Abstract
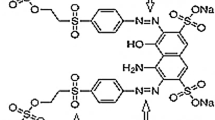
The studies were performed with 100 mg/L of Reactive Black 5 dye (RB5) in batch reactors on Ti/PtOx–RuO2–SnO2–Sb2O5 electrodes prepared indigenously. The electrodes were fabricated using titanium substrate (plate) and coated with mixed metals of a fixed composition by standard thermal decomposition (STD) and polymeric precursor thermal decomposition (PPTD) methods. The electrolysis up to 1 h was accomplished at a constant current density of 50 mA/cm2. For the process, an initial pH of 2 was maintained with the dosage of 4 g/L NaCl as supporting electrolyte. The effects of electrodes on colour, chemical oxygen demand, and total organic carbon removal were investigated for destruction of RB5. In this comparative study, while complete decolourizations of water containing RB5 were achieved on the electrodes prepared by STD and PPTD methods, but COD and TOC removal was found to be different for different electrodes at operating conditions. The 87% COD and 83% TOC removals were achieved on Ti/PtOx–RuO2–SnO2–Sb2O5 electrode prepared by PPTD method, in comparison to 83% COD and 80% TOC removals on the electrode prepared by STD method. The two types of electrodes were compared based on removal rate constant, mass transfer coefficient, instantaneous current efficiency, energy consumption, and accelerated life test. The energy demand for RB5 removal in this study was less than 250 kWh/kg COD, which is much lower than the reported values in similar studies. Further, the performance of electrodes was compared employing scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray diffraction analysis for coating deposited on Ti substrate, and also by cyclic voltammetry.



Similar content being viewed by others
References
Adams B, Tian M, Chen A (2009) Design and electrochemical study of SnO2-based mixed oxide electrodes. Electrochim Acta 54:1491–1498
American Public Health Association APHA (2005) Standard methods for the examination of water and wastewater. APHA, Washington, DC
Canizares P, Gomez JG, de Marcos I, Rodrigo M, Lobato J (2006) Measurement of mass-transfer coefficients by an electrochemical technique. J Chem Educ 83:1204–1207
Cerón-Rivera M, Dávila-Jiménez MM, Elizalde-González MP (2004) Degradation of the textile dyes Basic yellow 28 and Reactive black 5 using diamond and metal alloys electrodes. Chemosphere 55(1):1–10
Chatzisymeon E, Xekoukoulotakis NP, Coz A, Kalogerakis N, Mantzavinos D (2006) Electrochemical treatment of textile dyes and dye house effluents. J Hazard Mater 137:998–1007
Chen G, Chen X, Yue PL (2002) Electrochemical behavior of Novel Ti/IrOx–Sb2O5–SnO2 anodes. J Phys Chem B 106:4364–4369
de Oliveira-Sousa A, da Silva MAS, Machado SAS, Avaca LA, de Lima-Neto P (2000) Influence of the preparation method on the morphological and electrochemical properties of Ti/IrO2-coated electrodes. Electrochim Acta 45(27):4467–4473
Feng D, Li Y, Cao H, Wang Y, Crittenden J, Zhang Y (2015) Activated carbon electrodes: electrochemical oxidation coupled with desalination for wastewater treatment. Chemosphere 125:205–211
Fuat O, Bunyamin K (2015) Treatment of pretreated coke wastewater by electrocoagulation and electrochemical peroxidation processes. Sep Purif Technol 150:268–277
Hu CC, Lee CH, Wen TC (1996) Oxygen evolution and hypochlorite production on Ru-Pt binary oxides. J Appl Electrochem 26:72–82
Iranildes DS, Sinara BG, Júlio CA, Achilles JB (2011) Preparation and characterization of Ti/SnO2–Sb electrode by Pechini’s method for phenol oxidation. J Mater Res 4:408–416
Kim K, Lee EH, Kim JS, Shin KH, Kim KH (2001) Study on the electro-activity and non-stochiometry of a Ru-based mixed electrode. Electrochim Acta 46:915–921
Lin SM, Wen TC (1993) A mixture design approach to the service life and the oxygen evolving catalytic activity of Ru–Sn–Ti ternary oxide coated electrodes. J Appl Electrochem 23:487–494
Martínez-Huitle CA, Brillas E (2009) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: a general review. Appl Catal B Environ 87(3–4):105–145
Martinez CA, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect process Critical review. Chem Soc Rev 35:1324–1340
Martinez EJ, Rosas JG, Gonzalez R, Garcia D, Gomez X (2018) Treatment of vinasse by electrochemical oxidation: evaluating the performance of boron-doped diamond (BDD)-based and dimensionally stable anodes (DSAs) Int. J Environ Sci Technol 6:1159–1168
Montilla F, Morallon E, Battisti A, Vazques JL (2004) Preparation and characterization of antimony-doped tin dioxide electrodes. Part-1. Electrochemical characterization. J Phys Chem B 108:5036–5043
Park Y, Lee B, Kim C, Oh Y, Nam S, Parka B (2009) The effects of ruthenium-oxidation states on Ru dissolution in Pt Ru thin-film electrodes. J Mater Res 24:2762–2766
Pieczyńska A, Ossowski T, Bogdanowicz R, Siedlecka E (2018) Electrochemical degradation of textile dyes in a flow reactor: effect of operating conditions and dyes chemical structure. Int J Environ Sci Technol 1–14. https://doi.org/10.1007/s13762-018-1704-0
Profeti LPR, Profeti D, Olivi P (2009) Pt–RuO2 electrodes prepared by thermal decomposition of polymeric precursors as catalysts for direct methanol fuel cell applications. Int J Hydrog Energy 34:2747–2757
Rajkumar D, Kim JG (2006) Oxidation of various reactive dyes with in situ electro-generated active chlorine for textile dyeing industry wastewater treatment. J Hazard Mater B136:203–212
Rajkumar D, Palanivelu K (2004) Electrochemical treatment of industrial wastewater. J Hazard Mater B113:123–129
Rajkumar D, Song BJ, Kim JG (2007) Electrochemical degradation of Reactive Blue 19 in chloride medium for the treatment of textile dyeing wastewater with identification of intermediate compounds. Dyes Pigm 72:1–7
Sahu OP, Gupta V, Chaudhari PK, Srivastava VC (2015) Electrochemical treatment of actual sugar industry wastewater using aluminum electrode. Int J Environ Sci Technol 12:3519–3520
Sakalis A, Fytianos K, Nickel U, Voulgaropoulos A (2006) A comparative study of platinised titanium and niobe/synthetic diamond as anodes in the electrochemical treatment of textile wastewater. Chem Eng J 119:127–133
Shieh DT, Hwang BJ (1993) Morphology and electrochemical activity of Ru–Ti–Sn ternary-oxide electrodes in 1 M NaCl solution. Electrochim Acta 38:2239–2246
Soni BD, Ruparelia JP (2012a) Effects of preparation and methods on Ti/NiO–RuO2–SnO2–Sb2O5 anode for removal of reactive Black-5 Dye. J Environ Res Dev 8:1–12
Soni BD, Ruparelia JP (2012b) Studies on effects of electrodes for decontamination of dyes from wastewater. J Environ Res Dev 6:973–980
Soni BD, Patel UD, Agrawal A, Ruparelia JP (2017) Application of BDD and DSA electrodes for the removal of RB 5 in batch and continuous operation. J Water Process Eng 17:11–21
Szpyrkowicz L, Claudia J, Kaula SN (2001) A comparative study on oxidation of disperse dyes by electrochemical process, ozone, hypochlorite and Fenton reagent. Water Res 35:2129–2136
Szpyrkowicz L, Radaelli M, Daniele S (2005) Electro-catalysis of chlorine evolution on different materials and its influence on the performance of an electrochemical reactor for indirect oxidation of pollutants. Catal Today 100:425–429
Szpyrkowicz L, Juzzolino C, Kaul SN, Daniele S, De Faveri MD (2000) Electrochemical oxidation of dyeing baths bearing disperse dyes. Ind Eng Chem Res 39(9):3241–3248
Terezo AJ, Pereira EC (1999) Preparation and characterization of Ti/RuO2–Nb2O5 electrodes obtained by polymeric precursor method. Electrochim Acta 44:4507–4513
Vercesi GP, Rolewicz J, Comninellis C, Hinder J (1991) Characterization of DSA® type oxygen evolving electrodes. Choice of base metal. Thermochim Acta 176:31–47
Wang B, Kong W, Hongzhu M (2007) Electrochemical treatment of paper mill wastewater using three-dimensional electrodes with Ti/Co/SnO2–Sb2O5 anode. J Hazard Mater 146:295–301
Wu M, Zhao G, Li M, Liu L, Li D (2009) Applicability of boron-doped diamond electrode to the degradation of chloride-mediated and chloride-free wastewaters. J Hazard Mater 163:26–31
Zhou M, Särkkä H, Sillanpää M (2011) A comparative experimental study on methyl orange degradation by electrochemical oxidation on BDD and MMO electrodes. Sep Purif Technol 78:290–297
Acknowledgments
The financial assistance provided by the Department of Science and Technology (DST), Government of India, to Dr. Jayesh Ruparelia as Young Scientist under Fast Track Scheme (Award No. SR/FTP/ETA-028/2009) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Agnieszka Galuszka.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soni, B.D., Patel, U.D., Agrawal, A. et al. Electrochemical destruction of RB5 on Ti/PtOx–RuO2–SnO2–Sb2O5 electrodes: a comparison of two methods for electrode preparation. Int. J. Environ. Sci. Technol. 17, 903–916 (2020). https://doi.org/10.1007/s13762-019-02393-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-019-02393-5