Abstract
Background
Nemaline myopathy, the most common of the congenital myopathies, is caused by various genetic mutations. In this study, we attempted to investigate the clinical features, muscle pathology and genetic features of 15 patients with nemaline myopathy.
Results
Among the 15 patients, there were 9 (60.00%) males and 6 (40.00%) females, and 9 (60.00%) of them came from three families respectively. The age of seeing a doctor ranged from 9 to 52 years old, the age of onset was from 5 to 23 years old, and the duration of disease ranged from 3 to 35 years. Ten out of the 15 patients had high arched palate and elongated face. Only one patient had mild respiratory muscle involvement and none had dysphagia. Muscle biopsies were performed in 9 out of the 15 patients. Pathologically, muscle fibers of different sizes, atrophic muscle fibers and compensatory hypertrophic fibers could be found, and occasionally degenerated and necrotic muscle fibers were observed. Different degrees of nemaline bodies aggregation could be seen in all 9 patients. The distribution of type I and type II muscle fibers were significantly abnormal in patients with nemaline myopathy caused by NEB gene, however, it was basically normal in patients with nemaline myopathy caused by TPM3 gene and ACTA1 gene. Electron microscopic analysis of 6 patients showed that nemaline bodies aggregated between myofibrils were found in 5(83.33%) cases, and most of them were located near the Z band, but no intranuclear rods were found. The gene analysis of 15 NM patients showed that three NM-related genes were harbored, including 11 (73.33%) patients with NEB, 3 (20.00%) patients with TPM3, and 1 (6.67%) patient with ACTA1, respectively. A total of 12 mutation sites were identified and included 10 (83.33%) mutations in exon and 2(16.67%) mutations in intron.
Conclusions
The clinical phenotype of nemaline myopathy is highly heterogeneous. Muscle pathology shows that nemaline bodies aggregation is an important feature for the diagnosis of NM. NEB is the most frequent causative gene in this cohort. The splicing mutation, c.21522 + 3A > G may be the hotspot mutation of the NEB gene in Chinese NM patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Nemaline myopathy (NM) is one of the most common forms of congenital myopathy. NM is defined by the presence of rod-like structures in skeletal muscle fibers, which was first described by Shy and Conen in 1963 [1, 2]. The main clinical manifestations include hypotonia and muscle weakness, and respiratory muscles may be involved in severe cases. Some are accompanied by facial and bone deformities. Under the light microscope, dark blue rods can be found in fibers on skeletal muscle biopsy, which is the characteristic histologic feature of NM. Fourteen NM causative genes have been reported in the literature, including NEB, ACTA1, TPM3, TPM2, TNNT1, TNNT3, CFL2, KBTBD13, KLHL40, KLHL41, LMOD3, MYO18B, MYPN, RYR3 [3,4,5,6,7,8,9,10,11,12,13,14,15,16], among which NEB gene is the most commonly reported gene [17]. Recently, it has been reported that rods can be found in myopathy caused by the CAP2 gene [18]. Here, we systemically review the clinical, pathological and genetic characteristics of 15 patients with NM in our neuromuscular Center, and discuss the clinical phenotype of NM in Chinese population and the mutation characteristics of three common NM pathogenic genes, which are NEB, ACTA1 and TPM3.
Patients and methods
Patients
A total of 15 patients were included in the retrospective study. From January 1997 to January 2021, muscle biopsies were performed on patients with suspected myopathy in our neuromuscular Center, and 15 patients were diagnosed with NM according to the characteristic pathological features. Detailed clinical data were collected from 15 patients, which included gender, age at onset, clinical course, initial symptom, distribution and grade of muscle weakness, dysmorphic features, creatine kinase (CK), Electromyography (EMG) results and family history. Written informed consent was obtained from each patient, and this study was approved by the Medical Ethics Committee of Jiaozuo People's Hospital.
Pathological analysis
Open muscle biopsies were performed in 9 patients under local anesthesia in our cohort, which included quadriceps femoris muscle (n = 5), tibialis anterior muscle (n = 2) and gastrocnemius muscle (n = 2). A portion of the muscle specimens was fixed with liquid nitrogen and frozen into sections for routine histological and histochemical staining. The staining included hematoxylin and eosin (H&E), modified Gomori trichrome (MGT), Periodate Schiff reaction (PAS), oil red "O" (ORO), and adenosine triphosphatase (ATP) at varying pHs (pH = 4.2, 4.3, 10.3, 10.4) nicotinamide adenine dinucleotide tetrazolium reductase (NADH-TR). The stained slides were examined under the light microscope. The other portion of the muscle biopsy was fixed in 2.5% glutaraldehyde and osmium acid, dehydrated and embedded in plastic, and made ultrathin sections which were observed under the Transmission Electron Microscope (TEM).
Molecular analysis
Genetic testing was performed on 15 patients with the informed consent of the patients and their families. Genomic DNA was extracted from peripheral blood samples (n = 12) or muscle tissues (n = 3) from all patients using standard procedures, to screen genome-wide exon single-gene genetic disease, which was performed at the Genetic Testing Center.
Results
Clinical features
Among the 15 patients, there were 9 (60.00%) males and 6 (40.00%) females, of whom 9 (60.00%) came from three families, respectively. The age of seeing a doctor ranged from 9 to 52 years old, with a mean age of 31.53 ± 10.94 years the age of onset was ranged from 5 to 23 years old, with a mean age of 13.73 ± 4.33 years. The duration of disease ranged from 3 to 35 years, with a mean disease course of 17.80 ± 9.49 years. All patients presented with weakness of lower limbs mainly manifested as difficulty in squatting and standing up and weakness in foot dorsiflexion. Neurological examination showed normal cranial nerves, muscle tone of both upper and lower limbs was normal or decreased and deep tendon reflexes was reduced. The proximal muscle strength was ranged from 3/5 to 5/5, and the distal was ranged from 4/5 to 5/5. The muscle tone of both lower limbs was reduced and the tendon reflex at knee and ankle was absented. The proximal muscle strength was ranged from 2/5 to 4/5 and the distal was ranged from 2/5 to 5/5. Ten out of the 15 patients had high arched palate and elongated face. Only one patient had mild respiratory muscle involvement. The creatine kinase was normal or slightly elevated in all patients. Details of the clinical manifestations of the 15 patients were shown in Tables 1, 2.
Pathological features
Muscle biopsies were performed in 9 patients, and the muscle fiber sizes in 5 (55.56%) patients were significantly variable, while in 4 (44.44%) patients were slightly variable, and some fibers had nuclear inward migration (Fig. 1a). The eosinophilic substances assembled subsarcolemmal or in the center were observed in 4 (44.44%) patients (Fig. 1b). A few degenerated and necrotic fibers with a small amount of phagocyte infiltrating were occasionally observed in 3 (33.33%) patients. Dark stained rods were observed in muscle fibers of all 9 patients (Fig. 1c, d), and the involved myofibrillar structure was disarranged in 4 (44.44%) patients. ATPase staining indicated that the distribution of type I and type II fibers in patients with NM caused by NEB was abnormal, of which 5 (55.56%) patients were type I fiber predominance (Fig. 1e) and 2 (22.22%) patients were type II fiber predominance. The other 2 (22.22%) patients with NM caused by TPM3 and ACTA1 gene mutations respectively, had a normal checkerboard pattern of Type I and Type II fibers (Fig. 1f). Electron microscopic analysis showed that multiple focal myofibrillar structures disorganized, Z disc disappeared or disrupted, rods mostly located near the Z line accumulated in myofibril were found in 6 (66.67%) cases (Fig. 1g, h). No Intranuclear rods were found. Pathological changes in 9 patients with biopsies were shown in Table 3.
a HE staining showed that the muscle fibers were of different sizes, round or strip atrophic muscle fibers and compensatory hypertrophic muscle fibers (HE, × 400); b eosinophilic substance assembled in some muscle fibers, most of which were located under the sarcolemma (HE, × 400); c, d MGT staining showed that dark blue rod-shaped deposits (nemaline bodies) were observed in different muscle fibers (MGT, × 400); e ATP staining indicated that the distribution of typeI and typeII muscle fibers was abnormal, and type 1 fibers were significantly predominant, type II fibers were rare (ATPase10.3, × 200); f ATP staining revealed that type I and type II fibers were distributed in a mosaic pattern (ATPase10.3, × 200); g, h Electron microscopy showed many rods aggregated among subsarcolemmal and myofibril, and the rods were mostly located near Z line (EM lead-axis double staining, × 20,000)
Genetic analysis results
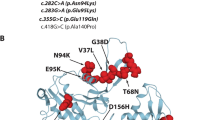
All 15 patients underwent genetic analysis and were identified to carry variants in one of three NM related genes including NEB (n = 11, 73.33%), ACTA1 (n = 1, 6.67%), TPM3 (n = 3, 20.00%). In total, 9 mutations were harbored in NEB genes of which 8 (88.89%) in exon and 1(11.11%) in the intron. The splicing mutation in the intron region carried by 10 patients were all c.21522 + 3A > G (Fig. 2). The other 8 mutations were in exon and included frameshift mutation c.1623delT in 3 (37.50%) patients, nonsense mutation c.17611C > T in 3(37.50%) patients, nonsense mutation c.4417C > T in 2 (25.00%) patients, which both had been reported previously. The remaining 6 mutations were novel, including c.17611C > T in 3 patients, and c.2549delA, c.3520G > A, c.21065dupA, c.20943G > A, c.192G > A, each in 1 patient respectively. We identified 2 novel mutations in 3 patients with TPM3, among which one was splicing mutation c.642 + 5G > A in the intron, the other was missense mutation c.*25G > A in exon. A de novo missense mutation c.745G > A in exon was identified in 1 patient with ACTA1.
Discussion
The clinical phenotype of NM has great heterogeneity. The proximal limb muscles are usually weak at onset, and the clinical symptoms progress slowly. Their enrollment was based on the following inclusion criteria [1, 2, 19]: (1) they clinically presented with muscle weakness,amyotrophy,hypotonia,delayed motor milestones and dysmorphic features; (2) muscle biopsy showed the presence of rods in muscle fibers after light and electron microscopy analysis, and no other characteristic pathological findings; (3) they did not have other diseases, such as muscular dystrophy, congenital myasthenic syndromes, or immuno-/metabolic myopathy. However, in recent years, some studies had reported the onset of distal muscle weakness, especially in NM with NEB gene mutation [20,21,22]. In our study, 2 patients with NEB gene mutation (Case1 and Case10) all developed distal limb weakness, manifested as dorsiextension weakness and inability to walk fast, and proximal limb muscle weakness gradually developed as the disease progressed, which was consistent with the report of Vilma-Lotta et al. [21]. Different from previous literature, this group of patients mainly had weakness in both lower limbs, and muscle weakness in both upper limbs gradually appeared 20 years after onset, and the clinical course progressed very slowly. And 10 out of 15 patients (66.7%) had arch palate and elongated faces, which indicates that the two are important features in NM patients.
The rod aggregates observed in muscle fibers in muscle fibers in muscle biopsy is an important pathological feature for the diagnosis of NM. MGT staining is the main staining method for the diagnosis of NM, and rod-shaped granular material stained purple and blue can be found in muscle fibers, which is the characteristic pathological change of NM. The rods mainly gather in subsarcolemmal and the center of muscle fibers, but can also be observed in normal and atrophic muscle fibers. The percentage of fibers containing rods was calculated more than 50% in 5/9 cases, and greater than 90% in 3/9 cases. The remaining four cases were 40%, 30%, 30% and 20%, respectively. None of the characteristic pathological changes of other muscle diseases were observed in any patients. The number of rods increased with age but there was no obvious correlation with the severity of disease. HE staining showed that dark red eosinophilic deposits mostly located under the sarcolemma could be seen in fibers of some patients, which were consistent with the positions of rods seen in fibers of MGT staining. ATP staining revealed that 7 out of 9 cases had abnormal distribution of fibers type, of which type I predominance in 5 cases and type II predominance in 2 cases. It also confirmed that most NM patients had aberrant distribution of fibers. However, the histology of patients with NM caused by TPM3 and ACTA1 indicated that type I and II fibers still presented mosaic distribution, and no obvious groupings were found, which suggested that NM caused by NEB gene was more prone to aberrant distribution and groupings of type I and II fibers. The mechanism of this phenomenon was still unclear. Electron microscopic analysis of 6 patients showed that rods mostly located near the Z band were observed in the myofibrils or subsarcolemmal in 5 cases. And this might be explained by the main component of the rods was α-actinin, which was the same as that of the Z line.
Until now, 14 genes have been reported to be related with NM. Among them, NEB is the most frequent and may account for up to 50% of cases [23]. NEB gene was identified in 11 out of 15 patients in our cohort, accounting for about 73%, which was consistent with previous reports [24]. The autosomal recessive mutation was most common in NEB, and it could also be sporadic, and autosomal dominant mutations was rare but have been reported [22]. The 11 patients with NEB mutation came from 7 families respectively, of which 6 patients were from two families. However, the 6 patients were all from the same generation, and the rest of their families were all healthy, which is consistent with autosomal recessive inheritance. The 11 NEB related NM patients were found to have compound heterozygous mutation. A total of 9 mutation sites were identified, including 8 mutations in exon and 1 mutation in the intron. In addition, the splicing mutation c.21522 + 3A > G in NEB, carried by 10 patients, was predicted to affect the splicing function of mRNA and lead to changes in amino acids, and it was classified as a pathogenic mutation [25]. At present, the mutation c.21522 + 3A > G has been reported in China and South Korea, which can cause childhood-onset NM [26, 27]. Yin et al. [28] found that c. 21522 + 3A > G was the most common mutation of NEB gene in a group of NM cases reported, which was consistent with our study. Wang et al. [29] reported in a study that the NEB gene mutation C.21417 + 3A > G firstly reported in South Korea may be a mutational hotspot in East Asian populations. Based on the results of this study, we consider that c.21522 + 3A > G may be another mutational hotspot of NEB gene in Chinese and East Asian populations.
The most common types of mutations observed in the NEB gene were missense, frameshift, and nonsense mutations, while the rarest types were the copy number and synonymous mutations [30]. There were 8 mutation sites in exons, including 3 frameshift mutations, 2 missense mutations, 1 nonsense mutations, 2 synonymous mutations, and no copy number variation was found. Except for one nonsense mutation c.4417C > T and one frameshift mutation c.1623delT, which both had been reported before [29, 30]. The remaining six mutation sites, c.17611C > T, c.2549delA, c.3520G > A, c.21065dupA, c.20943G > A and c.192G > A, have not been reported, so they should be novel variants of NEB gene.
ACTA1 gene mutation is the second most common one that causes NM. The majority of reported mutations are autosomal dominant and rare can be autosomal recessive [23]. The clinical manifestations of nemaline myopathy caused by ACTA1 gene mutations is often severe, more rarely, mutations in ACTA1 may cause the intermediate, mild, or typical Forms of nemaline myopathy [28, 31, 32]. The clinical subtype of the case in our cohort is mild, which is a rare one. A single heterozygous missense mutation, c.745G > A, in exon region of ACTA1 was harbored, which could result in disrupted protein structure and finally impaired muscle contraction. The patients showed no remarkable family history; hence, we also speculated that these were de novo mutations.
Mutations in TPM3 account for only a small percentage of patients with NM, which have been associated with both autosomal dominant NM, and rarely with autosomal recessive NM [33]. We identified 2 novel heterozygous mutations in 3 patients with TPM3 mutations that came from the same family. One was c.642 + 5G > A, a splice mutation in the intron region, which was predicted to affect the splicing function of mRNA and lead to changes in amino acids. Another was c.*25G > A, a missense mutation in the exon region, which caused amino acid alterations. The two mutations in TPM3 could be rated as meaningless mutations (VUS) according to the ACMG guidelines.
Conclusions
In conclusion, the clinical phenotype of NM is highly heterogeneous, and the age of onset in NM is mostly in childhood, with proximal limb muscle weakness as the main clinical manifestation. Some patients first present with distal lower extremity weakness that gradually progress to involve the proximal muscle. The rod aggregates observed in muscle biopsy is an important pathological feature ©for the diagnosis of NM. NEB is the most common NM causative gene in the present cohort of Chinese patients, and splicing mutation c.21522+3A>G in NEB is the most frequently found mutation in our patients. This is a novel finding and may indicate a new hotspot mutation in Chinese NM patients.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shy GM, Engel WK, Somers JE, Wanko T (1963) Nemaline myopathy. A new congenital myopathy. Brain 86:793–810. https://doi.org/10.1093/brain/86.4.793
Chue WL, Murphy EG, Donohue WL (1963) Light and electron microscopic studies of ‘myogranules’ in a child with hypotonia and muscle weakness. Can Med Assoc J 89:983–986
Pelin K, Hilpelä P, Donner K et al (1999) Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci USA 96:2305–2310. https://doi.org/10.1073/pnas.96.5.2305
Nowak KJ, Wattanasirichaigoon D, Goebel HH et al (1999) Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet 23:208–212. https://doi.org/10.1038/13837
Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, Blumbergs P, White S, Watkins H, Love DR (1995) A mutation in the alphatropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat Genet 9:75–79. https://doi.org/10.1038/ng0195-75
Donner K, Ollikainen M, Ridanpää M, Christen HJ, Goebel HH, de Visser M, Pelin K, Wallgren-Pettersson C (2002) Mutations in the betatropomyosin (TPM2) gene-a rare cause of nemaline myopathy. Neuromuscul Disord 12:151–158. https://doi.org/10.1016/s0960-8966(01)00252-8
Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, Koch T, Schäffer AA, Francomano CA, Biesecker LG (2000) A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet 67:814–821. https://doi.org/10.1086/303089
Agrawal PB, Greenleaf RS, Tomczak KK, Lehtokari VL, Wallgren-Pettersson C, Wallefeld W, Laing NG, Darras BT, Maciver SK, Dormitzer PR, Beggs AH (2007) Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am J Hum Genet 80:162–167. https://doi.org/10.1086/510402
Sambuughin N, Yau KS, Olivé M et al (2010) Dominant mutations in KBTBD13, a member of the BTB/Kelch family, cause nemaline myopathy with cores. Am J Hum Genet 87:842–847. https://doi.org/10.1016/j.ajhg.2010.10.020
Ravenscroft G, Miyatake S, Lehtokari VL et al (2013) Mutations in KLHL40 are a frequent cause of severe autosomal-recessive nemaline myopathy. Am J Hum Genet 93:6–18. https://doi.org/10.1016/j.ajhg.2013.05.004
Gupta VA, Ravenscroft G, Shaheen R et al (2013) Identification of KLHL41 mutations implicates BTB-Kelch-mediated ubiquitination as an alternate pathway to myofibrillar disruption in nemaline myopathy. Am J Hum Genet 93:1108–1117. https://doi.org/10.1016/j.ajhg.2013.10.020
Yuen M, Sandaradura SA, Dowling JJ et al (2014) Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy. J Clin Invest 124:4693–4708. https://doi.org/10.1172/JCI75199
Malfatti E, Böhm J, Lacène E, Beuvin M, Romero NB, Laporte J (2015) A premature stop codon in MYO18B is associated with severe nemaline myopathy with cardiomyopathy. J Neuromuscul Dis 2:219–227. https://doi.org/10.3233/JND-150085
Miyatake S, Mitsuhashi S, Hayashi YK et al (2017) Biallelic mutations in MYPN, encoding myopalladin, are associated with childhood-onset, slowly progressive nemaline myopathy. Am J Hum Genet 100:169–178. https://doi.org/10.1016/j.ajhg.2016.11.017
Sandaradura SA, Bournazos A, Mallawaarachchi A et al (2018) Nemaline myopathy and distal arthrogryposis associated with an autosomal recessive TNNT3 splice variant. Hum Mutat 39:383–388. https://doi.org/10.1002/humu.23385
Nilipour Y, Nafissi S, Tjust AE et al (2018) Ryanodine receptor type 3 (RYR3) as a novel gene associated with a myopathy with nemaline bodies. Eur J Neurol 25:841–847. https://doi.org/10.1111/ene.13607
Sewry CA, Laitila JM, Wallgren-Pettersson C (2019) Nemaline myopathies: a current view. J Muscle Res Cell Motil 40:111–126. https://doi.org/10.1007/s10974-019-09519-9
Gurunathan S, Sebastian J, Baker J, Abdel-Hamid HZ, West SC, Feingold B, Peche V, Reyes-Múgica M, Madan-Khetarpal S, Field J (2022) A homozygous CAP2 pathogenic variant in a neonate presenting with rapidly progressive cardiomyopathy and nemaline rods. Am J Med Genet A 188:970–977. https://doi.org/10.1002/ajmg.a.62590
Laitila J, Wallgren-Pettersson C (2021) Recent advances in nemaline myopathy. Neuromuscul Disord 31:955–967. https://doi.org/10.1016/j.nmd.2021.07.012
Wallgren-Pettersson C, Lehtokari VL, Kalimo H, Paetau A, Nuutinen E, Hackman P, Sewry C, Pelin K, Udd B (2007) Distal myopathy caused by homozygous missense mutations in the nebulin gene. Brain 130:1465–1476. https://doi.org/10.1093/brain/awm094
Lehtokari VL, Pelin K, Herczegfalvi A et al (2011) Nemaline myopathy caused by mutations in the nebulin gene may present as a distalmyopathy. Neuromuscul Disord 21:556–562. https://doi.org/10.1016/j.nmd.2011.05.012
Kiiski KJ, Lehtokari VL, Vihola AK et al (2019) Dominantly inherited distal nemaline/cap myopathy caused by a large deletion in the nebulin gene. Neuromuscul Disord 29:97–107. https://doi.org/10.1016/j.nmd.2018.12.007
Malfatti E, Romero NB (2016) Nemaline myopathies: state of the art. Rev Neurol (Paris) 172:614–619. https://doi.org/10.1016/j.neurol.2016.08.004
Ottenheijm CA, Hooijman P, DeChene ET, Stienen GJ, Beggs AH, Granzier H (2010) Altered myofilament function depresses force generation in patients with nebulin-based nemaline myopathy (NEM2). J Struct Biol 170:334–343. https://doi.org/10.1016/j.jsb.2009.11.013
Bean LJH, Funke B, Carlston CM, Gannon JL, Kantarci S, Krock BL, Zhang S, Bayrak-Toydemir P, ACMG Laboratory Quality Assurance Committee (2020) Diagnostic gene sequencing panels: from design to report-a technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet Med 22:453–461. https://doi.org/10.1038/s41436-019-0666-z
Wen Q, Chang X, Guo J (2020) A childhood-onset nemaline myopathy caused by novel heterozygote variants in the nebulin gene with literature review. Acta Neurol Belg 120:1351–1360. https://doi.org/10.1007/s13760-019-01230-3
Lee JM, Lim JG, Shin JH, Park YE, Kim DS (2017) Clinical and genetic diversity of nemaline myopathy from a single neuromuscular center in Korea. J Neurol Sci 383:61–68. https://doi.org/10.1016/j.jns.2017.10.020
Yin X, Pu C, Wang Z, Li K, Wang H (2022) Clinico-pathological features and mutational spectrum of 16 nemaline myopathy patients from a Chinese neuromuscular center. Acta Neurol Belg 122:631–639. https://doi.org/10.1007/s13760-020-01542-9
Wang Q, Hu Z, Chang X, Yu M, Xie Z, Lv H, Zhang W, Xiong H, Yuan Y, Wang Z (2020) Mutational and clinical spectrum in a cohort of Chinese patients with hereditary nemaline myopathy. Clin Genet 97:878–889. https://doi.org/10.1111/cge.13745
Lehtokari VL, Kiiski K, Sandaradura SA et al (2014) Mutation update: the spectra of nebulin variants and associated myopathies. Hum Mutat 35:1418–1426. https://doi.org/10.1002/humu.22693
Moreno CAM, Abath Neto O, Donkervoort S, Hu Y, Reed UC, Oliveira ASB, Bönnemann C, Zanoteli E (2017) Clinical and histologic findings in ACTA1-related nemaline myopathy: case series and review of the literature. Pediatr Neurol 75:11–16. https://doi.org/10.1016/j.pediatrneurol.2017.04.002
Jungbluth H, Sewry CA, Brown SC, Nowak KJ, Laing NG, Wallgren-Pettersson C, Pelin K, Manzur AY, Mercuri E, Dubowitz V, Muntoni F (2001) Mild phenotype of nemaline myopathy with sleep hypoventilation due to a mutation in the skeletal muscle alpha-actin (ACTA1) gene. Neuromuscul Disord 11:35–40. https://doi.org/10.1016/s0960-8966(00)00167-x
Wallgren-Pettersson C, Sewry CA, Nowak KJ, Laing NG (2011) Nemaline myopathies. Semin Pediatr Neurol 18:230–238. https://doi.org/10.1016/j.spen.2011.10.004
Acknowledgements
We are grateful to the patients for their participation.
Funding
This study had no funding sources.
Author information
Authors and Affiliations
Contributions
LH and LY contributed equally to this work. QQ participated in the design of the study. CPZX, and QQ collected data and all authors were involved in analysis and interpretation of the data. LH drafted the manuscript. Liu Yin helped to draft the manuscript. MX, LZ, CW, and ZY revised it critically for important intellectual content. The corresponding author attests that all listed authors meet authorship criteria and no others meeting the criteria have been omitted. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
This study was approved by the ethics committee of the Jiaozuo People’s Hospital of Henan Province, Henan, China. Written informed consent was obtained from all participants for the publication of data and any accompanying images.
Consent for publication
All authors agreed on the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haidong, L., Yin, L., Ping, C. et al. Clinico-pathological and gene features of 15 nemaline myopathy patients from a single Chinese neuromuscular center. Acta Neurol Belg 124, 91–99 (2024). https://doi.org/10.1007/s13760-023-02333-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-023-02333-8