Abstract
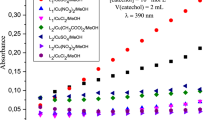
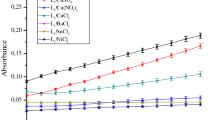
In the present work, catecholase activity is presented. The complexes were prepared by condensation of the organic ligand pyrazolyl L 1 –L 4 and copper(II) ion in situ. The pyrazolyl compounds L 1 –L 4 used in this study are: L 1 is (3,5-dimethyl-pyrazol-1-ylmethyl)-(4-methyl-pyridin-2-yl)-pyrazol-1-ylmethyl-amine; L 2 is 1-{4-[(3,5-dimethyl-pyrazol-1-ylmethyl)-pyrazol-1-ylmethyl-amino]-phenyl}-ethanone; L 3 is 1-{4-[(3,5-dimethyl-pyrazol-1-ylmethyl)-[1,2,4]triazol-1-ylmethyl-amino]-phenyl}-ethanone, and L 4 is 2-[(3,5-dimethyl-pyrazol-1-ylmethyl)-[1,2,4]triazol-1-ylmethyl-amino]-6-methyl-pyrimidin-4-ol, and copper ions salts Cu(II) are (Cu(CH3COO)2, CuCl2, Cu(NO3)2 and CuSO4). In order to determine factors influencing the catecholase activity of these complexes, the effect of ligand nature, ligand concentration, nature of solvent and nature of counter anion has been studied. The best activity of catechol oxidation is given by the combination formed by one equivalent of ligand L 2 and one equivalent of Cu(CH3COO)2 in methanol solvent which is equal to 9.09 µmol L−1 min−1. The Michaelis–Menten model is applied for the best combination, to obtain the kinetic parameters, and we proposed the mechanism for oxidation reaction of catecholase.












Similar content being viewed by others
References
K.D. Karlin, Science 261, 701–708 (1993)
E.I. Solomon, U.M. Sundaram, T.E. Machonkin, Chem. Rev. 96, 2563–2605 (1996)
B.B. Mishra, S. Gautam, A. Sharma, Food Chem. 134, 1855–1861 (2012)
C. Eicken, B. Krebs, J.C. Sacchettini, Curr. Opin. Struct. Biol. 9, 677–683 (1999)
I.A. Koval, K. Selmeczi, C. Belle, C. Philouze, E.S. Aman, I.G. Luneau, A.M. Schuitema, M.V. Vliet, P. Gamez, O. Roubeau, M. Lüken, B. Krebs, M. Lutz, A.L. Spek, J.L. Pierre, J. Reedijk Chem. Eur. J. 12, 6138–6150 (2006)
L. Gasque, V.M.U. Saldívar, I. Membrillo, J. Olguín, E. Mijangos, S. Bernès, I. González, J. Inorg. Biochem. 102, 1227–1235 (2008)
S.J. Smith, C.J. Noble, R.C. Palmer, G.R. Hanson, G. Schenk, L.R. Gahan, M.J. Riley, J. Biol. Inorg. Chem. 13, 499–510 (2008)
T. Csay, B. Kripli, M. Giorgi, J. Kaizer, G. Speier, Inorg. Chem. Commun. 13, 227–230 (2010)
S. Sarkar, S. Majumder, S. Sasmal, L. Carrella, E. Rentschler, S. Mohanta, Polyhedron 50, 270–282 (2013)
L.G. Sebastián, V.M.U. Saldívar, E. Mijangos, M.R.M. Quijano, L.O. Frade, L. Gasque, J. Inorg. Biochem. 104, 1112–1118 (2010)
Á. Kupán, J. Kaizer, G. Speier, M. Giorgi, M. Réglier, F. Pollreisz, J. Inorg. Biochem. 103, 389–395 (2009)
S. Mandal, J. Mukherjee, F. Lloret, R. Mukherjee, Inorg. Chem. 51, 13148–13161 (2012)
R. Bakshi, M. Rossi, F. Caruso, P. Mathur, Inorg. Chim. Acta 376, 175–188 (2011)
M.K. Panda, M.M. Shaikh, R.J. Butcher, P. Ghosh, Inorg. Chim. Acta 372, 145–151 (2011)
M.R. Malachowski, M.G. Davidson, J.N. Hoffman, Inorg. Chim. Acta 157, 91 (1989)
S. Calancea, S.G. Reis, G.P. Guedes, R.A. AllaoCassaro, F. Semaan, F. Lopez-Ortiz, M.G.F. Var, Inorg. Chim. Acta 453, 104–114 (2016)
F. Khaleghi, M.A. Khalilzadeh, J.B. Raoof, M. Tajbakhsh, H. Karimi-Maleh, J. Appl. Electrochem. 39, 1651–1654 (2009)
D.D. Daugherty, S.F. Karel, Degradation of 2,4-dichlorophenoxyacetic acid by pseudomonas cepacia DBOl(pRO101) in a dual-substrate chemostat. Appl. Environ. Microbiol. 60, 3261–3267 (1994)
D.L. Daubaras, K. Saido, A.M. Chakrabarty, Purification of hydroxyquinol 1,2-dioxygenase and maleylacetate reductase: the lower pathway of 2,4,5-trichlorophenoxyacetic acid metabolism by Burkholderia cepacia AC1100. Appl. Environ. Microbiol. 62, 4276–4279 (1996)
A. Kahru, L. Pollumaa, R. Reiman, A. Ratsep, M. Liiders, A. Haloveryan, The toxicity and biodegradability of eight main phenolic compounds characteristic to the oil-shale industry wastewaters: a test battery approach, Inc. Environ. Toxicol. 15, 431–442 (2000)
F. Hamaguchi, T. Tsutsui, Assessment of genotoxicity of dental antiseptics: ability of phenol guaiacol, p-phenolsulfonic acid, sodium hypochlorite, p- chlorophenol, m-cresol or formaldehyde to induce unscheduled dna synthesis in cultured syrian hamster embryo cells. Jpn. J. Pharmacol. 83, 273–276 (2000)
N. Okada, K. Satoh, T. Atsumi, M. Tajima, M. Ishihara, Y. Sugita, I. Yokoe, H. Sakagami, S. Fujisawa, Anticancer Res. 20, 2955–2960 (2000)
Y.J. Wang, Y.S. Ho, J.H. Jeng, H.J. Su, C.C. Lee, Different cell death mechanisms and gene expression in human cells induced by pentachlorophenol and its major metabolite, tetrachlorohydroquinone. Chem. Biol. Interact. 128, 173–188 (2000)
N. Schweigert, R.W. Hunziker, B.I. Escher, R.I.L. Eggen, Environ. Toxicol. Chem. 20(2), 239–247 (2001)
A. Zerrouki, R. Touzani, S. El Kadiri, Arab. J. Chem. 4, 459–464 (2011)
M. El Kodadi, F. Malek, R. Touzani, A. Ramdani, Catal. Commun. 9, 966–969 (2008)
I. Bouabdallah, R. Touzani, I. Zidane, A. Ramdani, Catal. Commun. 8, 707–712 (2007)
A. Mouadili, A. Zerrouki, L. Herrag, B. Hammouti, S. El Kadiri, R. Touzani, Res. Chem. Intermed. 38, 2427–2433 (2012)
R. Saddik, M. Khoutoul, N. Benchat, B. Hammouti, S. El Kadiri, R. Touzani, Res. Chem. Intermed. 38, 2457–2470 (2012)
R. Saddik, F. Abrigach, N. Benchat, S. El Kadiri, B. Hammouti, R. Touzani, Res. Chem. Intermed. 38, 1987–1998 (2012)
A. Mouadili, S. Attayibat, S. El Kadiri, S. Radi, R. Touzani, Appl. Catal. A Gen. 454, 93–99 (2013)
M. Khoutoul, Extraction liquide-liquide des métaux par des nouveaux absorbants à base du pyrazole, pyrane et triazole avec des calculs théoriques DFT et TD-DFT, Ph.D. Thesis, Oujda, Morocco, 2017
K.S. Banu, M. Mukherjee, A. Guha, S. Bhattacharya, E. Zangrando, D. Das, Polyhedron 45, 245–254 (2012)
T. Klabunde, C. Eicken, J.C. Saccettini, B. Krebs, Nat. Struct. Biol. 5, 1084–1090 (1998)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boyaala, R., El Ati, R., Khoutoul, M. et al. Biomimetic oxidation of catechol employing complexes formed in situ with heterocyclic ligands and different copper(II) salts. J IRAN CHEM SOC 15, 85–92 (2018). https://doi.org/10.1007/s13738-017-1211-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1211-0