Abstract
The related neurologic complications of SARS-CoV-2 infection in COVID-19 patients and survivors comprise symptoms including depression, anxiety, muscle pain, dizziness, headaches, fatigue, and anosmia/hyposmia that may continue for months. Recent studies have been demonstrated that chemokines have brain-specific attraction and effects such as chemotaxis, cell adhesion, modulation of neuroendocrine functions, and neuroinflammation. CCL11 is a member of the eotaxin family that is chemotactic agents for eosinophils and participate in innate immunity. Eotaxins may exert physiological and pathological functions in the central nerve system, and CCL11 may induce neuronal cytotoxicity effects by inducing the production of reactive oxygen species (ROS) in microglia cells. Plasma levels of CCL11 elevated in neuroinflammation and neurodegenerative disorders. COVID-19 patients display elevations in CCL11 levels. As CCL11 plays roles in physiosomatic and neuroinflammation, analyzing the level of this chemokine in COVID-19 patients during hospitalization and to predicting post-COVID-19-related neurologic complications may be worthwhile. Moreover, using chemokine modulators may be helpful in lessening the neurologic complications in such patients.
Similar content being viewed by others
Introduction
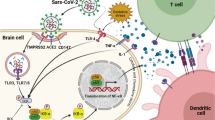
Neurological complications in previous coronavirus epidemics, severe acute respiratory syndrome coronavirus (SARS) and Middle East respiratory syndrome coronavirus (MERS) infections, were demonstrated in a high prevalence [1, 2]. Different forms of myopathy and prolonged muscle weakness have been reported among survivors of SARS-CoV-1 infection [3]. SARS complications related to involvement of the nervous system and the related effects on mood like chronic fatigue, even years after the initiation of infection, was reported in patients of previous SARS epidemics [4]. Many records of prolonged neurological-related complications such as symptoms of varying degrees of depression, sleep impairment, anxiety, headaches, dizziness, muscle pain, fatigue, and anosmia/hyposmia, myopathies that sometimes continuing for were reported in COVID-19 patients [5,6,7,8]. Chemokines as biomarkers that involved in psychiatric disorders can be used as targets for treatment of depressive disorders. Elevated levels of serum chemokines in major bipolar disorder, depressive disorder, and schizophrenia have previously been reported [9]. Increased levels of CCL11 in serum and lung tissue have been demonstrated in COVID-19 patients, in spite of eosinopenia [9, 10]. Although some reports demonstrated the increase and activation of peripheral eosinophils in severe cases of COVID-19 but the role of eosinophils in COVID-19 inflammation still remains obscure [11,12,13]. CCL11 leads to the degranulation of human eosinophils and release of eosinophil-derived neurotoxin (EDN) which could potentially clarify about the eosinopenia and secondary elevation of eosinophil-related granule contents [9, 14,15,16]. As physiosomatic symptoms are related to eotaxins, and especially CCL11, eotaxin-1 may play a role in COVID-19 neurologic syndrome.
Chemokines
The chemokine family consists of low molecular weight cytokines (7–12 kDa) that involved in the direct chemotaxis, leukocytes trafficking migration, inflammatory responses and immune system functions [17,18,19]. Chemokines are involved in neuroendocrine, neurotransmission, and neurodegeneration [20]. Chemokines are classified in four families according to the relative position of their cysteine residues and their functions. Chemokines bind G protein-coupled receptors and activate cell signaling cascades, and by changes in cell shape and movement induce directed chemotaxis [20, 21]. Recent studies have demonstrated that chemokines have specific effects on the neuroinflammation, chemotaxis, and modulation of neuroendocrine functions. In addition to chemokines and chemokine receptors residing in the brain system, the molecules have critical roles in the maintenance of the CNS homeostasis through autocrine or paracrine activity [22]. Elevated serum levels of chemokines in major bipolar disorder, depressive disorder, and schizophrenia have previously been reported [9]. Chemokines are suggested as biomarkers in pathophysiology of psychiatric disorders [23].
Chemokines and neuroinflammation
Inflammation in the brain, known as neuroinflammation, is a process that lead to activation of microglia that secrete inflammatory cytokines, free oxidative radicals, and chemokines [24, 25]. Chemokines recruit peripheral immune cells into damaged areas of the CNS, subsequently amplifying inflammation in the CNS and triggering adaptive immune responses [26]. Blood source chemokines can increase the permeability of the blood–brain barrier (BBB), and may destroy its integrity to accelerate the entrance of peripheral leukocytes to the brain inflammation site [20, 27]. The activation of chemokine receptors impairs neuronal activity in the CNS by affecting neurotransmitter releasing mainly through inhibiting calcium channels in nerve terminals [28].
CCL11 (eotaxin-1)
The eotaxin family comprises chemokines including CCL11 (eotaxin-1), CCL24 (eotaxin-2), and CCL26 (eotaxin-3), which are chemotactic agents for eosinophils and take part in innate immunity. CCL11, or eosinophil chemotactic protein (eotaxin-1), can selectively lead to the recruitment of eosinophils into inflammatory sites. Elevations in CCL11 levels occur in allergic reactions, allergic rhinitis, asthma, and other eosinophil-related conditions [29]. T-helper 2 cytokines, such as IL-4, IL-10 (Interleukin-10), IL-13, complement factors, and immune complexes, induce CCL11 production by eosinophils, T and B cells, macrophages, endothelial cells, fibroblasts, epithelial cells, chondrocytes, microglia, keratinocytes, and smooth muscle cells [29,30,31]. CCL11 is synthesized by microglia, and in addition transported to the brain by BBB [32]. Previous studies have shown that CCL11 reduces neurogenesis and is related to aging [33]. Elevations in CCL11 have been demonstrated in neuroinflammatory disorders like multiple sclerosis (MS), in neurodegenerative situations such as Alzheimer’s disease, and in psychiatric disorders such as major bipolar disorder, depression, and schizophrenia [29, 30, 34,35,36]. CCL11 binds to its main receptor; CCR3 is expressed on mast cells, eosinophils, Th2 lymphocytes, and keratinocytes and is demonstrated as one of the most important cytokines involved in tissue inflammation. CCL11 may be important in the pathophysiology Alzheimer’s disease and depression in age-old persons [33, 37]. As the association between psychiatric disorders and CCL11 and its possible pathologic role in these situations, it may be used as a neuroinflammation biomarker and therapeutic target in these disorders.
CCL11 in neuroinflammation and neurodegeneration diseases
CCL11 concentration is raised in the CSF and sera of patients suffering from neuroinflammatory disorders but the exact function of CCL11 in the CNS is not completely clear [33]. Activated astrocytes and microglia predominantly release and express CCL11 and its main receptor (CCR3), respectively [38]. CCR2 and CCR5 are the other CCL11 receptors with a lower affinity than CCR3 [39]. Microglia as macrophage-like cells in the CNS are capable to initiating inflammatory response [40]. Glial activation contributes to neuronal death in neurodegenerative diseases [41, 42]. CCL11 released from activated astrocytes or BBB crossed source in systematic infections and inflammation can promote the upregulation of nicotinamide adenine dinucleotide phosphate-oxidase 1 (NOX1) and production of ROS by microglia that induce neuronal death [43, 44]. Blood originated CCL11 can be produced by leukocytes, fibroblasts, chondrocytes, and endothelial cells, in systemically inflammation [45]. Parajuli et al. showed a possible mechanism independent of eosinophil recruitment of CCL11-mediated neuronal dysfunction through activated glial cells. [46].
Previous studies have indicated that increased (sera or CSF) CCL11 levels may contribute to the pathogenesis of MS by promoting eosinophil infiltration and subsequent neural damage in the affected areas [47, 48]. The CCL11 is related to MS duration and is a potential biomarker for the disease progression. Elevated localization of eosinophils and various eotaxins concentration was found in disease-associated lesions [49,50,51,52,53].
In Parkinson’s disease (PD), neuroinflammation and loss of neuronal connections are the main cause of neural impairment, and loss of brain cells. The accumulation of misfolded α-synuclein protein in the damaged cells, proteasomal and lysosomal dysfunction and reduced mitochondrial activity [53,54,55]. Iron accumulation with the misfolded α-synuclein protein could be due to oxidative stress, protein aggregation, or neuronal death [56]. In the regulation of the release and transmission of neurotransmitters and in the growth and development of related neurons some chemokines demonstrated have major roles. In addition, PD is related to immune and inflammatory mechanisms and suggested the role of chemokines in the underlying mechanisms. Administration of anti-CCL11 neutralizing antibody reduced the production of pro-inflammatory factors and the CD4 + /CD8 + T cells infiltration in the substantia nigra of mice, and improves motor symptoms in PD mice [57].
In Alzheimer’s disease (AD), microglial activation and neuroinflammation contribute to hippocampal atrophy. The hippocampus region is critical in learning and memory processes and is one of the strongest predictors of disease progression [58]. Eotaxin-1 levels increase throughout life and contribute to the possibility that CCL11 is an effector molecule in aging, the main risk factor for developing AD. Previous studies have reported the elevation and importance of CCL11 with age at the onset of AD [33]. Among carriers of the CCL11, A23T mutation in the region of eotaxin-1 binding and activation of its receptor CCR3 modulate eotaxin-1 signaling and neuroinflammation [59].
CCL11 and COVID-19 infection
Elevation of CCL11 levels in the sera and CSF of patients with neuroinflammatory disorders such as neuromyelitis optica, multiple sclerosis, and HTLV-1-associated myelopathy have been previously demonstrated [49, 60]. Many reports have shown the elevation of chemokines in COVID-19 patients [50, 61]. Determination of CCL11 with CBC indexes may be helpful in the early prediction of the severity, diagnosis, and follow-up of critical COVID-19 patients in the course of the disease [62]. In COVID-19 infection, immune responses mediated by T-helper 2 are related mainly to CCL11 and IL-4 and the recruitment of NK cells, eosinophils, and macrophages, a similar immune response to that seen with other respiratory viruses [63]. Analysis of soluble biomarker levels in COVID-19 cases ranging from mild/moderate to critically severe revealed that elevations in CCL11 and CCL26 levels were correlated with disease severity [62]. As physiosomatic symptoms are related to eotaxins, they, especially CCL11, may play a role in COVID-19 neurologic syndrome. Previous evidence supports this hypothesis, with special emphasis on the role of eotaxin-1 in neural demyelination [50]. It is recommended that eotaxin-11 levels be monitored as a predictor for COVID-19 neurologic syndrome.
Conclusion
It has been documented that in systematic inflammation, cytokines may damage the BBB and potentially play a role in neurological complications. CCL11 is synthesized by microglia, and in inflammatory and cytokine storm conditions, its blood-originated source could transport across the BBB and enter the brain [32]. Previous studies have shown that CCL11 reduces neurogenesis and is related to aging [33]. Elevated CCL11 levels have been demonstrated in neuroinflammatory disorders like multiple sclerosis (MS), in neurodegenerative situations such as Alzheimer’s disease, and in psychiatric illnesses such as major depression, bipolar disorder, and schizophrenia [29, 30, 34,35,36]. Considering the recent COVID-19 pandemic, it seems to be too soon to describe the full clinical features of COVID-19 neurological syndrome; however, published evidence shows an increased number of patients with neuropsychological effects of COVID-19, such as various degrees of depression, sleep impairment, and anxiety, in the survivors, irrespective of severity [7]. With regard to the role of CCL11 in physiosomatic and neuroinflammation, it may be valuable to analyze the level of this chemokine in the sera or SCF of COVID-19 patients. Elevated levels of CCL11 may be used to predict neuroinflammation related post-COVID-19 complications, and using the chemokine modulators may help to lessen the neurologic complications in these patients.
Data availability
Not applicable.
References
Hong KS, Lee KH, Chung JH, Shin KC, Choi EY, Jin HJ et al (2020) Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in Daegu, South Korea: a brief descriptive study. Yonsei Med. J 61(5):431
Lee SM, Kang WS, Cho A-R, Kim T, Park JK (2018) Psychological impact of the 2015 MERS outbreak on hospital workers and quarantined hemodialysis patients. Compr Psychiatry 87:123–127
Chan K, Zheng J, Mok Y, Li Y, Liu YN, Chu C et al (2003) SARS: prognosis, outcome and sequelae. Respirology 8:S36–S40
Lam MH-B, Wing Y-K, Yu MW-M, Leung C-M, Ma RC, Kong AP et al (2009) Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch Intern Med 169(22):2142–7
Serrano-Castro P, Estivill-Torrús G, Cabezudo-García P, Reyes-Bueno J, Petersen NC, Aguilar-Castillo M et al (2020) Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurología (English Edition) 35(4):245–251
Heneka MT, Golenbock D, Latz E, Morgan D, Brown R (2020) Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimer’s Res Ther 12(1):1–3
Junhua M, Qi Z, Xue G, Lijuan L, Zhongwen Z, Jing W (2020) Analysis of psychological and sleep state of medical stuff with novel coronavirus pneumonia. Herald Med 39(3):345–349
Goërtz YM, Van Herck M, Delbressine JM, Vaes AW, Meys R, Machado FV et al (2020) Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 6(4).
Horváth S, Mirnics K (2014) Immune system disturbances in schizophrenia. Biol Psychiat 75(4):316–323
Gebremeskel S, Schanin J, Coyle KM, Butuci M, Luu T, Brock EC et al (2021) Mast cell and eosinophil activation are associated with COVID-19 and TLR-mediated viral inflammation: implications for an anti-siglec-8 antibody. Front Immunol 12:641
Mitamura Y, Schulz D, Oro S, Li N, Kolm I, Lang C et al (2022) Cutaneous and systemic hyperinflammation drives maculopapular drug exanthema in severely ill COVID-19 patients. Allergy 77:595–608
Lindsley AW, Schwartz JT, Rothenberg ME (2020) Eosinophil responses during COVID-19 infections and coronavirus vaccination. J Allergy Clin Immunol 146(1):1–7
Lucas C, Wong P, Klein J, Castro TB, Silva J, Sundaram M et al (2020) Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584(7821):463–469
Lippi G, Henry BM (2020) Eosinophil count in severe coronavirus disease 2019 (COVID-19). QJM: An Int J Med 511–512
Shamri R, Melo RC, Young KM, Bivas-Benita M, Xenakis JJ, Spencer LA et al (2012) CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J 26(5):2084–2093
Melo RC, Spencer LA, Perez SA, Ghiran I, Dvorak AM, Weller PF (2005) Human eosinophils secrete preformed, granule-stored interleukin-4 through distinct vesicular compartments. Traffic 6(11):1047–1057
El-Shazly A, Masuyama K, Nakano K, Eura M, Samejima Y, Ishikawa T (1998) Human eotaxin induces eosinophil-derived neurotoxin release from normal human eosinophils. Int Arch Allergy Immunol 117(Suppl. 1):55–58
Foxman EF, Campbell JJ, Butcher EC (1997) Multistep navigation and the combinatorial control of leukocyte chemotaxis. J Cell Biol 139(5):1349–1360
Murphy PM, Baggiolini M, Charo IF, Hébert CA, Horuk R, Matsushima K et al (2000) International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 52(1):145–76
Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76(2):301–314
Réaux-Le Goazigo A, Van Steenwinckel J, Rostène W, Parsadaniantz SM (2013) Current status of chemokines in the adult CNS. Prog Neurobiol 104:67–92
Ivanovska M, Abdi Z, Murdjeva M, Macedo D, Maes A, Maes M (2020) CCL-11 or eotaxin-1: an immune marker for ageing and accelerated ageing in neuro-psychiatric disorders. Pharmaceuticals 13(9):230
Cardona AE, Li M, Liu L, Savarin C, Ransohoff RM (2008) Chemokines in and out of the central nervous system: much more than chemotaxis and inflammation. J Leukoc Biol 84(3):587–594
Milenkovic VM, Stanton EH, Nothdurfter C, Rupprecht R, Wetzel CH (2019) The role of chemokines in the pathophysiology of major depressive disorder. Int J Mol Sci 20(9):2283
Le Thuc O, Blondeau N, Nahon JL, Rovère C (2015) The complex contribution of chemokines to neuroinflammation: switching from beneficial to detrimental effects. Ann N Y Acad Sci 1351(1):127–140
Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR et al (2013) MHCII is required for α-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33(23):9592–9600
Kannarkat GT, Boss JM, Tansey MG (2013) The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis 3(4):493–514
Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20(1):197–216
Liu J-Q, Chu S-F, Zhou X, Zhang D-Y, Chen N-H (2019) Role of chemokines in Parkinson’s disease. Brain Res Bull 152:11–18
Teixeira AL, Gama CS, Rocha NP, Teixeira MM (2018) Revisiting the role of eotaxin-1/CCL11 in psychiatric disorders. Front Psych 9:241
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2019) A new schizophrenia model: immune activation is associated with the induction of different neurotoxic products which together determine memory impairments and schizophrenia symptom dimensions. CNS Neurol Disord-Drug Targets (Formerly Current Drug Targets-CNS Neurological Disorders) 18(2):124–40
Kindstedt E, Holm CK, Sulniute R, Martinez-Carrasco I, Lundmark R, Lundberg P (2017) CCL11, a novel mediator of inflammatory bone resorption. Sci Rep 7(1):1–10
Sirivichayakul S, Kanchanatawan B, Thika S, Carvalho AF, Maes M (2019) Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res 35(1):122–138
Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G et al (2011) The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477(7362):90–94
Sørensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA et al (1999) Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Investig 103(6):807–815
Eyre H, Baune BT (2012) Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology 37(9):1397–1416
Stuart MJ, Corrigan F, Baune BT (2014) Knockout of CXCR5 increases the population of immature neural cells and decreases proliferation in the hippocampal dentate gyrus. J Neuroinflammation 11(1):1–9
Baruch K, Ron-Harel N, Gal H, Deczkowska A, Shifrut E, Ndifon W et al (2013) CNS-specific immunity at the choroid plexus shifts toward destructive Th2 inflammation in brain aging. Proc Natl Acad Sci 110(6):2264–2269
Bertrand CP, Ponath PD (2000) CCR3 blockade as a new therapy for asthma. Expert Opin Investig Drugs 9(1):43–52
Martinelli R, Sabroe I, LaRosa G, Williams TJ, Pease JE (2001) The CC chemokine eotaxin (CCL11) is a partial agonist of CC chemokine receptor 2b. J Biol Chem 276(46):42957–42964
Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T et al (2018) Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci 12:488
Aloisi F (2001) Immune function of microglia. Glia 36(2):165–179
Hanisch UK (2002) Microglia as a source and target of cytokines. Glia 40(2):140–155
Choi D-H, Cristóvão AC, Guhathakurta S, Lee J, Joh TH, Beal MF et al (2012) NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxid Redox Signal 16(10):1033–1045
Cristóvão AC, Guhathakurta S, Bok E, Je G, Yoo SD, Choi D-H et al (2012) NADPH oxidase 1 mediates α-synucleinopathy in Parkinson’s disease. J Neurosci 32(42):14465–14477
Pease JE, Williams TJ (2001) Eotaxin and asthma. Curr Opin Pharmacol 1(3):248–253
Parajuli B, Horiuchi H, Mizuno T, Takeuchi H, Suzumura A (2015) CCL11 enhances excitotoxic neuronal death by producing reactive oxygen species in microglia. Glia 63(12):2274–2284
Erickson MA, Morofuji Y, Owen JB, Banks WA (2014) Rapid transport of CCL11 across the blood-brain barrier: regional variation and importance of blood cells. J Pharmacol Exp Ther 349(3):497–507
Michael B, Elsone L, Griffiths M, Faragher B, Borrow R, Solomon T et al (2013) Post-acute serum eosinophil and neutrophil-associated cytokine/chemokine profile can distinguish between patients with neuromyelitis optica and multiple sclerosis; and identifies potential pathophysiological mechanisms–a pilot study. Cytokine 64(1):90–96
Tanaka M, Matsushita T, Tateishi T, Ochi H, Kawano Y, Mei F-J et al (2008) Distinct CSF cytokine/chemokine profiles in atopic myelitis and other causes of myelitis. Neurology 71(13):974–981
Correale J, Fiol M (2011) Chitinase effects on immune cell response in neuromyelitis optica and multiple sclerosis. Mult Scler J 17(5):521–531
Comabella M, Fernández M, Martin R, Rivera-Vallvé S, Borrás E, Chiva C et al (2010) Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 133(4):1082–1093
Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM et al (2002) A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain 125(7):1450–1461
Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M et al (2010) Missing pieces in the Parkinson’s disease puzzle. Nat Med 16(6):653–661
Schulz-Schaeffer WJ (2010) The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120(2):131–143
Davie CA (2008) A review of Parkinson’s disease. Br Med Bull 86(1):109–127
Hirsch E (2009) Iron transport in Parkinson’s disease. Parkinsonism Relat Disord 15:S209–S211
Chandra G, Rangasamy SB, Roy A, Kordower JH, Pahan K (2016) Neutralization of RANTES and eotaxin prevents the loss of dopaminergic neurons in a mouse model of Parkinson disease. J Biol Chem 291(29):15267–15281
Wenk GL (2003) Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry 64:7–10
Lalli M, Bettcher B, Arcila M, Garcia G, Guzman C, Madrigal L et al (2015) Whole-genome sequencing suggests a chemokine gene cluster that modifies age at onset in familial Alzheimer’s disease. Mol Psychiatry 20(11):1294–1300
Khalil BA, Elemam NM, Maghazachi AA (2021) Chemokines and chemokine receptors during COVID-19 infection. Comput Struct Biotechnol J 19:976–988
Oliviero A, de Castro F, Coperchini F, Chiovato L, Rotondi M (2021) COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? Neuroscientist 27(3):214–221
Katar M (2021) Could eosinophil chemotactic factor (CCL11) be a useful biomarker of Covid-19? Journal of Surgery and Medicine 5(2):168–173
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors contributed to search and write the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
As the current study was a review and hypothesis, there was no ethics committee to approve the study.
Consent for publication
The authors have consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazarinia, D., Behzadifard, M., Gholampour, J. et al. Eotaxin-1 (CCL11) in neuroinflammatory disorders and possible role in COVID-19 neurologic complications. Acta Neurol Belg 122, 865–869 (2022). https://doi.org/10.1007/s13760-022-01984-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-022-01984-3