fish.
Similar content being viewed by others
Introduction
Organophosphorus pesticides are widely utilized in extensive crop farming along with aquaculture to get rid of insects (Sharbidre et al. 2011). These pesticides can enter the aquatic ecosystems either through surface runoff of rain or via leakage of toxicant from the soil (Hoffman et al. 1995). Chemical affects a colossal magnitude of non-target entities, for instance, invertebrates and fish species living in the aquatic environment besides the targeted pests (Peter et al. 2013; Saravanan et al. 2011). Application of organophosphorus chemicals are found to be increasing day by day since they are recyclable and consequently stay in the environment for a minimum period. As a results of their low persistence, once more and once more applications of those endocrine-disrupting chemicals are being executed for the management of insects in agrarian lands and after they enter the aquatic environments (Jyothi and Narayan 1999).
Among the organophosphates, profenofos is widely utilized in different countries for agricultural applications (Wilson and Tisdell 2001). It enters the aquatic system by permeating the soil as most rural plot is placed close to a body of water. If artificial compounds enter water bodies like lakes, waterways, and streams, this will be fatal to off-target aquatic life forms notably fish (Palmer et al. 2007). Additionally, profenofos eradicates helpful microorganisms and algae in the body of water, therefore devastating the photosynthesis process, and eliminating production of O2, and nourishment. As these are the fundamental elements sustaining aquatic life, several fish are killed as a result of a scarcity of oxygen in the contaminated water by the production of an abundance of ammonia through decay vegetation that eventually destroy the aquatic organic network (Miller 2004). Additionally, through direct contact with chemicals in polluted water, fish could pass away and even once solely a brief period of exposure irregularities from the standard can be produced in the blood cells of fish (Grisolia and Starling 2001).
Use of blood parameters as biomarkers of stress provides important information regarding the physiological reaction of fish in a changing environment and provides a wonderful tool for toxicological studies. The compositions of blood of fish fluctuate with changing conditions of the environment and respond quickly to any change in water quality as result of their close contact through gill surface. The danger of pesticides to aquatic life forms can be investigated by assessing adjustments within the enzymological, hematological, and biochemical parameters (Saravanan et al. 2011). Organophosphorus chemical causes many harmful consequences for fish erythrocytes at the nuclear and cellular levels (Sadiqul et al. 2016). A few examinations are accessible for groupings of pesticides in the bodies of fish (James and Sampath 1996) that cause numerous alterations in blood parameters and changes to the erythrocytic nuclear and cellular structures (Sadiqul et al. 2016; Svoboda et al. 2001).
The analysis of blood parameters could be a biomarker for distinctive identification of the outcomes of toxic contamination. The alteration of blood parameters can be used as health indicators of the aquatic environment and also provide early warning tools for monitoring environment quality (Pimpao et al. 2007). Additionally, a perception of change and recovery could be a basic instrument in the environment of risk examination (Du et al. 2009; Wu et al. 2005). Information on the duration of full recuperation of endocrine-disrupting-chemical-exposed fish helps the proper well-being of fish, henceforth secondarily human (Adhikari et al. 2004). The studies of the recuperation of endocrine-disrupting-chemical-exposed fish on specific parameters are very scanty. Consequently, the current investigation was aimed at evaluating the erythrocytic variations from the standard structure (nuclear and cellular), and studying the plausibility and examples of recuperation of those biomarkers in a very characteristic setting victimization in vivo method on a model fish of rice-field systems, silver barb (Barbonymus gonionotus) exposed to profenofos. Succeeding recuperation patterns were to boot calculable admitting the exposed fish to the profenofos liberated water for the equivalent time (7, 15 and 30 days) so that they were exposed to profenofos.
Materials and methods
Robust and disease-free silver barbs (Barbonymus gonionotus) were gathered from fish ranches in the instructional neighborhood, each having a length of 11.13 ± 1.44 cm and a body weight of 6.5 ± 2.52 g. The fish were permitted to acclimate to the exploration facility environments for 2 weeks to get rid of the assumed annoying subjects at a temperature starting from 24.0 to 25.0 °C. Two hundred individual fish were arbitrarily nominated and moved into a solidified tank containing tap water furnished with an air circulation system. The fish were nurtured with food consistently on two occasions each day with zooplankton containing protein and a basic supplement (pelleted feed, Mega Fish Feed Co. Ltd.). Starvation of fish was inaugurated 24 h before the trial run and throughout the investigation, and also the tank water was adjusted on each alternative two days. The trial approaches were completed following the principles of the Animal Welfare and Experimental Ethical Committee of Bangladesh Agricultural University, Bangladesh [Approval Ref. No. AWEEC/BAU/2019 (10)]. An endocrine-disrupting chemical, profenofos 98% [O-(4-bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate], an organophosphate, was obtained from a regional supplier in original sealed container.
Exposure assessment of fish to profenofos
The exploratory tests (range detection test) were performed to explicate the imprecise operational concentration range of profenofos on the test fish, as declared by OECD (1992). The check medium was developed over a vast range of concentrations. These experiments were conducted in glass aquarium (72 × 43 cm2) by exposing 10 specimens of silver barb in 25 L freshwater containing numerous concentrations of profenofos. The dead fish were recorded and separated promptly. The nominal dose was chosen where no death was documented in 24 h and also the highest fatal concentration was where 100% death was acknowledged in 24 h. Static bioassay methodology (APHA 1985) was followed to check the toxicity of profenofos. For acute toxicity test, 10 fish were unveiled to a serially diluted profenofos (0.2, 0.1, 0.05, 0.06, 0.07, and 0.08 ppm). Stock solutions (1.0 ppm) were prepared by dissolving profenofos in distilled water. For the trial and control test, dechlorinated tap water was assigned in the examination. The experiments were conducted in two replicates along with a control at room temperature. Feeding was fully in remission throughout the experimentation. Probit analysis was employed to verify the concentration that caused 50% death response of silver barb.
The median lethal concentration (LC50) for profenofos was noted at 0.1 ppm. To check the biomarker reactions, 180 sound fish were brought into the aquaria (72 × 43 cm2) with 1/10th (0.01 ppm) and one half (1/2) (0.05 ppm) of LC50 of profenofos as sublethal concentrations for 7, 15 and 30 days, while a third gathering was regarded as a control (0% profenofos) and each having 3 replications. Air circulation was connected to the water tank for 2 h to urge a same grouping of the fatal compound and 30 haphazardly chosen fish were changed to each study tank (aquarium).
After exposure to 1/10th (0.01 ppm) and one half (1/2) (0.05 ppm) of LC50 of profenofos for 30 days, recuperation of the toxicity of profenofos was evaluated in isolated aquaria containing profenofos-free freshwater for three comparison periods (7, 15 and 30 days) with daily reestablishment of water. Throughout the experiment, feeding and new measurements of profenofos levels were done following the changing of the water on interchange day. The fish were checked twice daily for death response and different behavioral changes. At the termination of day 7, 15 and 30 post-exposures, at the minimum 7 fish from each test tank (aquarium) were captured and their recuperation was evaluated.
Assessment of erythrocyte deformities
The fish were aloof from the aquaria on every examination day and later asleep with oil of cloves (5 mg/l); slime and wet shown on the external surface of the fish were wiped with tissue paper. Blood samples were collected with disposable syringes from the posterior part of the body, spread onto medical glass slides and dried at room temperature for 10 min. The spread was settled with methanol for 10 min and dyed with 5% Giemsa stain and consequently cleansed with tap water. The glass slides were dehydrated at room temperature and after that mounted with DPX and viewed beneath a microscope (MICROS, MCX100) using the 100X lens. A minimum of three glass slides were set up for every individual fish, and 2,000 cells were counted from each glass slide with no less than five fish investigated in every gathering. At the best cell with untarnished cellular and nuclear films, coding and blind scoring were done. The micronuclei evaluation indicators were acknowledged from Fenech et al. (2003): the micronuclei (MN) need to be isolated from or possibly covered by a fundamental nucleus to that degree as there is clear characteristic proof of the nuclear limit, and micronuclei ought to have stain alike to the principal nucleus. Nuclear irregularities of the erythrocyte (NAE) totally different from MN were ordered by Carrasco et al. (1990). Architectural variations from the standard recognizable proof criteria of erythrocytes ought to be conflicting than the general erythrocyte cells structure which has an ovoid outline with a midway dense nucleus (Sadiqul et al. 2016).
Statistical inference
The ordinariness (Shapiro–Wilk test) and homogeneity (Levene's test) of difference trial have been executed prior to the factual examinations. Erythrocyte (cellular and nuclear) anomalies were tried by utilizing one-way ANOVA (analysis of variance). Post hoc test was done utilizing Duncan's different examination techniques. Importance was set at 95%. All the factual investigation was accomplished using SPSS 16.0. All qualities were communicated as mean ± SD.
Results and discussion
Results
Acute toxicity test
Acute toxicity test was performed to investigate the impact of profenofos on silver within brief period of exposure. A time-dependent decrease as well as a dose-reliant increase was perceived in death rate; since the exposure period enhanced from 12 to 120 h, the LC50 decreased. No mortality was documented for control group; 10% mortality was recorded in 0.06 ppm; at identical time 80% death response was ascertained in the highest concentration of 0.20 ppm. The profenofos pesticide for silver barb persisted a median lethal concentration (LC50 with 95% confidence limits) of 0.10 ppm (Table 1).
Irregularities of erythrocytes at different sublethal concentrations of profenofos
The nuclear abnormalities of erythrocyte (NAE) ascertained at numerous introduction terms in two sublethal concentrations (1/10th and 1/2 of LC50) of profenofos incorporating control are represented in Table 2. An ovoid-shaped erythrocyte with a regular oval-shaped nucleus at the middle of the cell was found in the control fish, whereas in the profenofos-exposed fish, variations from the standard structure (e.g., micronuclei, swollen nucleus, terminal nucleus, extended/elongated nucleus, and karyopyknosis) were seen (Fig. 1). In this study, aberrations in the erythrocyte cells of silver barb treated with various concentrations of profenofos were considerably diverse from those ascertained in control fish (p < 0.05). In the control cluster, the common variety of NAEs was concerning zero; but, the amount increased sharply at a fixated and span-dependent mildew once introducing profenofos (Table 2, Fig. 2). The mean qualities of the cellular irregularities of erythrocytes (CAE) at distinctive presentation lengths are presented in Table 3 and Fig. 3. A comparative design of the return of CAE was found in the uncovered gatherings because it was in the NAE. The distinctive varieties of CAE seen in the studied fish were demembranated, crescentic, spindle-shaped, twin, and almond-fashioned cells (Fig. 4).
Diverse nuclear modifications of erythrocyte cell of silver barb exposed to the sublethal groupings of profenofos, for example a control (consistent cell), b terminal nucleus, c swollen nucleus, d micronucleus, e Karyopyknosis, and f elongated nucleus. Two gatherings of fish were bestowed to 1/10th and 1/2 of LC50 of profenofos for 7, 15, and 30 days; at the same time, the third gathering was regarded as control (0% profenofos). Three glass slides were set up for each fish, and 2000 cells were counted from each slide and no less than 5 fish were examined in each gathering. Giemsa stain: 100×
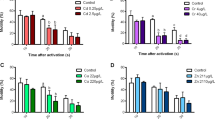
Alterations of the average total of all NAEs in the test fish treated with sublethal concentrations (1/10th and 1/2 of LC50) of profenofos and the corresponding controls at each time of exposure (7, 15 and 30 days) and recuperation in pesticide-free freshwater for comparable interims. Data represent mean ± SD (n = 7). Cont—control, Expo—exposure, Recov—recovery
Varieties of the average total of all CAEs in the test fish presented to sublethal concentrations (1/10th and 1/2 of LC50) of profenofos and the corresponding controls at every time of exposure (7, 15 and 30 days) and recuperation in freshwater for comparable interims. Data represent mean ± SD (n = 7). Cont—control, Expo—introduction, Recov—recovery
Diverse cellular modifications of erythrocyte cell of silver barb exposed to the sublethal groupings of profenofos for example a control, b demembranated, c spindle shaped, d almond formed, e twin formed, and f crescentic molded. Two gatherings of fish were presented to 1/10th and one half (1/2) of LC50 of profenofos for 7, 15, and 30 days; at the identical time, the third gathering was regarded as control (0% profenofos). Three glass slides were set up for each fish and 2000 cells were counted from each glass slide and no less than 5 fish were investigated in each gathering. Giemsa stain: 100×
Recuperation responses of NAE and CAE
The recuperation reactions of NAE are displayed in Table 2. The recuperation reactions of the fish treated with different concentrations of profenofos differed significantly (p < 0.05). All irregular nuclei in Fig. 1 (micronuclei, swollen nucleus, terminal nucleus, elongated nucleus, and karyopyknosis) and recuperation rate at 1/10th concentration of profenofos demonstrated relatively higher recuperation than the 1/2 concentration of profenofos (Figs. 2 and 5a). In the two focuses, a significant (p < 0.05) distinction between recuperation rates was seen in the 15-day recuperation groups than in the opposite two (7-day and 30-day) recuperation groups.
Remarkable dissimilarities in the recuperation reactions of CAE in reference to the control gatherings and among recuperation days are shown in Table 3. Every aberrant cell (demembranated, crescentic, spindle-shaped, almond-molded and twin-molded cells) at 1/10th concentration showed a higher recuperation rate like NAE than at the 1/2 concentration of profenofos (Figs. 3 and 5b).
Discussion
The present investigation inspected the cytotoxicity of profenofos on erythrocytes and its recuperation arrangements and recovery outlines in B. Gonionotus through two investigations: an easy and solid nuclear variation from the standard structure of erythrocytes (NAE) and a cytotoxic test of cellular anomalies of erythrocytes (CAE). Our principal objectives were to assess the appropriateness of those two criteria as bio-indicator of pesticide prevalence, employing a freshwater teleost, silver barb (B. Gonionotus), as a model organism. Here, it had been seen that profenofos causes numerous deformations in erythrocytes when absorbed into the fish body. Blood profile has been recognized a pathophysiological indicator of the health status of fish, and thus, hematological features like erythrocytes are imperative tool to detect the physiology and biochemistry of fish exposed to different endocrine-disrupting chemicals. Erythrocyte are capable to respond to a few environmental obsess and modifications of erythrocyte (cellular and nuclear) represent the most common reflection towards pesticide present in water bodies (Sawhney and Johal 2000).
Micronuclei and different nuclear variations from the standard are biomarkers of chromosomal variability and cytotoxic occurrences. Micronuclei (chromosome harm events) might increase the danger of formative and deteriorating conditions in fish. The development of micronuclei may be a marker of genomic harm. The micronuclei of fish are useful exploratory framework for evaluating the genotoxic attributes of chemicals prevailing in the aquatic ecosystem (Khan et al. 2018; Sadiqul et al. 2016; Bolognesi and Hayashi, 2011). Micronuclei were likewise answered to start out amid anaphase from slacking acentric chromosomes or chromatid sections produced through unrepaired DNA breakdowns or the misrepair of DNA breaks (Fenech et al. 2011). Outcomes of the present examination demonstrated fixation and term reliant escalation in the recurrence of micronuclei and a few different nuclear anomalies in silver barb's erythrocytes that backs the discoveries of Ahmed et al. (2011).
In a snakehead, the recurrence of micronuclei was observed to be basically larger extent attributable to arsenic introduction although it had been brought down in the control gathering of the fish (Patowary et al. 2012). In the present examination, micronuclei expanded following seven days of pesticide exposure. Micronucleated erythrocytes in fish appeared from one to five days resulting from introduction to genotoxic and, in addition, cytotoxic specialists (Udroiu 2006). A 7-day recuperation period demonstrated the foremost astounding recuperation reaction, and also the decreasing micronuclei arrangement could show overhaul of harmed DNA, damage of intensely injured cells, or both as conferred by Grover et al. (2001). This reverse connection between the time of application and DNA harm could be attributable to the danger of xenobiotics that might irritate enzymatic procedures in the arrangement of DNA harm (Rank and Jensen 2003).
Every erythrocyte seems oval accompanied by an elongated nucleus under the microscope. The intense assault of the chemical profenofos prompts erythrocytic layer disturbances and nuclear retrogressions in the experimental fish, specifically, micronuclei, binucleus, declined nucleus, notched nuclei, nuclear scaffold, and nuclear bud. Various investigations delineate the introduction of genotoxic substances and also the distance of varied NAE (Khan et al. 2018; Sadiqul et al. 2016; Ergene et al. 2007; Barˇsiene et al. 2006; Cavas and Ergene-Gozukara 2005; Kammann et al. 2004). Pesticides delivered deeply responsive free radicals inside the fish body. These free radicals respond with DNA and generate nuclear budding in the interphase section of cell division in this manner; as a result, blabbed, lobed, binucleated, declined, notched, nuclear bridges, and nuclear buds are formed (Hussain et al. 2012). Levin et al. (2004) likewise conjectured that variations from the norm emerge because harm occurred to the hereditary substance due to free radicals delivered during oxidative stress of the endocrine disruptive chemicals. Lobed, notched, vacuolated, dense, and divided erythrocytes with blabbed nucleus might likewise be known with the frustration of tubulin polymerization attributable to fatal impacts (Walia et al. 2013; Campos-Pereira et al. 2012). Most astounding, in the present examination binucleated declined nuclei, notched nuclei, nuclear bud, and nuclear scaffold were seen each at 7-day and 15-day periods of treatment.
As found in some other examinations, here we observed that the NAE number was conditional on duration and profenofos concentration (e.g., Khan et al. 2018; Sadiqul et al. 2016; Osman 2014; Ergene et al. 2007). In the present examination, we found that the frequencies of every irregularity in each investigation were in the following trend: micronuclei < terminal nucleus < swollen nucleus < elongated nucleus < karyopyknosis. Moreover, we saw the most astounding recuperation for the 15-day time frame. At the 30-day exposure and recuperation periods, the two concentrations demonstrated the strength of variations from the standard structure. Nepomuceno et al. (1997) recommended that the excessive poison fixation may restrain typical cell division, harm erythrocyte chromosomes, and intrude on DNA duplication, making nuclear variations decay from the standard. The NAE has a tendency to try and out, and fish may elevate some protective component to lessen a portion of the pesticide deposits in the body with a specific end goal to balance out the poisonous quality (Nepomuceno et al. 1997).
The erythrocyte layer is by all accounts most influenced by profenofos and expanded porosity. These modifications of the erythrocyte membrane could be the disorganization of lipid microenvironment of the membrane and, moreover, because of expand of lipid per oxidation affected by profenofos, ensuing increase in the permeability and fluidity of membrane (Brecher and Bessis 1972). The increment in the grouping of pesticide for the present time frame enlisted an expansion in the quantity of CAE. CAE incorporates demembranated, crescentic, spindle-shaped, almond-molded, and twin-molded cells. These morphological changes occur because of adjustments in the plasma layer influencing surface twisting and making erythrocytes a lot of defenseless to blasts once crossing little vessels (Brecher and Bessis, 1972). Jha (2008) declared that varied endocrine troubled chemicals having oxidative pressure probably could attack DNA, bringing about clastogenic, morphological, and subnuclear harm. Additionally, Ateeq et al. (2002) explained the grouping of cell debasement under the impact of pesticides and proposed that pesticides might originate hypoxic status, which lead to the ill-being of ATP that prompts an unusual state of erythrocytes.
Our investigation uncovered that the most extreme frequencies of extended and combined cells discovered promptly once exposure for seven days are lowered. In addition, the most noteworthy recuperation reaction was ascertained at a 15-day time span before moderating downs. Comparable investigations were made by Das and Nanda (1986) in a period-conditional decline of CAE in the blood of stinging catfish. In their study, twin- and spindle-shaped erythrocytes were incontestable maxima during a 15-day period.
Conclusion
Alterations in the shape and size of erythrocyte parameters are generally vulnerable to a couple of stereotypic reactions to an assortment of natural fixates, which are considered as the major bioindicators of ecological contamination. The results of the current examination indicate that profenofos is altogether harmful to the nourishment of fish contingent upon the fixation and exposure periods. Accordingly, changes to erythrocytes could be considered as biomarkers in the poisonous quality appraisal of profenofos in fish and other aquatic life. Not long ago, little was thought about recuperation examples or periods of duration essential for the recuperation of erythrocytes of aquatic animals particularly in fish. Our findings showed that recuperation of the NAE and CAE is time and measurement dependent, as the parameters steadily normalized after exposure to pesticide-free water. In any case, more research is required to grasp the instruments associated with the harmfulness of organophosphate and with recuperation both in the short and long terms at various levels of the natural organization. Finally, any analysis can facilitate people to become cognizant concerning the impact of pesticides used for yield generation on the physiological health of fish and different aquatic life. Such studies could likewise discover a much better way of using this organophosphate in the field. From the eco-physiological perspective, the use of profenofos in the agriculture and aquaculture should be exactly assessed.
References
Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S (2004) Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol Environ Saf 58(2):220–226
Ahmed MK, Habibullah-Al-Mamun M, Hossain MA, Arif M, Parvin E, Akter MS, Khan MS, Islam MM (2011) Assessing the genotoxic potentials of arsenic in tilapia (Oreochromis mossambicus) using alkaline comet assay and micronucleus test. Chemosphere 84:143–149
Al-Sabti K, Metcalfe CD (1995) Fish micronuclei for assessing genotoxicity in water. Mutat Res 343:121–135
APHA (1985) Standard methods for the examination of water and waste water APHA. AWWA and WPCF, New York
Ateeq B, Abul FM, Niamat AM (2002) Induction of micronuclei and erythrocyte alternations in the catfish, Clarrias batrachus by 2, 4-dichlorophenoxyacetic acid and butachlor. Mutat Res 518:135–144
Barˇsiene J, Dedonyte V, Rybakovas A, Andreikenaite L, Andersen OK (2006) Investigation of micronuclei and other nuclear abnormalities in peripheral blood and kidney on marine fish treated with crude oil. Aquat Toxicol 78:99–104
Brecher G, Bessis M (1972) Present status of speculated red cells and their relationship to the discocyte–echinocyte-transformation: a critical review. Blood 40(3):333–344
Campos-Pereira FD, Camila AO, Acacio AP, Silva-Zacarin ECM, Barbieri R, Spatti EF, Marin-Morales MA, Severi-Aguiar GDC (2012) Early cytotoxic and genotoxic effects of atrazine on Wistar rat liver: a morphological immunohistochemical, biochemical, and molecular study. Ecotoxicol Environ Saf 78:170–177
Carrasco KR, Tilbury KL, Myers MS (1990) Assessment of the piscine micronucleus test as in situ biological indicators of chemical contaminant effects. Can J Fisher Aquat Sci 47:2123–2136
Cavas T, Ergene-Gozukara S (2005) Induction of micronuclei abnormalities Oreochromis niloticus following exposure of petroleum refinery and chromium processing plant effluents. Aquat Toxicol 74:264–271
Das RK, Nanda NK (1986) Induction of micronuclei in peripheral erythrocytes of fish Heteropneustes fossilis by mitomycin C and paper mill effluent. Mutat Res 175:67–71
Du Y, Shi X, Liu C, Yu K, Zhou B (2009) Chronic effects of water-borne PFOS exposure on growth, survival, and hepatotoxicity in zebrafish: a partial life-cycle test. Chemosphere 74(5):723–729
Ergene S, Cavas T, Celik A, Koleli N, Aymak N (2007) Evaluation of river water genotoxicity using the piscine micronucleus test. Environ Mol Mutagen 48(6):421–429
Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E (2003) HUMN project: detailed description of the scoring criteria for the cytokinesis-block micronucleus assay using isolated human lymphocyte cultures. Mutat Res 534:65–75
Fenech M, Kirsch-Volders M, Natarajan AT, Surralles J, Crott JW, Parry J, Norppa H, Eastmont DA, Tucker JD, Thomas P (2011) Molecular mechanisms of micronucleus, nucleoplasmic bridge and nuclear bud formation in mammalian and human cells. Mutagenesis 26:125–132
Grisolia CK, Starling FLRM (2001) Micronuclei monitoring of fishes from Lake Paranoa under influence of sewage treatment plant discharge. Mutat Res 491:39–44
Grover P, Banu BS, Devi KD, Begum S (2001) In vivo genotoxic effects of mercuric chloride in rat peripheral blood leucocytes using comet assay. Toxicology 164(3):191–197
Hoffman JD, Rattner BA, Burton GA, Cairns J (1995) Handbook of ecotoxicology. Lewis Publishers, London
Hussain R, Mahmood F, Khan A, Javed MT, Rehan S, Mehdi T (2012) Cellular and biochemical effects induced by atrazine on the blood of male Japanese quail (Coturnix japonica). Pest Biochem Physiol 103:38–42
James R, Sampath K (1996) Individual and combined effects of carbaryl and methyl parathion on leucocyte and their recovery in Heteropneustes fossilis. In: Mishra SR (ed) Assessment of water pollution. A.R.H. Publishing Corporation, Darya Ganj, New Delhi, pp 417–421
Jha AN (2008) Ecotoxicological applications and significance of the comet assay. Mutagenesis 23:207–221
Jyothi B, Narayan G (1999) Certain pesticide-induced carbohydrate metabolic disorders in the serum of freshwater fish Clarias batrachus (Linn.). Food Chem Toxicol 37:417–421
Kammann U, Biselli S, Huhnerfuss H, Reineke N, Theobald H, Vobach M, Wosniok W (2004) Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test. Environ Pollut 132(2):279–287
Khan MM, Moniruzzaman M, Mostakim GM, Khan MSR, Rahman MK, Islam M Sadiqul (2018) Aberration of the peripheral erythrocytes and its recovery patterns in a freshwater teleost, silver barb exposed to profenofos. Environ Pollut 234:830–837
Levin ED, Swain HA, Donerly S, Linney E (2004) Developmental chlorpyrifos effects on hatching zebrafish swimming behavior. Neurotoxicol Teratol 26(6):719–723
Miller GT (2004) Chapter. Sustaining the earth, 6th edn. Thompsoon Learning Inc., Pacific Grove, pp 211–216
Nepomuceno JC, Ferrarri I, Spano MA, Centeno AJ (1997) Detection of micronuclei in peripheral erythrocytes of Cyprinus carpio exposed to metallic. Environ Mol Mutagen 30(3):293–297
OECD (1992) OECD guideline for testing of chemicals. Fish Acute Toxicity 203:1–9
Osman AGM (2014) Genetoxicity tests and their contributions in aquatic environmental research. Environ Prot 5:1391–1399
Palmer WE, Bromley PT, Brandenburg RL (2007) Wildlife and pesticides—peanuts. North Carolina Cooperative Extension Service, Pittsboro
Patowary K, Hazarika NS, Goswami M (2012) Studies on the toxic impact of arsenic on some enzymes and chromosomes of Channa punctatus. Clarion 1:148–153
Peter VS, Babitha GS, Bonga SE, Peter MC (2013) Carbaryl exposure and recovery modify the interregnal and thyroidal activities and the mitochondria-rich cell function in the climbing perch Anabas testudineus Bloch. Aquat Toxicol 126:306–313
Pimpao CT, Zampronio AR, de Assis HCS (2007) Effects of deltamethrin on hematological parameters and enzymatic activity in Ancistrus multispinis (Pisces, Teleostei). Pestic Biochem Physiol 88:122–127
Rank J, Jensen K (2003) Comet assay on gill cells and hemocytes from the blue mussel Mytilus edulis. Ecotoxicol Environ Saf 54:323–329
Sadiqul IM, Zannatul F, Tanvir ANM, Mostalim GM, Khalilur RM (2016) Acute exposure to a quinalphos containing insecticide (convoy) causes genetic damage and nuclear changes in peripheral erythrocytes of the silver barb, Barbonymus gonionotus. Environ Pollut 219:949–956
Saravanan M, Prabhu KK, Ramesh M (2011) Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic Biochem Physiol 100(3):206–211
Sawhney AK, Johal MS (2000) Erythrocytes alternations induced by malathion in Channa punctatus (Bloch). Bull Environ Contam Toxicol 64:398–405
Sharbidre AA, Metkari V, Patode P (2011) Effect of methyl parathion and chlorpyrifos on certain biomarkers in various tissues of guppy fish Poecilia reticulate. Pestic Biochem Physiol 101(2):132–141
Svoboda M, Luskova V, Drastichova J, Ilabek V (2001) The effect of diazinon on hematological indices of common carp (Cyprinus carpio L.). Acta Veterinaria Brno 70:457–465
Udroiu I (2006) The micronucleus test in piscine erythrocytes. Aquat Toxicol 79(2):201–2014
Walia GK, Handa D, Kaur H, Kalotra R (2013) Erythrocytes abnormalities in a freshwater fish, Labeo rohita exposed to tannery industry effluent. Int J Pharm Biol Sci 3(1):287–295
Wilson C, Trisdell C (2001) Why farmers continue to use pesticide despite environmental health and sustainability costs. Ecol Econ 39(3):449–462
Wu RS, Siu WH, Shin PK (2005) Induction adaptation and recovery of biological responses: implications for environmental monitoring. Mar Pollut Bull 51(8–12):623–634
Acknowledgments
This work was supported by a grant of Impact of Aquaculture Drugs and Chemicals on Aquatic Ecology and Productivity Project (IADCAEPP) provided by Bangladesh Fisheries Research Institute (BFRI), Mymensingh-2201, Bangladesh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Islam, S.M., Khan, M.M., Moniruzzaman, M. et al. Recuperation patterns in fish with reference to recovery of erythrocytes in Barbonymus gonionotus disordered by an organophosphate. Int. J. Environ. Sci. Technol. 16, 7535–7544 (2019). https://doi.org/10.1007/s13762-019-02425-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02425-0