Abstract
An eco-friendly green corrosion inhibitor Commiphora Mukul was tested for its efficacy to control material loss in 6061 aluminum alloy under collective influence of mechanical erosion and electrochemical corrosion in a submerged jet impingement rig. Electrochemical techniques were utilized in the current investigation which consisted of potentiodynamic polarization and electrochemical impedance spectroscopy. The effect of temperature and flowrate of artificial seawater slurry on the inhibitory effect of Commiphora Mukul is investigated. Under the experimental conditions of 303 K temperature and 4 L min−1 flowrate, the inhibitor showed an efficiency of 54% as determined by the potentiodynamic polarization studies. With the increase in temperature and flowrate of artificial seawater slurry, the protection efficiency of the inhibitor decreased. Protection efficiency of 35% was observed. Possible reasons for this phenomenon were discussed. Electrochemical impedance studies reported that the process is both charge transfer and diffusion controlled. At 323 K, the diffusion component was prominent for all the studied flowrates of 4 L min−1, 8 L min−1, and 12 L min−1. It seems that the moving medium makes it challenging for the inhibitor molecules to adsorb on the metal surface in the presence of abrasive particles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Material damage due to erosion-corrosion is a severe problem faced by many industrial applications like slurry pumps, hydraulic turbines, and transport of solids in pipelines, pipelines, mining machinery components, marine and oil and gas industries, etc. Slurry erosion is a type of material loss encountered by any component, when it is exposed to a high velocity fluid stream or to a stream consisting of solid particles where the fluid is generally water. The service life of the equipment is considerably reduced due to slurry erosion resulting in huge economic losses [1]. Aluminum and its alloys have significant importance in the industry due to their high technological value [2]. Because of their excellent properties of high specific strength, good formability, and good corrosion resistance, Al6061 is extensively used. They show a wide application in the areas of subsea installations, deep sea mining, and in various naval underwater requirements [3, 4]. Therefore, when applied in such fields, aluminum alloy is often exposed to rain or seawater [5]. Aluminum alloys in seawater show corrosion resistance owing to the self-repairing protective oxide film, but their wear loss is comparatively larger [3].
In such aggressive environments, corrosion mitigation is of prime importance. One practical and economical method is the use of corrosion inhibitors. Adsorbed anti-corrosive component molecules isolate the surface from the corrosive environment alleviating corrosion. Nevertheless, their application is limited due to the environmental and human health hazards posed. The concern was related to the environmental poisoning and also to the contact of the protected metal for instance with food, beverage, medicals, during its lifecycle. These inhibitors may cause damage to the human organ system or may disturb the biochemical process at some site in the body [6]. These drawbacks have made many scientists to anticipate the use of eco-friendly inhibitors [7].
Zeng et al. investigated the application of imidazole ionic liquids as mild steel eco-friendly inhibitor in sulfuric acid. The weight loss studies confirmed that the inhibitor can exhibit good corrosion mitigation behavior with increasing temperature [8]. In another study, Yuan-Ting Du et al. selected baicalin derivatives synthesized from Scutellaria baicalensis Georgi as aluminum corrosion inhibitor in HCl solution. Maximum protection efficiency of 95% was obtained at 25 °C [9]. Based on potentiodynamic polarization results of AA2024-T3 alloy in NaCl solution, the pitting resistance was significantly enhanced in the presence of eco-friendly inhibitors namely, cysteine and histidine molecules [10]. The corrosion alleviation action of Brassica campestris extract on Cor-Ten steel in NaCl solution was investigated by Casaletto et al. [11]. Luo et al. [12] understood the mixture of green inhibitors (glucomannan and bis quaternary ammonium salt) can behave as strong inhibitors for mild steel corrosion in simulated seawater. The aqueous extract of Lawsonia inermis has been experimentally studied as a corrosion inhibitor for nickel, zinc, and carbon steel in neutral and alkaline solutions using electrochemical techniques [13].
Using plant extracts as good corrosion inhibitors for metals is one of their major applications. Plant extracts are extensively used as corrosion inhibitors in saline waters. Generally, inhibitor evaluations are based on experiments conducted under static conditions. Most of the industries operate under turbulent conditions and because of high mass and heat transfer realized the inhibitor fails to function efficiently due to its incompetence to remain at the surface. Recently reported work considers inhibitor performance in high shear conditions [14,15,16].
In view of the above facts, the current investigation focuses on the performance of green inhibitor, Commiphora Mukul in combatting erosion-corrosion of 6061 aluminum alloy.
Materials and methods
The study material in the current investigation is 6061 Aluminum alloy. The elemental composition of 6061 aluminum alloy is 0.96% magnesium, 0.80% silicon, 0.27% copper, 0.21% chromium, 0.40% is Iron and the balance is aluminum.
The machined substrate was embedded in epoxy resin exposing a working area of 1.1 cm2. The surface of the alloy was polished using emery paper of 600 grit size.
The testing solution, the artificial seawater, was prepared as per ASTM standards. The composition of artificial water is sodium chloride—24.53%, magnesium chloride—5.20%, sodium sulfate—4.09%, calcium chloride—1.16%, potassium chloride—0.695%, sodium bicarbonate—0.201%, potassium bromide—0.101%, boric acid—0.027%, strontium chloride—0.025%, sodium fluoride—0.003% [3]. Table 1 displays the experimental parameters of the study.
Electrochemical measurements
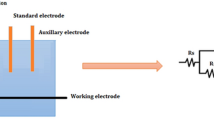
Electrochemical studies were accomplished with an electrochemical workstation (CH-600 D-Series US, with a beta software) consisting of three electrode system: platinum as the counter electrode, saturated calomel as the reference electrode, and AA 6061 as the working electrode [17]. The electrode potential that is measured with respect to the reference electrode when no current flows through it is known as the OCP. Potentiodynamic polarization curves were obtained by polarizing the electrode at ± 500 mv at a scan rate of 1 mV s−1. The frequency-domain chosen was 10 kHz to 0.1 Hz with an applied 10 mv AC voltage [18].
Inhibitor
Commiphora Mukul, also known as guggulu, is a highly valued drug in Ayurvedic medicine and is used for more than 2000 years. It belongs to Burseraceae family and is used in the treatment of various ailments [19]. Ethanolic extract of C. Mukul gum resin was obtained from the manufacturers and exporters of herbal extracts, Ms Revinto Life sciences Pvt. Ltd., Karnataka, India.
One of the major actives present in Commiphora Mukul is guggulusterones. Commiphora Mukul contains antioxidants, which might account for its corrosion inhibition property through their and chemical structure.
Experimental setup
The experiments were carried out in a submerged jet-impingement rig as illustrated in Fig. 1. The experimental rig consisted of a reservoir tank, a pump, flow rate controller, temperature controller, thermocouple, and valves. An electrochemical cell was incorporated into the rig to perform the electrochemical studies. The slurry was driven by a centrifugal pump through a recirculating loop which leaves the 8 mm diameter nozzle to impact in a direction normal to the specimen.
Results and discussion
Potentiodynamic polarization studies
Potentiodynamic polarization curves for aluminum alloy at various temperatures and flowrate in the absence and presence of 1000 ppm of inhibitor are presented in Fig. 2.
As is in the figure, the trend shows that as the flow rate increases, the current densities seem to increase. This indicates that the performance of Commiphora Mukul as a corrosion inhibitor tends to gradually weaken as the flowrate increases.
At 303 K—12 L min−1—the anodic current density increased as compared to 4 L min−1 and 8 L min−1, but the cathodic current density decreased.
At 313 K—the anodic and cathodic current densities increased at higher flowrates as compared to 4 L min−1. It is obvious that the metal became more active compared to that at 4 L min−1. At 313 K—4 L min−1, passivity is observed. At lower flowrates, the shear effects are not dominant that they can remove the protective layer formed on the metal surface. But with the increase in the flowrate, passivity disappears.
At 323 K—4 L min−1—passivation is observed. This passivation disappears with the rise in slurry flowrate.
At 323 K—12 L min−1—the cathodic current density increases as compared to the lower flowrates.
With the increase in slurry flowrate, the mechanical impact of sands contained in the solution is enhanced significantly. Hence, there develops a competition among activation and oxidation of the target material in the slurry. While activation is dominant due to the impact of sand, a negative shift of corrosion potential is observed [1]. Table 2 demonstrates the values of corrosion potential, corrosion current density, and protection efficiency at different flow rates and temperatures. After the addition of the inhibitor, the corrosion current density diminished at all experimental conditions.
Variation of Ecorr
If the shift in the Ecorr value varies more than ± 85 mV, the inhibitor is said to be cathodic or anodic. If the shift happens to be less than ± 85 mV, then the inhibitor is said to behave like a mixed inhibitor, where the inhibitor controls both the anodic and the cathodic reactions to different levels. A lesser corrosion potential value (i.e., a higher affinity to corrosion) and a higher corrosion rate could be witnessed in the slurry flow condition. The enhancement in oxygen supply causes an increase in the corrosion potential, while the removal of corrosion product film roots to the decrease in corrosion potential [11].
Effect of inhibitor concentration
Potentiodynamic polarization studies were carried out with 500 ppm and 1000 ppm Commiphora Mukul in artificial seawater slurry. The protection efficiency of the inhibitor increased with an increase in concentration. However, the trend of decreasing inhibition efficiency with respect to flow rate and temperature remained the same at both concentrations. When inhibitor concentration was at 500 ppm (data not presented here), adsorbed inhibitor molecules decreased the number of surface active sites by paralleled adsorption on the metal surface. As the concentration was increased, more inhibitor molecules adsorbed on the target blocking more active sites. Increase in the inhibitor concentration further did not indicate any significant change which may be due to mutual repulsion.
Hydrodynamics shows two opposite effects on inhibitor performance. Increased flow velocity may increase mass transport of inhibitor molecules to the vicinity of the target surface and increased shear stress may also result in the removal of inhibitor layers from the surface. This combined effect results in an increased concentration for a better performance of the inhibitor [21].
Effect of flowrate on corrosion rate
Figure 3 illustrates the variation of corrosion rate of 6061 aluminum alloy with respect to flowrate with and without the inhibitor. The decrease in the corrosion density in the presence of inhibitor indicates a decrease in corrosion rate. But the performance of the inhibitor deteriorates at higher flowrates. With increasing slurry flow rates, metals are put under an active state which is a consequence of the decrease in the diffusion layer and increased transport of oxygen [22, 23]. An increase in mechanical degradation leads to the availability of larger surface areas for attack by the corrosive media [24]. These processes occur simultaneously, significantly enhancing the corrosion and erosion rate, in case when corrosion product/inhibitor film does not adsorb to the surface [25]. Formation of Inhibitor film may be prohibited because of the local acidification in the pits. As a consequence, the corrosion current density and hence corrosion rate will increase [26].
The corrosion product layer formed at a lower flowrate may not be much protective but may adsorb the inhibitor molecules. Meanwhile, these corrosion product layers growing with inhibitor concentrations may exhibit a varied range of protectiveness. Both corrosion product layer and inhibitor layer are influenced by flow velocity and are expected to degrade exposing the bare metal surface. Few inhibitor molecules impacted by the sand were easily removed from the target surface, yet they may still adsorb back again to the target after the impacted stress was dismissed locally.
Also one of the factors for increased corrosion attack may be weak adsorption of the inhibitor molecules on the surface of the specimen prior to the formation of corrosion product [27]. The probable generation of bubbles during the impingement of the artificial seawater solution as a result of turbulence may also result in corrosion layer damage and increased corrosion rate. The passive layer that is formed on the 6061 aluminum alloy may be removed locally due to the impingement of sand particles. Increasing the flow rate to a larger value causes an increase in the particle energy leading to greater localized damage which further causes a higher plastic deformation resulting in more work hardening of the alloy surface. Accelerated dislocation density as a result of work hardening and localized removal turns the alloy more active consequently increasing the corrosion rate [28].
The influence of mass transfer on the behavior of the metals subjected to various flow rates can be accredited to either transfer of chemical species, inhibitor molecules, or corrosion products. Usually, increasing the flow rate increases the accessibility of the reacting species to the metal surface, enhances the movement of corrosion products or the inhibitor molecules and intensifies the impact energy. Most frequently, there is a movement of the chemical species to and away from the anode surface. Alternatively, the corrosion product may be transported from the alloy surface further causing difficulty in the formation of a protective layer [29].
Role of sand
The slurry flow containing suspended sand can weaken the metal surface fully or partially based on the velocity, angle of impingement, mechanical strength of the target, and shape of the solid particles involved in the erosion process [30].
At higher velocities, higher material loss is observed. This is due to the fact that particles carry higher kinetic energy at higher flowrates which leads to excessive material removal. It is understood that particles carry lower kinetic energies at lower velocities [31]. At an impingement angle of 90°, the energy transfer normal to the metal surface will be at its maximum. Partial exclusion of the protective surface film generates favorable conditions for accelerated pitting corrosion [32]. When the substrate is under the normal impact, minimum damage occurs due to the shear force exerted by the erodent particles on the sample which leads to the least damage, and therefore, losses due to abrasion are insignificant. The impacting particle energy is directly proportional to the velocity. Therefore, as the flow rate increased the wear rates increase [33].
Effect of temperature on corrosion rate
The consequence of temperature on the performance of the inhibitor is depicted in Fig. 3. The temperature has a significant effect on the corrosion rate of the metal. The data reveal that the corrosion current density increases with temperature. The corrosion process gets accelerated as the temperature rises. A rise in the temperature leads to the weakening of the adsorption forces between the inhibitor and the metal temperature [34]. Stable corrosion product film is formed at higher, whereas the film formed at lower temperature has a higher porosity and is not more stable [35].
In a neutral solution, the intensification of temperature has a favorable influence on the rate of oxygen diffusion and a decrease in its solubility. The weakening of physical adsorption may be one of the reasons for a decrease in inhibition efficiencies with a rise in temperature. The influence of slurry flowrate on corrosion enhances with the increase in temperature. Similar behavior was observed in the presence of inhibitors [23].
Electrochemical impedance spectroscopy
The EIS plots function as potent tool in giving a detailed information about the interfacial corrosion process. In artificial seawater medium along with 1000 ppm inhibitor (Fig. 4), the Nyquist plots are featured with a high frequency (HF) and medium frequency (MF) capacitive loop and a low-frequency (LF) diffusive tail. The capacitive loops indicate the resistance presented for corrosion to continue. The HF capacitive loop is often credited to charge transfer resistance for corrosion [36]. The diffusive tail is suggestive of the Warburg effect.
A continuous decrease in the radius of the semicircle with the increase in the flow rate is observed in this figure at all temperatures.
Diffusion created impedance is referred to as the Warburg impedance. This is dependent on the perturbation frequency. The Warburg impedance is usually small at HF domain since diffusing reactants do not move very far. The Warburg impedance increases at the LF domain since at low frequencies the reactants have to diffuse farther. On the impedance spectrum, the infinite Warburg impedance appears as a diagonal line with a slope of 0.5 [37].
During erosion corrosion, mass transfer of reactants to and from the metal surfaces plays a key role. Normally, dissolved ions or oxygen diffuses from the metal to the electrolyte and from the solution to the metal [38].
Inductive effect is caused by the continuous adsorption and desorption of inhibitor molecules. It is also an indication of inhibitor not being completely adsorbed on the electrode during the erosion corrosion process resulting in low inhibition efficiency [39].
Equivalent circuits
The impedance data were analyzed by fitting it into an equivalent circuit. Simulated circuit illustrated in Fig. 5 is a model fit for the following conditions: 313 K—4 L min−1, Fig. 6: 303 K-4 L min−1, 8 L min−1, 12 L min−1, 313 K-8 L min−1, 12 L min−1, 323 K-4 L min−1, 8 L min−1, 12 L min−1. The change in the equivalent circuit under various experimental conditions explains the changes undergoing on the metal surface during the tests process. Equivalent circuit in Fig. 5 consists of the following elements: solution resistance Rs, inductance L, resistance R1 and R2, constant phase element Q. The resistance between the working and the reference electrode is known as the solution resistance. A constant phase element present in the circuit indicates that a pure capacitor behavior is not exhibited by the film.
R1 denotes the porous layer resistance because it is continuously exposed to the electrolyte. Solution resistance within the pores of the film is dependent on the defective film structure. R2 refers to the resistances of the inner compact portion of the passive film.
In Fig. 5a, the Warburg element does not exist indicating that the pores are clogged due to the reaction components.
The equivalent circuit in Fig. 6b consists of the following elements: solution resistance Rs, resistance R1 and R2, capacitance C1, constant phase element Q, Warburg element W. The presence of W and CPE provides credibility to the circuit indicating that the inhibitor layer formed on the metal surface is not perfect and it involves electrochemical and diffusion process causing inhibitor film deterioration [40].
Various transport mechanisms occur in the outer and inner regions as the film develops. In the formation of a passive layer multilayered in nature, internal redox reactions play a key role. In various cases, the compositions of these layers may vary.
In Fig. 6b, R2 accounts for the resistance due to the corrosion products/inhibitor complex above the active corrosion sites. The active sites are represented by the parallel connection between the polarization resistance R2 connected in series with Warburg W and constant phase element Q.
The pores are no longer blocked with compounds from artificial sea water solution or with reaction products may be because, the rate of reaction is very low and the quantity of corrosion reaction products is very small [41].
Bode plot
In the Nyquist plot, when we proceed from left to right, the frequency decreases. The data collected over the frequency range are not displayed in the Nyquist plot, and therefore, the same data are expressed in Bode plots.
Two time constants are observed in the Bode plots as seen in Fig. 7. Peak at HF domain is attributed to passive layer and the peak at LF is ascribed to the charge transfer resistance. Two time constants are observed in Bode plot, and therefore, the chosen simulated circuit also consists of two time constants. Bode magnitude plots for the experiments conducted at various temperatures and flow rates are shown in Fig. 8. From Fig. 8, it can be observed that highest resistance to corrosion is offered by the lower flowrates irrespective of temperatures. At LF, the modulus |Z| indicates the barrier resistance offered by the film.
The presence of a protecting and thin isolated inhibitor film is a characteristic of the high frequency time constant, whereas the LF time constant relates to the process of corrosion at unprotected sites. The latter is a consequence of electrolyte penetrating into the pores present in the inhibitor film.
The impedance of the alloy at lower flow rate of 4 L min−1 is larger as compared to other higher flowrates which reveals that the inhibitor shows a better performance at lower flowrates.
SEM morphology
Figure 9a depicts the pits, craters formed due to corrosion and erosion. Ploughing mechanism and lip formation may be observed in Fig. 9b. The particles in the slurry slide over the electrode resulting in ploughing [42]. The lip formation can also be seen on the eroded surface. Impingement at an angle of 90° results in the formation of crater ‘‘lips’’ of deformed material due to fatigue. In nature, these lips are fragile and would be removed by successive impact by the particles [43]. Further, these are successively removed as platelets after successive particle impact [44]. When the slurry impinges at 90°, normal stress is applied at the electrode even though the film is broken, it still remains on the surface of the electrode, providing a certain level of protection [20].
Conclusion
-
1.
The results depict that enhancement in flowrate decreases the protection efficiency of Commiphora Mukul against erosion corrosion.
-
2.
The inhibitor Commiphora Mukul showed a highest inhibition efficiency of 54% at 1000 ppm concentration, temperature of 303 K, and flowrate of 4 L min−1.
-
3.
The low protection efficiency may be ascribed to drastic turbulence flow and greater wall shear stress throughout the test, which checks the inhibitor adsorption and harms the adsorbed inhibitor film.
-
4.
The impedance spectrum reveals that the erosion corrosion mitigation mechanism for 6061 aluminum alloy in artificial seawater is both charge and diffusion controlled.
References
C.S. Ramesh, R. Keshavamurthy, B.H. Channabasappa, S. Pramod, Mater. Des. 30(9), 3713–3722 (2009). https://doi.org/10.1016/j.matpr.2015.07.234
A.S. Fouda, A.A. Al-Sarawy, F.S. Ahmed, H.M. El-Abbasy, Prot. Met. Phys. Chem. 45(5), 635–643 (2009). https://doi.org/10.1134/S2070205109050244
M.I.U. Haq, A. Raina, K. Vohra, R. Kumar, A. Anand, Mater. Today Proc. 5, 3602–3609 (2018). https://doi.org/10.1016/j.matpr.2017.11.610
N.R. Rameshand, V.S. Senthil Kumar, Appl. Ocean Res. 98, 102121 (2020). https://doi.org/10.1016/j.apor.2020.102121
H. Ding, G. Zhou, Z. Dai, Y. Bu, T. Jiang, Wear 267, 292–298 (2009). https://doi.org/10.1016/j.wear.2008.11.031
S. Marzorati, L. Verotta, S. Trasatti, Molecules 24, 48 (2019). https://doi.org/10.3390/molecules24010048
G. Palumbo, K. Berent, E. Proniewicz, J. Banaś, Materials 12(16), 2620 (2019). https://doi.org/10.3390/ma12162620
X. Zeng, X. Zheng, L. Guo, Q. Xu, H. Huang, B. Tan, J. Mol. Liquids (2020). https://doi.org/10.1016/j.molliq.2020.115063
Y.T. Du, H.-L. Wang, Y.-R. Chen, H.-P. Qi, W.-F. Jiang, J. Environ. Chem. Eng. (2017). https://doi.org/10.1016/j.jece.2017.11.004
M. Izadi, A.R. Rad, T. Shahrabi, I. Mohammadi, Mater. Chem. Phys. (2020). https://doi.org/10.1016/j.matchemphys.2020.122997
M.P. Casaletto, V. Figà, A. Privitera, M. Bruno, A. Napolitano, S. Piacente, Corros. Sci. 136, 91–105 (2018). https://doi.org/10.1080/17518253.2019.1578997
X. Luo, X. Pan, S. Yuan, S. Du, C. Zhang, Corros. Sci. 125, 139–151 (2017). https://doi.org/10.1088/1742-6596/1378/3/032046
N. Patni, S. Agarwal, P. Shah, Chin. J. Chem. Eng. (2013). https://doi.org/10.1155/2013/784186
A. Neville, C. Wang, Wear 267(1–4), 195–203 (2009). https://doi.org/10.1016/j.wear.2009.01.038
J.L. Mora-Mendoza, S. Turgoose, Corros. Sci. 44(6), 1223–1246 (2002). https://doi.org/10.1016/S0010-938X(01)00141-X
C. Wang, A. Neville, Facil. Constr. 01, 1–10 (2009). https://doi.org/10.2118/114081-PA
M. Abedini, H.M. Ghasemi, Trans. Nonferrous Met. Soc. China 27, 2371–2380 (2017). https://doi.org/10.1016/S1003-6326(17)60263-2
M. Lavanya, V.R. Murthy, P. Rao, Chin. J. Chem. Eng. 28, 340–347 (2020). https://doi.org/10.1016/j.cjche.2019.07.016
S. Nakhaee, S. Ghasemi, K. Karimzadeh, N. Zamani, S. Alinejad-Mofrad, O. Mehrpour, Subst. Abuse Treat. Prev. Policy 15, 1–13 (2020). https://doi.org/10.1186/s13011-020-00272-8
G.A. Zhang, L.Y. Xuand, Y.F. Cheng, Corros. Sci. 51(2), 283–290 (2009). https://doi.org/10.1080/1478422X.2016.1141546
Q. Luo, Q. Zhang, Z. Qin, Z. Wu, B. Shen, L. Liu, W. Hu, J. Alloys Compd. 747, 861–868 (2018). https://doi.org/10.1016/j.jallcom.2018.03.103
X. Jiang, Y.G. Zheng, W. Ke, Corros. Sci. 47(11), 2636–2658 (2005). https://doi.org/10.1016/j.corsci.2004.11.012
S.B. Choe, S.J. Lee, Ocean Eng. 141, 18–24 (2017). https://doi.org/10.1016/j.oceaneng.2017.05.035
A.A. Khadom, J. Chil. 59(3), 2545–2549 (2014). https://doi.org/10.4067/S0717-97072014000300004
R. Ketrane, B. Saidani, O. Gil, L. Leleyter, F. Baraud, Desalination 249(3), 1397–1404 (2009). https://doi.org/10.1016/j.desal.2009.06.013
F.-A. Setta, A. Neville, Desalination 281, 340–347 (2011). https://doi.org/10.1016/j.desal.2011.08.021
M. Lavanya, V.R. Murthy, P. Rao, J. Bio Tribo Corros. 5, 93 (2019). https://doi.org/10.1007/s40735-019-0288-7
S. Yahya, N.K. Othman, M.C. Ismail, Eng. Fail. Anal. 100, 365–380 (2019). https://doi.org/10.1016/j.engfailanal.2019.02.036
O.O. Ige, S.R. Oke, O.E. Falodun, S. Aribo, K.M. Oluwasegun, O.O. Ajibola, J.O. Olawale, A. Ogunbadejo, O.T. Olalemi, P.A. Olubambi, Mater. Today Proc. 38(7), 1–8 (2020). https://doi.org/10.1016/j.matpr.2020.03.709
K.A. Said, M.A. Amin, Overview on the response surface methodology (RSM) in extraction processes. JASPE 2(1), 8–16 (2015). https://doi.org/10.33736/jaspe.161.2015
M.Y. Naz, N.I. Ismail, S.A. Sulaiman, S. Shukrullah, Sci. Rep. 5, 16583 (2015). https://doi.org/10.1016/j.measurement.2017.04.042
S. Aribo, A. Fakorede, O. Ige, P. Olubambi, Wear 376, 608–614 (2017). https://doi.org/10.1016/j.wear.2017.01.034
M.A. Islam, Z.N. Farhat, J. Bio Tribo Corros. 1(4), 26 (2015). https://doi.org/10.1007/s40735-015-0027-7
S. Das, Y.L. Saraswathiand, D.P. Mondal, Wear 261(2), 180–190 (2006). https://doi.org/10.1016/j.wear.2005.09.013
S.J. Amirfakhri, J.L. Meunier, D. Berk, Electrochim. Acta 114, 551–559 (2013). https://doi.org/10.1016/j.electacta.2013.10.094
H. Shahali, H.M. Ghasemi, M. Abedini, Mater. Chem. Phys. 233, 366–377 (2019). https://doi.org/10.1016/j.matchemphys.2019.05.051
M.A. Deyab, J. Mol. Liquids 271, 240–245 (2018). https://doi.org/10.1016/j.molliq.2018.09.002
S. Reddy, R. Du, L. Kang, N. Mao, J. Zhang, Appl. Catal. B Environ. 194, 16–21 (2016). https://doi.org/10.1002/smll.201600398
S.S. Rajahram, T.J. Harvey, R.J.K. Wood, Wear 267(1–4), 244–254 (2009). https://doi.org/10.1016/j.wear.2009.01.052
L. Zeng, G.A. Zhang, X.P. Guo, C.W. Chai, Corros. Sci. 90, 202–215 (2015). https://doi.org/10.1016/j.corsci.2014.10.011
S.K. Shetty, A.N. Shetty, J. Mol. Liquids 225, 426–438 (2017). https://doi.org/10.1016/j.molliq.2016.11.037
D.B. Nergis, P. Vizureanu, D. Țopa, M.G. Minciuna, M.M. Abdullah, Mater. Sci. Eng. C. 877, 1–1012044 (2020). https://doi.org/10.1088/1757-899X/877/1/012044
K. Goyal, World J. Eng. (2016). https://doi.org/10.1007/s12666-016-0956-y
R.C. Shivamurthy, M. Kamaraj, R. Nagarajan, S.M. Shariff, G. Padmanabham, Metall. Mater. Trans. A 41(2), 470 (2010). https://doi.org/10.1179/1743294413Y.0000000168
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mulky, L., Murthy, V.R. & Rao, P. An insight into inhibitory performance of Commiphora Mukul on corrosion of aluminum alloy under tribological conditions. J IRAN CHEM SOC 18, 2953–2963 (2021). https://doi.org/10.1007/s13738-021-02245-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02245-5