Abstract
The capacity of a synthetic (N1E, N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine Schiff base ligand to operate as a cation carrier in a poly(vinylchloride) (PVC) membrane (electrode I) and screen-printed ion-selective electrode (SPE) (electrode II) was investigated. The screen-printed and the fabricated poly(vinylchloride) membrane (PVC) electrodes displayed outstanding response properties for Al(III) ions. The electrodes had linear potential response with a slope of 17.95 ± 0.14 and 19.80 ± 0.46 mV decade−1 in the concentration range of 1.0 × 10−5–1.0 × 10−1 and 1.0 × 10−7–1.0 × 10−1 mol L−1 for electrode I and electrode II, respectively. The detection limit of the proposed sensors is 2.1 × 10−6 and 6.3 × 10−8 mol L−1, and it can be used over a period of 35 and 190 days for electrode I and electrode II, respectively. The suggested sensors showed strong selectivity against a wide range of other cations, including alkali, alkaline earth, heavy, and transition metals, and could be employed in pH ranges of 3.0–6.0 and 2.5–6.5 for electrode I and electrode II, respectively. The effect of several plasticizers has been studied. These electrodes had been successfully used to determine Al(III) in aqueous solution and various real water samples. They used as an indicator electrodes in aluminum ion potentiometric titration against standard EDTA solution. The devised approach was used to determine the concentration of Al(III) in several real water samples with high percentage recoveries and low standard and relative standard deviation values. The results were in good agreement with those obtained using atomic absorption spectrometry as indicated from the calculated t- and F-test values.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aluminum is the third most prevalent element on the planet and is widely employed in both industrial and home applications. As a result, aluminum is prevalent in environmental samples, and its quantification is critical due to its toxicity above a specific threshold. Dementia, anemia, myopathy, and bone and joint disease have all been linked to aluminum [1,2,3].
Several analytical techniques have been used to determine aqueous aluminum concentration, including liquid chromatography [4], fluorescence [5], spectrometry [6], electrothermal atomic absorption spectrometry [7, 8], inductively coupled plasma spectrometry [9], and inductively coupled optical emission spectrometry [10, 11]. These procedures are not ideal for routine or on-site analysis, despite the fact that they offer accurate data. They are time-consuming and labor-intensive because they require sample pretreatments and specialized and expensive instruments [12,13,14]. These published procedures are known to use hazardous solvents, take a long time to examine, and require sample pretreatments on occasion. They are probably still the most commonly used procedures for routine determination, but they required expert personnel, costly instrumentation [4,5,6,7,8,9,10,11,12,13,14], a complicated operating procedure, and a long detection period. As a result, it is critical to create reliable [15,16,17,18], simple, and low-cost aluminum determination methodologies [15].
The improvement in potentiometric measurements has been aided by the introduction of novel ion-selective membrane electrodes [16]. Easy handling, non-destructive analysis, accuracy, repeatability, fast relative response, cheaper costing, time-saving, affectivity, and facility in sample preparation are all advantages of ISEs over many other methods for cations and anions detection [17,18,19].
There is yet to be a decent commercial electrode for the ion [20, 21], so finding carrier chemicals that react with aluminum with high selectivity is critical. ISEs have been produced using a variety of organic and inorganic chemicals [22, 23]. The ability to act as a selective carrier with excellent responsiveness to a few metal ions is the most crucial prerequisite for an ionophore. New avenues for developing particular sensors have opened up thanks to the availability of enhanced highly selective materials. Efforts have begun to construct Al(III) selective electrodes employing Schiff base ligands as the sensor material [24].
Using diverse natural ligands as ion carriers, recently, multiple PVC-based membrane electrodes for Co(II) [25], Cu(II) [26], Cd(II) [27] and Ag(I) [28] were introduced. There were interests in developing a new solvent polymeric membrane electrode for selective monitoring of aluminum ion in solution due to the critical importance of aluminum determination in chemical, industrial, and clinical investigation. The screen-printed electrode (SPE) with Schiff base produces “environmentally friendly” analytical sensors with distinct advantages over macroelectrodes, including enhanced mass transport, catalysis, a large effective surface area, and control over the electrode microenvironment [29, 30].
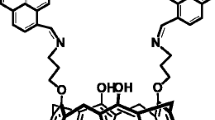
The potentiometric approach is one of the most common electrochemical techniques for quantifying distinct metal ions by measuring the potential (voltage) of cells under basically balanced conditions [20,21,22,23,24]. The utility of ion sensors is progressively being realized as a result of rapid industry and technology growth. Traditional techniques of analysis have numerous disadvantages when compared to good ion-selective electrodes (ISE), which give an accurate, repeatable, rapid, and frequently selective determination for diverse ion species. Not only have that, but the ISEs enable for online monitoring of a specific ion in a tiny sampled volume without the need for pretreatment. The ISEs, in particular, are not harmful. ISEs are utilized in physiology, process control, and environmental studies, among other fields. As a result, they are one of the most prominent chemical sensor groups [25,26,27,28,29,30]. In this study, a new Schiff base ligand was synthesized by combining 2-quinolinecarboxaldehyde with m-phenylenediamine in a 2:1 ratio. It was characterized using elemental spectroscopic methods, and antibacterial activity tests were discussed. In diverse petroleum water samples, a highly selective and sensitive PVC membrane and screen-printed electrodes were constructed for Al(III) ion determination based on a synthetic Schiff base of (N1E,N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine (BQMBD) as a carrier (Scheme 1). The results revealed that the PVC membrane and screen-printed electrodes had a high detection limit and outstanding selectivity for aluminum ions, allowing for direct determination of Al(III) concentration in various media without the need for prior separation processes. Different experimental conditions were optimized, and the accuracy and precision of the electrodes were discussed in terms of inter- and intra-days study. The estimated F- and t-test values indicated that the proposed potentiometric and atomic absorption spectrometry (AAS) procedures were not significantly different.
Experimental
Reagents
All chemicals were analytical grade, and the tests were conducted with bidistilled water. BDH provided the 2-quinolinecarboxaldehyde and m-phenylenediamine. Merck provided the aluminum chloride. Fluka and Alfa Aesar provided o-nitrophenyl octyl ether (o-NPOE) and tricresyl phosphate (TCP), respectively. Merck provided the dioctyl sebacate (DOS), dioctyl phthalate (DOP), and dibutyl phthalate (DBP). Aldrich provided graphite powder (synthetic 1–2 μm) for the electrode preparation. Interfering materials included cadmium, zinc, strontium, magnesium, ferrous, ferric, calcium, lead, copper, potassium, sodium, and barium chloride salts. Interfering ions included cerium sulfate and sodium bromide, carbonate, and bicarbonate.
Samples
Formation water (Amry deep (6), Western Desert, Agiba Petroleum Company (sample 1), Egypt, Karama, Al-Wahhat-Al-Bahhriyah, Qarun Petroleum Company (sample 2), Badr1 (sample 3), and Badr2 (sample 4) Western Desert, Badr Petroleum Company, Egypt) included water samples.
Instrumentation
A Jenway 3505 pH meter was used to assess potential in the laboratory. The reference electrode was a silver–silver chloride double-junction electrode (Metrohm 6.0726.100) in combination with a separate ion-selective electrode. Thermo-Orion, model Orion 3 stars, USA, was used to monitor pH. All glassware was meticulously cleansed with distilled water and dried in the oven before usage prior to analysis. Carbon, hydrogen, and nitrogen microanalyses were performed at Cairo University Microanalytical Center utilizing a CHNS-932 (LECO) Vario Elemental Analyzer. UV–Vis spectra were collected using a Shimadzu UV mini-1240 UV–Vis spectrophotometer. At the Microanalytical Center, National Research Center, Egypt, mass spectra were acquired using the EI method at 70 eV on an MS5988 GS-MS Hewlett–Packard instrument. As KBr pellets, FTIR spectra were collected on a Perkin-Elmer 1650 spectrometer (4000–400 cm−1). TMS was used as an internal standard to record 1H NMR spectra as a solution in DMSO-d6 on a 300 MHz Varian-Oxford Mercury at room temperature.
Solutions of Schiff base ligand
For analyzing the UV–Vis spectra in the wavelength range of 200 to 700 nm, ligand solution (10−4 M) was generated in DMF solvent.
Synthesis of Schiff base ligand
Condensation of 2-quinolinecarboxaldehyde with m-phenylenediamine yielded Schiff base ligand. In a ratio of 2:1, a solution of 2-quinolinecarboxaldehyde (0.4 g) dissolved in DMF was added drop by drop to m-phenylenediamine (1.06 g) dissolved in ethanol. The resultant mixture was agitated for about 3 h at reflux, then filtered, recrystallized, washed with diethyl ether, and vacuum-dried.
Preparation of electrodes
Screen-Printed electrode fabrication
The electrodes were fabricated as described before [31,32,33]. The working electrodes were printed using handmade printing carbon ink with (N1E,N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine (BQMBD) Schiff base as an ionophore to obtain varied compositions, then maintained for 30 min at 50 °C, and allowed to cool. A layer of insulator was then applied to the printed electrodes, leaving on the other side a rectangular-shaped (5 mm) working area and a corresponding area (for electrical contact). The electrodes were manufactured, stored at 4 °C, and used in potentiometric tests right away.
PVC membrane electrode fabrication
The PVC membranes were made by dissolving an adequate amount of 5–20 mg (BQMBD) ionophore + 240 mg different plasticizers + 6 ml THF + 240 mg PVC in a solution. Plasticizers of o-NPOE, TCP, DOP, DBP, and DOS were added to some compositions to produce membranes with various combinations. Following the addition of THF, this solution was rapidly agitated with a glass rod for easy PVC dissolution. When the solution had thickened, it was poured into a 2.5-cm-inner-diameter glass ring that was put on a smooth glass plate. To keep dust and air streams from ruining the combination, a filter paper was placed on top of the glass plate. The solution was then allowed to evaporate at room temperature for 24 h. The resulting membranes, which were trimmed to size and bonded to one end of a Pyrex glass tube with araldite, had a thickness of around 0.5 mm. Under titration, the PVC was filled with 1 × 10−2 mol L−1 KCl and 1 × 10−3 mol L−1 AlCl3. When not in use, the electrodes were conditioned for at least 24 h in a fresh 1 × 10−3 mol L−1 AlCl3 solution and washed thoroughly with distilled water. To generate reproducible, noiseless, and stable potentials, the ratio of membrane constituents, time of contact, and concentration of equilibrating solution were tuned [34].
The potentiometric measurements
The potentiometric measurements were carried out in the following manner. The modified PVC membrane and screen-printed electrode were immersed for a period of time in a stirred 50-mL 1.0 × 10−1 mol L−1 Al(III) solution until the potential reading became constant [35]. The electrodes response properties were investigated using the standard addition method [36]. Aluminum salts standard solutions were added, resulting in a range of Al(III) concentrations of 1.0 × 10−8–1.0 × 10−1 mol L−1. After each addition, when steady values were established, potential readings were taken (usually after 20–40 s). The matched potential method (MPM) was used to calculate the potentiometric selectivity of these electrodes toward distinct cations [37]. The activity of Al(III) was increased from aA = 1.0 × 10−3 mol L−1 (primary ion) to áA = 5.0 × 10−3 mol L−1 (secondary ion) in this way, and the resulting potential change (E) was measured. Then, until the same potential change (E) was recorded, a solution of an interfering ion (aB) in the range 1.0 × 10−2–1.0 × 10−4 mol L−1 was introduced to a new primary ion (áA). For each interferent, the selectivity factor, KpotA,B, was computed as follows: KpotA,B = (áA − aA)/aB.
Results and discussion
Schiff base ligand had a pale yellow color (melting point 200 °C; yield 74%), molecular weight 386 g/mol with molecular formula C26H18N4. The elemental analyses were as follows: %found (calcd.): C: 80.83 (80.93), H: 4.66 (4.76), N: 14.51 (14.81). FTIR (ν, cm−1): azomethine (C=N) 1665sh, C=N of pyridine ring 1619w. UV–Vis (DMF): (λmax nm) = 268 (π–π*) and 322 (n-π*). 1H NMR (300 MHz, DMSO-d6, δ, ppm): 7.38–8.21 (m, 18H, Ar–H), 10.09 (s, 1H, azomethine).
Preliminary potentiometric studies
Potentiometric sensors for Al(III) ions determination based on using (N1E,N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine) (BQMBD) Schiff base ligand as ionophore were fabricated [38]. The structural properties of Schiff bases enable geometric and cavity control of host–guest complexation as well as lipophilicity manipulation, resulting in extraordinary selectivity, sensitivity, and stability for a single ion. To test the suitability of the synthesized Schiff base compound as an ion carrier in modified screen-printed ion-selective electrodes (MSPE) (electrode II), it was employed to prepare MSPE for a range of cations in preliminary studies. After training the electrodes by soaking them in the chloride salt solution of each cation, the potentiometric responses of these electrodes were obtained.
Figure 1 shows the potential responses of the developed electrodes to various cations in the concentration range of 1.0 × 10−7–1.0 × 10−1 mol L−1. The slopes of the related potential against pMn+ plots, with the exception of the Al(III)–MSPE electrode, were substantially lower than the expected Nernstian slopes of 59.6, 29.8, and 19.8 mV decade−1 for univalent, bivalent, and trivalent cations, respectively. Over the concentration range tested, the MSPE based on the Schiff base ionophore gave the most sensitive (and Nernstian) potential response for the Al(III) ion, while the other cations give lower concentration range and low slope values than the recommended Nernstian slopes. As a result, this ionophore was chosen to create an Al(III)–MSPE, and additional research into the response properties of the resulting selective electrode was conducted. The interaction between the ionophore and target ions takes place through formation of trigonal bipyramidal complex as shown in scheme 1.
Effect of ionophore content
The slope was determined after various electrodes (MPVC and MSPE) with varied quantities of (N1E,N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine (BQMBD) ionophore were built and calibrated using Al(III) solution. The proportions of (BQMBD) ionophore in the fabricated electrodes were 2, 4, 6.5, 8.5, 10, and 11.5 mg using TCP plasticizer. The slopes of the above electrodes were observed to be 9.12 ± 0.36, 12.70 ± 0.59, 15.20 ± 0.33, 16.54 ± 0.24, 17.95 ± 0.14 and 15.76 ± 0.34 mV decade−1 and 12.40 ± 0.56, 16.58 ± 0.52, 17.67 ± 0.21, 19.80 ± 0.46, 16.81 ± 0.30 and 15.64 ± 0.39 mV decade−1 for the six MPVCE membrane and MSPE, respectively. Figure 2 and Table 1 show that the electrodes respond well to Al(III) ions in the concentration range from 1.0 × 10−7 to 1.0 × 10−1 mol L−1. Based on these findings, 10.0 (MPVCE, electrode I) and 8.5 mg (MSPE, electrode II) of BQMBD ionophore were chosen as the optimal quantity for further research. The detection limit of a potentiometric sensor was calculated by extrapolating the two segments of the calibration graph, and the intersection is the detection limit (2.1 × 10−6 and 6.3 × 10−8 mol L−1) for electrode I and electrode II, respectively.
Effect of addition of plasticizer
Plasticizer is a critical component in electrode performance because it is responsible for (BQMBD) ionophore salvation and distribution in the membrane matrix, which controls the detection limit, affects selectivity and sensitivity, and gives the plastic membrane its proper elasticity and strength [39]. By running the sensor at varied concentrations of Al(III) ion, the performance characteristics of five different plasticizers (o-NPOE, TCP, DOP, DBP, and DOS) were examined. Table 2 shows the operating concentration range for various electrodes. The electrodes with membranes and paste containing o-NPOE plasticizer and active (BQMBD) ionophores, respectively, were clearly the best. In comparison with the other plasticizers, this was owing to a practically perfect slope of 17.95 and 19.80 mV decade−1 for electrode I and electrode II, respectively. The operating concentration range of these sensors was (1.0 × 10−5–1.0 × 10−1 and 1.0 × 10−7–1.0 × 10−1 mol L−1) and the detection limit was (2.1 × 10−6 and 6.3 × 10−8 mol L−1) for electrode I and electrode II, respectively. These membranes and paste slopes were similar to Nernstian slopes, and the working concentration ranges were wide. This suggested that o-NPOE solvent medium provided the optimum complexation environment for aluminum ions and their respective carriers. As a result, more research was done with the cells using electrodes I and II.
Surface characterization
Surface interaction between the selected (N1E, N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine (BQMBD) Schiff base ligand (BQMBD ionophore) and the Al(III) ions is important for selective extraction of the target ion from the sample solution into the paste during potential measurements.
As a consequence, a scanning electron microscope was used to characterize the surface morphology of the suggested SPE electrode(II), which is a suitable instrument for evaluating the surface morphology of an ion-selective electrode. Figure 3 shows SEM images of the manufactured electrode before and after soaking in 1.0 × 10−3 mol L−1 Al(III) ion solution (a).
After soaking the suggested SPE in Al(III) ions solution, less gaps between surface components were detected as a result of the interaction between the Al(III) ions and the active sites groups of the Schiff base (BQMBD) that occupied the space between the surface components (Fig. 3b). The EDX analysis of the paste with BQMBD ionophore after soaking in Al(III) ion solution (Fig. 3c) gives Al(III) peak confirming the formation of Al complex as previously discussed in Scheme 1.
Dynamic response time
The reaction time of a PVC membrane (electrode I) and screen-printed electrode (electrode II) is one of the most important factors. When predicting a greater ion concentration after monitoring lower ion concentrations, the reaction time was rather fast, but the opposite procedure took much longer. It was studied to measure the practical reaction time necessary to obtain a 90% value of stable potential following consecutive immersion of the constructed sensor in a series of interesting Al(III) solutions ranging from 1.0 × 10−5 to 1.0 × 10−1 mol L−1 and from 1.0 × 10−7 to 1.0 × 10−1 mol L−1 for electrodes I and II, respectively, according to the IUPAC definition. In all solutions, the average dynamic response durations for electrodes I and II were 8 and 5 s, respectively, as shown in Fig. 4. The rapid exchange kinetics of Al(III) ions with the sensing element at the test solution–membrane interface, as well as the lack of an internal reference solution, might explain this phenomenon [40].
Effect of pH
The effect of the pH value of the Al(III) solution on the potentiometric response of the proposed ISE was evaluated at two Al(III) concentrations (1.0 × 10−5 and 1.0 × 10−3 mol L−1) throughout a pH range of 1 to 8. Figure 5 shows that the potential response for PVC membrane (electrode I) and screen-printed electrode (electrode II) remains nearly constant across the pH ranges of 3.0- 6.0 and 2.5–6.5, respectively, beyond which a progressive shift in potential may be detected. As a result, this pH range may be considered the recommended electrodes operating range. The development of certain hydroxyl complexes of Al(III), such as Al(OH)+ and Al(OH)3, leads to a reduction in Al(III) concentration at higher pH values, but at lower pH, the plentiful H+ ions can protonate the N atoms of BQMBD and even induce the decomplexation of the Al(III)–BQMBD complex. The H+ ions might interfere with the electrode response at the same time. Both of them have the ability to increase the potential. The MSPE (electrode II) has a wide pH range than the MPVCE (electrode I) because MSPE has many advantages over MPVCE such as simplicity, small size, very low Ohmic resistance, very short response time in addition to the ease of fabrication and regeneration as well as long functional lifetime.
Lifetime
The electrodes lifespan may be determined by tracking the change in slope and detection limit of the electrodes over time. After 1 and 6 months, the findings shown graphically in Fig. 6 revealed a very modest progressive decline in the slope (17.95 and 19.80 mV decade−1) for electrodes I and II, respectively, with changes in the slope from 17.95 to 15.75 mV decade−1 and from 19.80 to 17.49 mV decade−1 of activity. The electrodes were utilized for 1 h per day, 3 days per week during this time. When not in use, the electrodes were kept dry in an opaque closed jar and re-equilibrated by dipping into 1.0 × 10−3 mol L−1 Al(III) solution for 15 min when ready to use.
Effect of temperature
The temperature of the test solution generally affects the potential of electrodes I and II, respectively. To investigate the electrodes thermal stability, calibration graphs (Eelect. versus pAl(III)) were created at various test solution temperatures ranging from 10 to 70 °C. For the determination of the isothermal coefficient (dE°/dt) of the electrodes, the standard electrode potentials (E°) against the normal hydrogen electrode at different temperatures were obtained from calibration graphs as the intercepts at p[Al(III)] = 0 (after subtracting the values of the standard electrode potential of the Ag/AgCl electrode at these temperatures) and were plotted versus (t − 25), where t is the temperature of the test solution in °C. A straight-line plot is obtained according to Antropov’s equation:
where E°(25) is the standard electrode potential at 25 °C, T is the temperature in unit cellulous, and the isothermal coefficient of the electrode is represented by the slope of the straight-line produced, which was determined to be 0.000127 and 0.000153 V/ºC for electrode I and electrode II, respectively (Fig. 7). The electrodes measured isothermal coefficients reveal that electrodes I and II have good thermal stability up to 60 ºC without deviating significantly from Nernstian behavior.
Potentiometric selectivity
One of the most important properties of PVC membrane (electrode I) and screen-printed electrode (electrode II) is the response to the primary ion in the presence of other ions present in the solution, which is described in terms of potentiometric selectivity coefficients. The matched potential method (MPM) [37] and the fixed interference technique (FIM) [41] were used to determine the selectivity coefficient of the PVC membrane and screen-printed electrodes toward various cationic species (Mn+). The selectivity coefficient was calculated using the matched potential method, which involved measuring the change in potential as the primary ion activity was increased from aA to aA', where aB represents the activity of an interfering ion added to the reference solution of primary ion activity aA, which causes the same potential change. It is expressed as follows:
where aA and aA´ were held at 1.0 × 10−3 and 1.0 × 10−4 mol L−1 Al(III) in this work, while aB was determined empirically. A variety of cations were investigated for possible interferences. The selectivity coefficient in the FIM, on the other hand, was calculated using potential measurements on solutions with a fixed concentration of interfering ion (1.0 × 10−3 mol L−1) and variable amounts of Al(III) ions. The following equation is used to determine the selectivity coefficient:
where aA (DL) is the primary ion activity at the limit of detection, aB is the activity of the interference ion (1.0 × 10−3 mol L−1), and ZA and ZB are the primary charge and the charge of interference ions, respectively. It can be seen from Table 3 that the proposed electrodes are highly selective with regard to a range of cations toward Al(III) ions. A satisfactory agreement exists between the two sets of results obtained by the methods of MPM and FIM. The MSPE (electrode II) has a better selectivity values than the MPVCE (electrode I) because MSPE has many advantages over MPVCE.
Analytical application
Potentiometric titration
In a potentiometric titration of 10 ml Al(III) (1.0 × 10−4 mol L−1) with EDTA (1.0 × 10−3 mol L−1) solution at pH 5.0, the membrane and paste assembly were utilized as the indicator electrodes. As demonstrated in Fig. 8, the change in potential with titrant volume has a well-defined cutoff point. The resulting titration curve and related dE/dV vs volume graph for the titration of Al(III) ion solution with EDTA are shown in Fig. 8, proving that the quantity of Al(III) can be determined reliably using both electrodes I and II. These electrodes can be used as an indicator electrode in Al(III) titrations with appropriate chelating or precipitating agents, as well as for direct measurement of Al(III) ions.
Real petroleum water samples
The electrodes were successfully used to determine Al(III) in a variety of water samples from a variety of sources. Each sample was diluted to 100 mL with distilled water and the necessary amount of HNO3 to achieve a pH of 4.0. The concentration of Al(III) was then determined using the calibration curve and the potential of the diluted solution. Atomic absorption spectrometry (AAS) was used to measure the concentration of Al(III) samples, and the findings are presented in Table 4. The sensors results were found to be in good agreement with those obtained by AAS.
Accuracy and precision
The suggested Al(III) sensors were evaluated by doing five repeated experiments at varied concentrations of Al(III) in pure and spiked actual sample Nos. 2 and 4 to assess the repeatability, validity, and application of the proposed technique. Furthermore, intra- and inter-day studies on various concentrations of samples revealed small RSD percent values (0.94–2.51 and 0.73–1.82%) and high recovery percent values (97.21–99.69 and 98.26–100.11%) for electrodes I and II, respectively, indicating the proposed Al(III) electrodes reasonable repeatability and accuracy (Table 5). To test the electrodes reproducibility, five preparations of pastes with the optimum composition were produced, and their responses were evaluated at an Al(III) ion concentration of 1.0 × 10−3 mol L−1, yielding an RSD percent value of 1.76 and 1.03 for electrodes I and II, respectively.
Comparison with other electrodes
The suggested Al(III)–PVC membrane (electrode I) and Al(III)–MSPE electrodes (electrode II) primary working conditions were compared to those of existing Al(III) ion sensors in the literature [15, 34, 42,43,44,45,46]. The slope and applicability range of the suggested electrodes are in good accord with those published in the literature, as shown in Table 6. The linear dynamic range and the detection limit of the present electrodes seem to be higher than some of the reported ones. This can be attributed to the type of electrode used, the constituent, and the ionophore used.
Conclusion
By using (N1E,N3E)-N1,N3-bis(quinolin-2-ylmethylene)benzene-1,3-diamine (BQMBD) as a neutral carrier, a novel Al(III) ion Al(III)–PVC membrane (electrode I) and Al(III)–MSPE (electrode II) were created. The results revealed that these sensors are an excellent complement to the current Al(III) potentiometric sensor family, since it improved selectivity and sensitivity. These simple, cheap, and renewable Al(III) sensors may be used as an indicator electrodes in potentiometric titrations of Al(III) against EDTA, as well as for sensitive and selective Al(III) detection in actual petroleum water samples. The electrodes were successfully applied for the determination of Al(III) ions in petroleum water sample without significant differences from the reported method as indicated from the t- and F-test values. Acceptable recovery values, low standard, and relative standard deviations were obtained.
References
A. Sajwani, Y. Nielsen, Int. J. Geomate 12, 1 (2017)
C. Exley, Morphologie 100, 51 (2016)
E. Cerme, S. Seven, E. Vural, S. Mercan, I. Bavunoglu, Medicine 9, 790 (2020)
E. Öztürk, S. Yıldırım, A. Akyol, Eur. J. Clin. Nutr. 75, 567 (2021)
J.-C. Qin, X.-Y. Cheng, R. Fang, M.-F. Wang, Z.-Y. Yang, T.-R. Li, Y. Li, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 152, 352 (2016)
F. Guzmán-Osorio, V. Domínguez-Rodríguez, R. Adams, C. Lobato-García, A. Guerrero-Peña, J. Barajas-Hernández, Egypt. J. Petrol. 30, 63–67 (2021)
N. Şatıroğlu, İ Tokgöz, Int. J. Environ. Anal. Chem. 90, 560 (2010)
M.A. Qadir, M. Ahmed, S. Shahzad, Anal. Lett. 48, 147 (2015)
T. Samarina, D. Volkov, I. Mikheev, M. Proskurnin, Anal. Lett. 51, 659 (2018)
P. Masson, T. Dalix, S. Bussiere, Commun. Soil Sci. Plant Anal. 41, 231 (2010)
E.J. Drobyshev, N.D. Solovyev, N.B. Ivanenko, M.Y. Kombarova, A.A. Ganeev, J. Trace Elem. Med. Biol. 39, 14 (2017)
H. Landis, K. Hicks, I. McCall, J. Henry, B. Whipker, III International Symposium on Growing Media, Composting and Substrate Analysis, p. 13052019
X. Du, Y. Xu, L. Qin, X. Lu, Q. Liu, Y. Bai, Am. J. Anal. Chem. 2014 (2014).
S. Abbasi, A. Farmany, M.B. Gholivand, A. Naghipour, F. Abbasi, H. Khani, Food Chem. 116, 1019 (2009)
M. Arvand, S. Asadollahzadeh, Talanta 75, 1046 (2008)
T.A. Ali, Z.F. Akl, J. Radioanal. Nucl. Chem. 328, 267 (2021)
T.A. Ali, A. Al-Sabagh, Egypt. J. Chem. 64, 9 (2021)
T.A. Ali, A.A. Abd-Elaal, G.G. Mohamed, Microchem. J. 160, 105693 (2021)
E.S. Gad, T.A. Ali, A.A. Elsayed, G.G. Mohamed, H. El-Bary, Int. J. Electrochem. Sci. 15, 11904 (2020)
M. Esmaelpourfarkhani, G.H. Rounaghi, M.H. Arbab-Zavar, J. Braz. Chem. Soc. 26, 963 (2015)
D.S. Tyagi, A. Singh, Anal. Bioanal. Electrochem. 5, 588 (2013)
X. Liu, R. Yuan, W. Xu, Y. Ma, Y. Chai, J. Chin. Chem. Soc. 58, 482 (2011)
T.A. Ali, G.G. Mohamed, H. Eldessouky, A.-E. Adeeb, Egypt. J. Petrol. 28, 233 (2019)
S. Rana, S.K. Mittal, N. Singh, J. Singh, C.E. Banks, Sens. Actuat. B Chem. 239, 17 (2017)
V. Gupta, A.K. Jain, M. Al Khayat, S. Bhargava, J. Raisoni, Electrochim. Acta 53, 5409 (2008)
S. Chandra, P.K. Tomar, A. Kumar, A. Malik, A. Singh, J. Saudi Chem. Soc. 20, S293 (2016)
O. Özbek, Ö. Isildak, M.B. Gürdere, C. Berkel, Int. J. Environ. Anal. Chem. 1, 1–16 (2020)
Ö. Isildak, O. Özbek, J. Chem. Sci. 132, 1 (2020)
T.A. Ali, G.G. Mohamed, Sens. Actuat. B Chem. 216, 542 (2015)
T.A. Ali, G.G. Mohamed, Anal. Methods 7, 6280 (2015)
Z.F. Akl, T.A. Ali, RSC Adv. 6, 77854 (2016)
T.A. Ali, G.G. Mohamed, A.H. Said, Chem. Eng. Commun. 203, 724 (2016)
T.A. Ali, M.H. Soliman, G.G. Mohamed, A.B. Farag, M.K. Samah, Int. J. Electrochem. Sci. 10, 3192 (2015)
H. Yao, S. Wang, X. Ma, L. Ren, F. Yan, Int. J. Electrochem. Sci 9, 2158 (2014)
T.A. Ali, G.G. Mohamed, G.A. Yahya, Iran. J. Pharmaceut. Res. 16, 498 (2017)
T.A. Ali, G.G. Mohamed, M.M. El-Dessouky, R.M. Ragheb, Int. J. Electrochem. Sci. 10, 4820 (2015)
K. Tohda, D. Dragoe, M. Shibata, Y. Umezawa, Anal. Sci. 17, 733 (2001)
F. Nworie, F. Nwabue, N. Elom, S. Eluu, J. Basic Appl. Res. 2, 295 (2016)
T.A. Ali, G.G. Mohamed, M. Omar, N.M. Hanafy, J. Ind. Eng. Chem. 47, 102 (2017)
T.A. Ali, G.G. Mohamed, A.R. Othman, Int. J. Electrochem. Sci. 10, 7275 (2015)
Y. Umezawa, P. Bühlmann, K. Umezawa, K. Tohda, S. Amemiya, Pure Appl. Chem. 72, 1851 (2000)
M. Arvand, M. Kermanian, Food Anal. Methods 6, 578 (2013)
A. Abbaspour, A. Esmaeilbeig, A. Jarrahpour, B. Khajeh, R. Kia, Talanta 58, 397 (2002)
A. Yari, L. Darvishi, M. Shamsipur, Anal. Chim. Acta 555, 329 (2006)
M. Mousavi, M. Arvand-Barmchi, M. Zanjanchi, Electroanal. Int. J. Devot. Fund. Pract. Aspects Electroanal. 13, 1125 (2001)
V.K. Gupta, A.K. Jain, G. Maheshwari, Talanta 72, 1469 (2007)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Missing Open Access funding information has been added in the Funding Note.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, T.A., El-Henawy, S.B. & Mohamed, G.G. Electroanalytical determination of Al(III) ion in petroleum water samples using symmetric 1,3-diamine-based Schiff base as a carrier. J IRAN CHEM SOC 19, 3549–3560 (2022). https://doi.org/10.1007/s13738-022-02549-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-022-02549-0