Abstract
Background
The potential impact of insulin resistance on stroke prognosis after IV thrombolysis is poorly understood. This study aimed to assess the effect of insulin resistance and metabolic syndrome on the outcome of IV thrombolysis in non-diabetic patients with acute ischaemic stroke.
Methods
This prospective observational study was conducted on 70 non-diabetic acute ischaemic stroke patients who received rt-PA within 3 h of stroke onset. Patients were subjected to baseline and follow-up NIHSS measurements at 24 h and 3 months post-treatment. Stroke outcome was assessed after 3 months using the Modified Rankin Scale (mRS). The homeostasis model assessment–insulin resistance (HOMA-IR) was calculated for the included patients at stroke onset.
Results
The mean age of included patients was 57.04 ± 14.39 years. Patients with unfavourable outcome had a significantly higher frequency of insulin resistance and metabolic syndrome, higher values of baseline NIHSS, insulin, HOMA-IR, uric acid and lower levels of HDL than those with favourable outcome (P value = 0.035, 0.007, ≤ 0.001, 0.001, ≤ 0.001, 0.002, 0.033, respectively). Each point increase in NIHSS before rt-PA increased the odds of an unfavourable outcome by 2.06 times (95% CI 1.22 − 3.478). Also, insulin resistance increased the odds of the unfavourable outcome by 11.046 times (95% CI 1.394–87.518). There was a statistically significant improvement in NIHSS 3 months after receiving rt-PA in all patients, significantly higher in patients who did not have insulin resistance or metabolic syndrome.
Conclusion
Insulin resistance and metabolic syndrome were associated with worse functional outcomes in non-diabetic stroke patients after receiving rt-PA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the main leading cause of mortality and disability in adults [1]. Nowadays, intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) is the primary reperfusion therapy with proven efficacy for managing acute ischaemic stroke, saving cerebral ischaemic tissue and reducing neurologic sequelae [2]. Unfortunately, the functional outcome after rt-PA thrombolysis is not always optimal, as some patients may still have some neurological deficits.
Insulin resistance (IR) is recognized as an impaired response of target tissues to insulin. Insulin resistance impairs glucose metabolism, leading to a compensatory increase in insulin production and hyperinsulinemia and may subsequently lead to metabolic syndrome [3].
Both insulin resistance and diabetes may lead to a prothrombotic and proinflammatory state through impairment of endogenous fibrinolysis and increased platelet activation, explaining their known association with a higher incidence of stroke [4].
Several tools exist for the quantitative assessment of insulin resistance. The homeostasis model assessment-insulin resistance (HOMA-IR) serves as a reliable method of estimating insulin resistance widely used in clinical studies [5].
While previous studies have confirmed the association between diabetes and poor functional outcome of stroke after thrombolysis [6, 7], studies regarding insulin resistance in this area are scarce [8, 9].
This study investigates the effect of insulin resistance and metabolic syndrome on the outcome of IV thrombolysis in non-diabetic patients with acute ischaemic stroke.
Methods
This prospective observational study was carried out at the Stroke Unit of Beni-Suief University Hospital from January 2021 to December 2021. We included 70 patients presenting with acute ischaemic stroke who were in the therapeutic window for treatment with rt-PA (within the first 3 h) and had no contraindication for rt-PA injection according to guidelines set by The American Heart Association/American Stroke Association [10]. The included patients should be over ≥ 18 years.
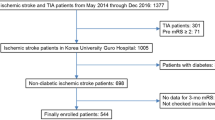
Diabetic patients whose diagnosis was made by either fasting blood glucose (FBG) levels ≥ 126 mg/dL or 2-h plasma glucose ≥ 200 mg/dL or HbA1c ≥ 6.5% [11], patients with premorbid Modified Rankin Scale (MRS) ≥ 2, patients who underwent mechanical thrombectomy were excluded. Also, patients on drugs known to affect the lipid profile or insulin sensitivity were excluded (statins, fibrates, steroids, oral contraceptives, antipsychotics, B blockers, and thiazide diuretics). A flow diagram for the included and the excluded patients was illustrated in Fig. 1
Study measures
Demographic information and stroke risk factors evident by history and supported by appropriate investigations were collected. Patients were coded with substance use disorder (SUD) if they were fulfilling the criteria of the Diagnostic and statistical manual of mental disorders (DSM-V) [12]. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Waist circumference (to the nearest 0.5 cm) was measured using a plastic tape measure midway between the last rib and the iliac crest at the end of normal expiration.
The stroke subtype was determined according to the Org 10,172 trial in the acute stroke treatment (TOAST) categories [13]. The National Institute of Health Stroke Scale (NIHSS) [14] was recorded for the included patients at stroke onset and 24 h post-treatment.
Size of the infarction was measured by the pure ellipsoid model of ABC/2. It was found by Sims et al. to be the best model for a rapid and accurate clinical estimation of stroke volume [15]. In addition to the initial brain imaging, computerized tomography (CT) scan of the brain was performed 24 h following the treatment (and if there was any deterioration in NIHSS) to assess the occurrence of symptomatic intracerebral haemorrhage (sICH). In line with the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria [16], sICH is defined as a neurological deterioration of ≥ 4 points on the NIHSS from baseline within 24 h combined with haemorrhage on follow-up by neuroimaging.
According to the definition of metabolic syndrome provided by the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) report [17], three or more of the following were required to diagnose metabolic syndrome in the included patients: (1) FBG ≥ 100 mg/dL; (2) elevated blood pressure (≥ 130/80 mmHg) (3) HDL cholesterol of < 40 mg/dL for men and < 50 mg/dL for women; (4) triglycerides > 150 mg/dL, and (5) a waist circumference of > 90 cm for men and > 80 cm for women.
Laboratory work-up
-
High-density lipoprotein cholesterol (HDL-C) and Triglycerides (TGs) levels.
-
Fasting glucose.
-
Fasting insulin
-
HOMA-IR was calculated based on the following equation: fasting insulin (μU/mL) × fasting glucose (mmol/L)/22.5 [18]. The state of insulin resistance was established if the HOMA-IR value > 2.5 [19].
Serum samples venous blood samples of 4 mL were withdrawn within 24 h from stroke onset. Samples were allowed to clot for 1 h at room temperature before centrifugation for 15 min at 3000×g. Serum was separated, and samples were divided. One part was sent for chemical analysis (triglycerides, total cholesterol, HDL), and 2nd part was stored at – 80 °C until the end of the study to measure serum fasting insulin by quantitative sandwich enzyme immunoassay technique ELISA kit Thermo Fisher.
Study outcome
According to the hospital policy, patients were re-evaluated by NIHSS and the Modified Rankin Scale (mRS) score during a face-to-face interview 90 days following stroke. A favourable outcome at 90 days following stroke is defined as mRS scores of 0–2, whereas scores of 3–6 define the unfavourable outcome [20].
Sample size calculation
The sample size was calculated using Epi calc 2000, version 1.02, 1997. Based on an alpha level of significance of 0.05, and 40.3% prevalence rate of metabolic syndrome in stroke patients with good outcome after IV thrombolysis and 88.7% prevalence rate in those with poor outcome after IV thrombolysis [21], a total sample size of at least 60 patients was required to achieve a statistical power of 99%.
Ethical statement
Written informed consent was obtained from each patient or their first-degree relatives. The study was performed in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committees of the Beni-Suef University; the approval number was FWA00015574.
Statistical analysis
IBM SPSS (Statistical Package of Social Science) Version 25 was used to analyze the data. Categorical variables were expressed as numbers and percentages. Normally distributed quantitative variables were expressed as mean and standard deviation (SD), while non-normally distributed variables were expressed as the median and interquartile range (IQR). Chi-squared test was used for comparison between patients with favourable and unfavourable outcome in categorical variables. The Independent sample t test was used to compare the two groups in normally distributed quantitative variables. In contrast, the Mann–Whitney test was used to compare them in non-normally distributed quantitative variables. A mixed ANOVA test was used to compare NIHSS before and 3 months after thrombolytic therapy in patients with and without metabolic syndrome and in those with and without insulin resistance. A binary logistic regression model was done to identify predictors of poor outcome from thrombolytic therapy after being adjusted for their potential mutual confounding effect. P value ≤ 0.05 was considered statistically significant. All tests were two-tailed.
Results
Demographics, clinical and laboratory characteristics of the included patients
This prospective observational study was conducted on 70 acute ischaemic stroke patients eligible for rt-PA treatment (45 males and 25 females). The mean age of the included patients was 57.04 ± 14.39 years. The mean BMI and waist circumference values in cm were 29.75 ± 4.66 and 94.67 ± 12.48, respectively. Regarding stroke risk factors, 72.9% of the included patients were hypertensive, 47.1% had AF, 44.3% were smokers, 8.6% were drug abusers, and 25.7% had a family history of stroke.
Regarding stroke subtype, cardio-embolic stroke represented the most frequent subtype that was reported in 45.7% of the whole study population, followed by small artery disease (34.3%), large artery disease (12.9%), other determined (4.3%), and lastly undetermined (2.9%).
The median value for door-to-needle time (DTN) was 32.5 min with IQR (30–45). The mean value for NIHSS before rt-PA was 13.61 ± 4.44; after 24 h was 11.24 ± 5.46; and after 3 months was 6.11 ± 4.83. The median value for infarction size was 9.365 cm3 with IQR (4.22–19.57).
After receiving rt-PA, eight patients (11.4%) experienced complications (5 patients had reperfusion injury and three patients had haemorrhagic transformation). At the 3-month follow-up, 47.1% of the included patients had a favourable outcome, while 52.9% had an unfavourable outcome. Forty percent of patients had insulin resistance, and 57.1% had metabolic syndrome (Table 1).
Outcome from thrombolytic therapy (assessed by mRS)
The group of patients with unfavourable outcome (n = 37) had a significantly higher frequency of males and smokers, higher mean values of baseline NIHSS, FBS, insulin, HOMA, uric acid, and lower mean values of HDL than the group of patients with favourable outcome (n = 33) (P value = 0.035, 0.007, ≤ 0.001, ≤ 0.001, 0.001, ≤ 0.001, 0.002, 0.033, respectively). The frequency of both insulin resistance and metabolic syndrome was significantly higher in patients with unfavourable outcomes than in those with favourable outcomes (P value ≤ 0.001, 0.019, respectively) (Table 2).
A binary logistic regression model was done to identify the predictors of unfavourable outcome from thrombolytic therapy. Sex, smoking, NIHSS before rt-PA, HDL, uric acid, insulin resistance and metabolic syndrome were used as the independent variables.
Each point increase in NIHSS before rt-PA increased the odds of an unfavourable outcome by 2.06 times (95% CI 1.22 − 3.478). Also, insulin resistance increased the odds of the unfavourable outcome by 11.046 times (95% CI 1.394–87.518) (Table 3).
Impact of insulin resistance and metabolic syndrome on the outcome from thrombolytic therapy (assessed by NIHSS)
There was a statistically significant improvement in the scores of NIHSS 3 months after receiving rt-PA in all included patients, whether they had insulin resistance or metabolic syndrome or not (P value ≤ 0.001 in all parameters). Such improvement was significantly higher in patients who didn’t have insulin resistance or metabolic syndrome (P value = 0.046, 0.005, respectively) (Table 4).
Impact of insulin resistance and metabolic syndrome on the occurrence of complications from thrombolytic therapy
Patients with metabolic syndrome had a significantly higher incidence of complications from rt-PA than those without (P value = 0.009). In contrast, there was no statistically significant difference between patients with and without insulin resistance regarding the incidence of complications (P value = 0.878) (Table 5).
Discussion
It has long been recognized that hyperglycaemia in acute ischaemic stroke patients is associated with unfavourable functional outcomes [22]. Therefore, stroke treatment guidelines [10] recommend the normalization of blood glucose levels. Yet, no recommendation has been made regarding insulin resistance.
This study emphasizes the negative effect of insulin resistance on functional outcome and dependency in stroke patients who received rt-PA (OR 11.046, 95% CI 1.394–87.518). Many studies showed significantly more favourable outcomes in patients with acute ischaemic stroke receiving rt-PA within 3 h than between 3 and 4.5 h of symptoms onset [23, 24]. For the purpose of eliminating possible confounding factors as much as possible, we excluded patients with acute ischaemic stroke who received rt-PA in the extended window (3–4.5 h).
Insulin resistance may promote various conditions that could worsen the response to intravenous thrombolysis and stroke outcome, such as defective endogenous fibrinolysis, increased platelet activation, endothelial dysfunction, and a chronic proinflammatory state [25]. Moreover, impaired synaptic plasticity, excessive reactive oxygen species and mitochondrial dysfunction mediated by insulin resistance may interfere with functional recovery from the stroke insult [26].
Similar work conducted by Yang, Li [9] showed that insulin resistance was significantly associated with a high probability of haemorrhagic transformation as well as a poor functional outcome at 90 days in non-diabetic ischaemic stroke patients treated with intravenous thrombolysis. Yet, the authors did not investigate the effect of metabolic syndrome or its components on the stroke outcome.
The current study found that improvement of NIHSS 3 months after receiving rt-PA was significantly higher in patients without metabolic syndrome than in those with metabolic syndrome. Furthermore, patients with metabolic syndrome had a significantly higher frequency of complications from rt-PA than those without metabolic syndrome. It is well established that the state of metabolic syndrome is characterized by defective endogenous fibrinolysis with an enhancement of fibrinolysis inhibitors like plasminogen activator inhibitor-1 (PAI-1) [27], which may account for decreased efficacy of clot lysis after rt-PA administration. It should be noted that metabolic syndrome represents a fundamental risk factor not only for atherosclerosis but also for cardioembolic strokes. It has been found that chronic hypertension, dyslipidaemia and elevated leptin levels promote atrial fibrosis and angiotensin II-evoked AF [28].
On studying the other components of metabolic syndrome individually in relation to poor functional outcome, the only factor significantly associated with the unfavourable outcome was lower mean values of HDL. This can be explained by the fact that HDL has well-known action of reverse cholesterol transport in which HDL transports cholesterol from the arterial wall to the liver for excretion. Moreover, it has an anti-inflammatory, antioxidant and endothelial protective action. HDL may also reduce the thrombotic risk by inhibiting platelet functions and improving endothelial function by stimulating the release of the prostacyclin [29, 30].
On the other hand, other components of the metabolic syndrome, including serum triglycerides, hypertension, and waist circumference, were not significantly associated with the unfavourable outcome after intravenous thrombolysis, contrary to the previous studies [31,32,33].
Another significant independent predictor of unfavourable stroke outcome found in this study that has to be mentioned is higher baseline NIHSS, which is in line with previous studies [34,35,36,37]. The current study showed that each point increase in NIHSS before rt-PA administration nearly doubles the odds of an unfavourable outcome (OR 2.06, 95% CI 1.22 − 3.478).
Finally, it should be noted that the main limitation of our study is that we did not investigate the effect of insulin resistance and metabolic syndrome in relation to symptomatic cerebral haemorrhage after treatment with rt-PA due to the limited number of patients who developed this complication.
Conclusion
Insulin resistance and metabolic syndrome were associated with worse functional outcomes in non-diabetic stroke patients after receiving IV thrombolysis. These findings may strengthen the importance of screening and proper management of insulin resistance and metabolic syndrome in patients at risk of acute ischaemic stroke.
Availability of data and materials
Authors report that the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8:355–369
Tsivgoulis G, Katsanos AH, Alexandrov AV (2014) Reperfusion therapies of acute ischemic stroke: potentials and failures. Front Neurol 5:215
Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26:19–39
Alessi MC, Juhan-Vague I (2008) Metabolic syndrome, haemostasis and thrombosis. Thromb Haemost 99:995–1000
Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A (2015) Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab 19:160–164
Desilles J-P, Meseguer E, Labreuche J, Lapergue B, Sirimarco G, Gonzalez-Valcarcel J et al (2013) Diabetes mellitus, admission glucose, and outcomes after stroke thrombolysis. Stroke 44:1915–1923
Pajo AT, Diestro JDB (2020) Thrombolysis outcomes in patients with diabetes and previous stroke: a meta-analysis. Can J Neurol Sci 47:486–493
Calleja AI, García-Bermejo P, Cortijo E, Bustamante R, Rojo Martínez E, González Sarmiento E et al (2011) Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care 34:2413–2417
Yang X, Li C, Li J, Hou D, Luo Y, Zhang S et al (2021) Insulin resistance is significantly related with worse clinical outcomes in non-diabetic acute ischemic stroke patients treated with intravenous thrombolysis. J Stroke Cerebrovasc Dis 30:105526
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K et al (2019) Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 50:e344–e418
American Diabetes Association (2011) Diagnosis and classification of diabetes mellitus. Diabetes Care 34(Suppl 1):S62–S69
American Psychiatric Association Group (2013) Diagnostic and statistical manual of mental disorders, 5th edn. American Psychiatric Association, Arlington
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL et al (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24:35–41
National Institute of Neurological Disorders and Stroke (U.S.) (2011) NIH stroke scale. Dept of Health and Human Services, USA
Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH et al (2009) ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 72:2104–2110
Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W et al (2007) Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet (London, England) 369:275–282
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 106(25):3143–3421
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Owei I, Umekwe N, Provo C, Wan J, Dagogo-Jack S (2017) Insulin-sensitive and insulin-resistant obese and non-obese phenotypes: role in prediction of incident pre-diabetes in a longitudinal biracial cohort. BMJ Open Diabetes Res Care 5:e000415
Banks JL, Marotta CA (2007) Outcomes validity and reliability of the modified ranking scale: implications for stroke clinical trials. Stroke 38:1091–1096
Arenillas JF, Sandoval P, de la Ossa NP, Millán M, Guerrero C, Escudero D et al (2009) The metabolic syndrome is associated with a higher resistance to intravenous thrombolysis for acute ischemic stroke in women than in men. Stroke 40:344–349
Lindsberg PJ, Roine RO (2004) Hyperglycemia in acute stroke. Stroke 35:363–364
Lansberg MG, Bluhmki E, Thijs VN (2009) Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: a metaanalysis. Stroke 40:2438–2441
Cheng NT, Kim AS (2015) Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist 5:101–109
Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C (2018) Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol 17:122
Chang Y, Kim CK, Kim MK, Seo WK, Oh K (2021) Insulin resistance is associated with poor functional outcome after acute ischemic stroke in non-diabetic patients. Sci Rep 11:1229
Russo I (2012) The prothrombotic tendency in metabolic syndrome: focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica (Cairo) 2012:525374
Szczepańska-Szerej A, Kurzepa J, Grabarska A, Bielewicz J, Wlizło-Dyś E, Rejdak K (2017) Correlation between CH(2)DS(2)-VASc score and serum leptin levels in cardioembolic stroke patients: the impact of metabolic syndrome. Int J Endocrinol 2017:7503763
Sanossian N, Saver JL, Navab M, Ovbiagele B (2007) High-density lipoprotein cholesterol: an emerging target for stroke treatment. Stroke 38:1104–1109
Podrez EA (2010) Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clin Exp Pharmacol Physiol 37:719–725
Jung M-H, Yi S-W, An SJ, Yi J-J, Ihm S-H, Han S et al (2022) Associations between the triglyceride-glucose index and cardiovascular disease in over 150,000 cancer survivors: a population-based cohort study. Cardiovasc Diabetol 21:52
Kim JH, Choi KH, Kang KW, Kim JT, Choi SM, Lee SH et al (2019) Impact of visceral adipose tissue on clinical outcomes after acute ischemic stroke. Stroke 50:448–454
Tork MA, Aref HM, El-Khawas HM, Khalil MF, ElSadek A (2020) Outcome predictors of intravenous thrombolytic therapy in acute ischemic stroke patients: an Egyptian center experiences. Egypt J Neurol Psychiatry Neurosurg 56:103
Ali SF, Siddiqui K, Ay H, Silverman S, Singhal A, Viswanathan A et al (2016) Baseline predictors of poor outcome in patients too good to treat with intravenous thrombolysis. Stroke 47:2986–2992
Bhardwaj A, Sharma G, Raina SK, Sharma A, Angra M (2017) Advanced age and higher national institutes of health stroke scale score as predictors of poor outcome in ischemic stroke patients treated with alteplase: a study from a tertiary care centre in rural North-west India. J Neurosci Rural Pract 8:236–240
Mehrpour M, Afrakhte M, Shojaei SF, Sohrabi A, Ashayeri R, Esmaeili S et al (2019) Factors predicting the outcome of intravenous thrombolysis in stroke patients before rt-PA administration. Caspian J Intern Med 10:424–430
Wouters A, Nysten C, Thijs V, Lemmens R (2018) Prediction of outcome in patients with acute ischemic stroke based on initial severity and improvement in the first 24 h. Front Neurol 9:308
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors did not receive any funding for this work.
Author information
Authors and Affiliations
Contributions
MA participated in the study design and collection of data. MH participated in study design, analysis and interpretation of data, and helped to draft manuscript. RM participated in interpretation of data and helped to draft manuscript. AK participated in study design and collection of data. AO participated in collection of data and helped to draft manuscript. SA participated in doing the laboratory work up and helped to draft manuscript. WO participated in interpretation of data and helped to draft manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Ethical approvals
Written informed consent was obtained from each patient or their relatives. The study was performed in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committees of the Beni Suief University; the approval number was FWA00015574.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, M., Hussein, M., Magdy, R. et al. The potential role of insulin resistance in predicting outcome from intravenous thrombolytic therapy. Acta Neurol Belg 123, 885–892 (2023). https://doi.org/10.1007/s13760-022-02060-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-02060-6