Abstract
In the first part of the work, anodic oxidation of some selected low-permittivity liquids (1,4-dioxane, diethyl ether, diisopropyl ether, dichloromethane, trichloromethane, tetrahydrofuran, carbon disulfide, triethylamine, trihexylamine, ethyl acetate, 1-pentyl acetate) was investigated on platinum electrode in acetonitrile which provided a higher-permittivity medium to enable the electrode processes. The pure solvents containing supporting electrolyte were also studied, and in several solvents, very low currents flowed when their cyclic voltammograms were recorded between 0 and 4 V. Contrarily, dissolving them in acetonitrile, high currents could be recorded and their anodic peaks appeared. The most remarkable difference between the pure liquid and acetonitrile solution was produced by the amines. Trihexylamine was immiscible with acetonitrile, so its solubility was determined by using the cosolvent calibration method (mixture of acetonitrile and 1-propanol) for the voltammetric determination, and the saturated concentration of the amine was 44.253 ± 0.564 mM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dielectric constant of solvents basically determines their usefulness for electrochemical investigations. As it is known, when a supporting electrolyte with lipophilic character is dissolved in the liquids, the extent of its dissociation into ions is highly influenced by the solvent permittivity [1, 2]. Due to the low concentration of ions and therefore the significant ohmic potential drop, a problem is encountered in low-permittivity solvents when thermodynamic properties of compounds or solvents themselves are in focus. In practice, the ohmic potential drop results in shift of anodic peaks to more positive potential and in shift of cathodic peaks to more negative potentials. In these cases, the use of a higher-permittivity solvent provides an appropriate solution for the study of the selected compounds. To minimize the ohmic potential drop occurring in very low-conductivity environments, ultramicroelectrodes are applied in media with lower-dielectric constant [3,4,5], but their usefulness is also limited if it is extremely low (between 1 and 5).
Acetonitrile is an organic solvent with high permittivity (ε = 36 [6]) which is miscible with a series of other organic liquids. It provides an excellent medium for electrooxidation processes in a wide anodic potential window. Anodic oxidation of rare gases [7] and short-chain alkanes [8] was carried out at ultramicroelectrodes in this solvent at extremely high potentials.
In this article, low-permittivity solvents were studied by anodic oxidation in media with higher permittivity making possible charge transfer processes at significantly lower potentials. The experiment was carried out for making visible the differences between the liquids and their solutions prepared with acetonitrile according to the appearance of their peaks.
Experimental
Experiments were carried out by using analytical-grade chemicals. Platinum disk (1 mm in diameter) sealed in polyetheretherketone was the working electrode, Ag wire served as reference electrode, and Pt wire was the counter electrode. Thorough polishing was applied before all experiments for the Pt disk with 0.05 μm alumina on a polishing cloth and finally washed with deionized water. All electrochemical experiments were carried out with a potentiostat (DropSens, Spain). Tetrabutylammonium perchlorate (TBuClO4) was used as supporting electrolyte.
Results and discussion
Voltammetric investigation of pure solvents
In order to establish the electrochemical properties of low-permittivity solvents, cyclic voltammetric studies were carried out between 0 and 4 V in their own phase with scan rate of 0.1 V/s and in the presence of 0.2 M supporting electrolyte. The corresponding voltammograms are displayed in Fig. 1.
It is clearly seen that carbon disulfide, triethylamine, and trihexylamine did not show any detectable current signal in the whole anodic potential range due to their very low permittivity and consequently the very low concentration of ions presenting in the liquid phase. The permittivities of the selected solvents were mainly below 5, and the dielectric constants of ethyl acetate, dichloromethane, and tetrahydrofuran were only a little higher. In solvents with lowest permittivity, the solid crystals of TBuClO4 could not dissolve completely. The crystals of supporting electrolyte could be evidently seen also after thorough mixing. As a consequence, its concentration presenting in the solution phase (solubility) was well below 0.2 M.
The current signals recorded in the ethers (diethyl and diisopropyl ether, dioxane) and esters (ethyl- and 1-pentylacetate) indicate ohmic behavior. The slopes of the current–potential curves reflect the conductivity of the liquids. In these situations, they can be treated as the electroactive material whose concentration is as high as possible (the pure material). When the conductivity of the medium is not satisfactory, the anodic peaks or waves by electrooxidation reactions are shifted to more positive potentials by increasing the concentration of electroactive material. Due to the extremely low conductivity and very high concentration of liquids, the raising part of the voltammograms (mainly charging currents flow where the I-E curve is linear) lies on a very wide potential window. In these cases, the shapes of voltammograms are independent of the electrode size except for cases where it is extremely small (ultramicroelectrodes). Diisopropyl ether gave higher current signal than diethyl ether due to the higher solubility of the supporting electrolyte. The difference could be established also with naked eye. Trichloromethane and ethyl acetate gave similar magnitude signals due to their similar permittivities. They could dissolve the supporting electrolyte as their permittivities are a little higher than the accessible limit for use in electrochemistry. Ethyl acetate itself provides an appropriate medium for electrochemical investigations when microelectrodes are used in the presence of high supporting electrolyte concentration as it was shown by works of Agüí in determination of vanillin [9] and thiram [10].
Regular voltammograms could be recorded only in tetrahydrofuran and in dichloromethane due to their higher permittivities when compared with the other liquids.
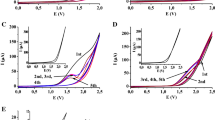
Studies of low-permittivity liquids in acetonitrile
Estimation of electrochemical activity of the low-permittivity liquids was also carried out in acetonitrile dissolved in 0.2 M concentration and containing 0.2 M TBuClO4. The related voltammograms taken between 0 and 4 V are shown in Fig. 2. The only exception was trihexylamine (part B) due to the appearance of its anodic peak at a mildly anodic potential (1.25 V), and therefore, the potential window was between 0 and 2 V and its concentration was limited by the solubility in acetonitrile. It is clearly seen that all compounds resulted in remarkable current signals due to the enhanced permittivity of the solutions whose peaks or waves appeared mainly between 3 and 4 V. These signals overlapped with that of the solvent acetonitrile (see Fig. 1, part C), and its maximum current was approximately 60 μA. This value is small compared with most of the compounds investigated here. The contribution of background currents attributable to the solvent close to 4 V resulted in current plateaus in some cases instead of peaks as the latter is usual by macroelectrodes.
Really surprising differences were observed in case of many liquids concerning the current magnitude taking into account their voltammograms in solutions and in their own phase. The current plateaus of trichloromethane and dichloromethane are similar and the height of anodic peak of the solvent acetonitrile prevails in case of trichloromethane indicating that it oxidizes weakly in accordance with the previous experiments. On the other hand, electroactivity of dichloromethane and acetonitrile is comparable as signal magnitude obtained here is almost identical to that of pure dichloromethane.
Trihexylamine is the only liquid of the selected ones which is not miscible with acetonitrile. The solution became saturated with trihexylamine by thorough stirring and ultrasonicating for 5 min. After the separation of the two liquid phases, the experiments were done at its equilibrium concentration. Its determination needed an additional procedure which will be discussed in the following section. The anodic peak of triethylamine shifted also significantly to a lower potential (2.45 V) resulting in the highest signal of the investigated liquids (~ 800 μA) showing its susceptibility to oxidation similarly to trihexylamine. Both tertiary amines start to oxidize around 1 V. These results indicate that trisubstituted amines might be oxidized more easily than the other selected compounds.
In the literature, there are a large numbers of electrochemical reactions of ethers. In the review of Dunach [11], many organic syntheses are summarized. These studies were accomplished mostly in methanol solvent as its nucleophile attack on the positively charged carbon atom resulted in the desired methoxylated product. Aliphatic ethers oxidize on the carbon atom in the neighborhood of ether oxygen [12]. There are attempts also in dry acetonitrile [13]. By the ethers investigated here, the carbon atom in neighborhood of oxygen is oxidized followed by formation of a positively charged radical cation. There is a small difference between diisopropyl ether and diethyl ether. The height of the current plateau of diisopropyl ether was a little higher probably due to the larger diffusion coefficient. Isopropyl groups have a secondary carbon atom in the neighborhood of oxygen, and the formed positive charge during the oxidation can be stabilized. Therefore, its oxidation starts at a little smaller potential than in case of diethyl ether which has shorter alkyl chains. In case of tetrahydrofuran, a little higher currents could be measured possibly due to oxidation on a carbon atom bound to oxygen. 1,4-dioxane has two oxygen atoms and one carbon atom in their neighbourhood can be oxidized producing similar current plateau as by tetrahydrofurane. Probably due to the higher diffusion coefficient their plateau currents were higher than that of aliphatic ethers.
Esters can be oxidized on their ether oxygen atom as it was demonstrated in case of an ester cavitand [14]. A radical cation can also form and electrooxidation produced also a radical with positively charged oxygen by ethyl acetate and 1-pentil-acetate.
Carbon disulfide is a compound widely used in organic chemistry by synthesis of certain sulfur-containing materials. Previously, it was shown that the pure liquid has poor conductivity for carrying out voltammetric measurements in it. Its electroreduction was investigated in some works in dimethyl formamide solvent [15]. The intermediate radical anion transformed into carbon disulfide heterocycle [16]. On mercury cathode, the primarily formed radical anion dimerized into a dianion followed by the subsequent transformation into sulfur-containing organic molecules [17]. In the literature, nothing is found about carbon disulfide electrooxidation in nonaqueous environments. As the voltammogram taken in its solution prepared with acetonitrile shows it begins to oxidize around 3 V forming also a cation radical bearing the positive charge on one sulfur atom. For additional information, electrolysis of its solution at 3.5 V was carried out, but during the procedure, no color change was observed.
Determination of the solubility of trihexylamine in acetonitrile
In many cases, it is important to know the solubility of solute materials in different solvents. In order to find the saturated concentration of trihexylamine in acetonitrile, a binary solvent was used at a temperature of 25 °C (298 K). As trihexylamine is highly soluble in 1-propanol, it was selected as cosolvent. On the other hand, dielectric constant of 1-propanol is 20.8 [6], so this solvent itself is also favorable to electrochemical investigations. A stock solution of the amine was prepared with 1-propanol without supporting electrolyte. The saturated concentration was reached by adding pure trihexylamine to pure acetonitrile without any added material, and the mixture was stirred followed by ultrasonication until an opalescent solution could be obtained. One day was necessary until all trihexylamine droplets settled down due to its higher density. At the same time, a binary solution series was prepared with the same solvent composition for calibration containing trihexylamine in different concentrations containing 0.1 M TBuClO4 supporting electrolyte. In total, 5 cm3 of acetonitrile was transferred to 10-cm3 volumetric flasks, and the necessary volumes of the stock solution prepared with 1-propanol were added to it and mixed to minimize the error coming from the volume change of mixing. Finally, the flask was filled with 1-propanol to the mark followed by mixing. Due to this procedure, a uniform solvent composition could be set ensuring the same diffusion characteristics in each solution which is essential by the voltammetric methods. A solution for the solubility determination was prepared by adding 5 cm3 of acetonitrile saturated with trihexylamine into a 10-cm3 volumetric flask and filled with 1-propanol to the mark. Cyclic voltammograms were recorded between 0 and 2 V with scan rate of 0.1 V/s in each solution. Acetonitrile was supplied after taking 5 cm3 volume from the saturated solution. The saturated concentration was reached again according to the procedure described above.
By using an anodic peak current–concentration calibration plot, a linear relationship could be obtained between 0 and 80 mM trihexylamine: Ipa (μA) = 0.6785 + 1.19168 c (mM). With the aid of this equation and taking the average of three parallel determinations, the concentration of trihexylamine can be obtained. As the saturated solution was doubly diluted, the saturation concentration is the double of the concentration calculated from the calibration curve so the solubility of trihexylamine in acetonitrile is 44.253 ± 0.564 mM at 298 K. The low scattering shows the good reproducibility of the determination procedure.
Conclusion
The electrochemical oxidation of low-permittivity liquids in acetonitrile showed that some of them (especially tertiary amines) are able to undergo anodic oxidation at lower positive potentials in higher-permittivity solvents, while in their own phase, they are unable to oxidize also at extremely high potentials. The tertiary amines gave regular and reproducible voltammograms showing that they can be investigated in higher-permittivity solvents.
References
K.B. Oldham, T.J. Cardwell, J.H. Santos, J. Electroanal. Chem. 430, 25 (1997)
K.B. Oldham, T.J. Cardwell, J.H. Santos, J. Electroanal. Chem. 430, 39 (1997)
L. Geng, A.G. Ewing, J.C. Jernigan, R.W. Murray, Anal. Chem. 58, 852 (1986)
D.L. Goldfarb, H.R. Corti, Electrochem. Commun. 2, 663 (2000)
A.P. Abbott, C.A. Eardley, J.C. Harper, E.G. Hope, J. Electroanal. Chem. 457, 1 (1998)
D.R. Lide, CRC Handbook of Chemistry and Physics, Chapter 6, 76th edn. (CRC Press, Inc., Boca Raton, 1995–1996), p. 173
T. Dibble, S. Bandyopadhyay, J. Ghoroghchian, J.J. Smith, J. Phys. Chem. 90, 5275 (1986)
J. Cassidy, S.B. Khoo, S. Pons, J. Phys. Chem. 89, 3933 (1985)
L. Agüí, J.E. Lopez-Guzmán, A. González-Cortés, P. Yánez-Sedeno, J.M. Pingarrón, Anal. Chim. Acta 385, 241 (1999)
M.A. Hernández-Olmos, L. Agüí, P. Yánez-Sedeno, J.M. Pingarrón, Electrochim. Acta 46, 289 (2000)
E. Dunach, Second supplements to the 2nd edition of Rodd’s chemistry of carbon compounds, in Organic Electrochemistry, vol. V, ed. by M. Sainsbury (Elsevier, Amsterdam, 2002), p. 307
Y. Hou, T. Fuchigami, Tetrahedron Lett. 40, 7819 (1999)
T. Martens, F. Billon-Souquet, I. Gauthier, J. Royer, Tetrahedron Lett. 38, 4075 (1997)
A. Pailleret, D.W.M. Arrigan, Electrochem. Commun. 3, 24 (2001)
S. Wawzonek, S.M. Heilmann, J. Org. Chem. 39, 511 (1974)
M.F. Hurley, J.Q. Chambers, J. Org. Chem. 46, 775 (1981)
S. Wawzonek, H. Chang, W. Everett, M. Ryan, J. Electrochem. Soc. 130, 803 (1983)
Acknowledgements
Open access funding provided by University of Pécs (PTE). Financial support of the GINOP 2.3.2-15-2016-00022 Grant is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kiss, L. Electrooxidation of low-permittivity solvents in acetonitrile and solubility of trihexylamine in acetonitrile. J IRAN CHEM SOC 17, 67–71 (2020). https://doi.org/10.1007/s13738-019-01748-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01748-6