Abstract
Purpose of Review
This study is to examine potential micronutrient deficiencies and any need for supplementation in children following specific diet plans in the first 1000 days of life.
Recent Findings
Optimal nutrition in the first 1000 days of life has a lifelong positive impact on child development. Specific intrauterine and perinatal factors, pathological conditions, and dietary restrictions can represent potential risk factors for micronutrient deficiencies in the first 1000 days of life, which can have negative systemic consequences. Preterm and low-birth-weight infants are intrinsically at risk because of immature body systems. Children affected by cystic fibrosis are prone to malnutrition because of intestinal malabsorption. The risk of micronutrient deficiency can increase in various situations, including but not limited to children following selective dietary regimens (vegetarian and vegan diets and children affected by specific neuropsychiatric conditions) or specific dietary therapies (children affected by food allergies or specific metabolic disorders and children following restricted diet as a part of therapeutic approach, i.e., ketogenic diet for epilepsy). In light of this situation, the micronutrient status in these categories of children should be investigated in order to tailor strategies specific to the individual’s metabolic needs, with a particular focus on deficiencies which can impair or delay the physical and cognitive development of children, namely, vitamin B12, vitamin D and folic acid, as well as oligo-elements such as iron, zinc, calcium, sodium, magnesium, and phosphorus, and essential fatty acids such as omega-3.
Summary
Identification of micronutrient deficiency in the first 1000 days of life and timely supplementation proves essential to prevent their long-term consequences.
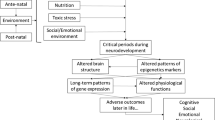
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The first 1000 days (conception, pregnancy, and the first two years of life) represent a period of enormous vulnerability to nutritional deficiency as well opportunity for promoting better outcomes in children’s lives. The fetuses, infants, and children experience unique physiological changes and have specific nutritional needs [1]. In this context, an optimal nutrition during this time acts as the first line of prevention against developmental shortfalls [2,3,4,5,6]. The traditional concept of optimal nutrition during the first 1000 days of life included only macronutrient and energy balance. This concept was recently extended to include an adequate supplementation of micronutrients, given the evidence that diet influences gene expression through epigenetic mechanisms during the first 1000 days of life. Adequate micronutrient intake is essential for neural, visual, and skeletal system development because of its role in early fetal organ development and cell differentiation [7, 8•, 9•, 10,11,12,13,14,15,16,17,18,19,20, 21•, 22,23,24,25,26]. Micronutrient can only be provided by the diet and act as coenzymes in the production of hormones and essential substances for proper growth. Hence, their role in phases of rapid growth as the first 1000 days is essential. There is no definitive evidence on the benefits of supplementation of other micronutrients without demonstrated deficiency, both for lactating mothers and both for breastfed and weaning infants. At the same time, a supplementation of micronutrients become necessary during the first 1000 days of life in infants or children adopting restricted or unbalanced diets and affected by particular diseases [16, 27, 28••]. In fact, infants or children affected by specific intrauterine and perinatal factors or pathological conditions, as well as preterm/low birth weight infants, could be particularly at risk of micronutrient deficiencies. This article explores potential early-life micronutrient deficiencies and their associated risks in the context of some of the most common chronic conditions during early childhood. Specifically, it aims to urge pediatricians to pay more attention to potential micronutrients deficiencies in these infants and children.
Importance of Micronutrients During the First 1000 Days
Micronutrients at significant risk of deficiency during the first 1000 days are reported in Table 1. Among those, deficiencies of omega-3 fatty acids, vitamins C, B9, B12, and D, and minerals such as iodine and iron seem the most involved in clinical syndromes [1]. It is important to note that unbalanced diets can lead to micronutrient deficiencies even when following an omnivorous diet due to maternal and child undernutrition and food insecurity especially in low-income and middle-income countries. In this paragraph, we have focused on dietary regimens that involve the conscious choice to exclude food groups or the therapeutic necessity to do so.
Vitamin C promotes the absorption and conservation of iron and stimulates the body’s immune system because of its antioxidant and anti-infective properties. Newborns with vitamin C deficiency may be irritable and may not gain weight as expected. Bone growth is impaired in infants and children, and bleeding and anemia may occur. Infections may develop with difficult-to-heal wounds [28••, 29••].
Vitamin B9, or folic acid, promotes the embryo’s neural tube closure; therefore, its deficiency may cause neural tube malformations. Folate requirements increase during pregnancy due to fetal and maternal tissue growth and active transfer of folate to the fetus. The benefit of daily folic acid supplementation for all females is well demonstrated in the periconceptional period [9•]. Choline insufficiency can result in neural tube defects as well [30].
Vitamin B12, or cobalamin, is involved in erythropoiesis, the synthesis of DNA and fatty acids, energy production, and functioning of the nervous system. A deficit results in megaloblastic anemia and nervous system, mood, and memory disorders. Neurological symptoms include tingling in feet and hands, loss of sensation, and weakness in arms and legs. In infants with nutritional vitamin B12 deficiency, early clinical symptoms are irritability, failure to thrive, and food refusal, accompanied, in worst cases, by central nervous system lesions, which can be irreversible [14,15,16, 31•].
Vitamin D promotes the absorption of calcium and phosphorus and bone remodeling. Studies also show its anti-inflammatory, anti-tumoral, and cardiovascular protection effects [22]. In vitamin D deficiency, the body absorbs less calcium and phosphate, causing bone disorders associated with bone weakness (rickets in children or osteomalacia in adults). Vitamin D deficiency during pregnancy can cause deficiency in the fetus; sometimes, the deficiency is severe enough to cause osteomalacia in women. Muscle spasms (tetany) caused by a low calcium level in the blood in people may be newborns’ first signs of rickets when severe vitamin D deficiency occurs. If the spasms are severe, they can lead to seizures. In younger children with rickets, the entire skull may be soft. Infants may have difficulty sitting up, crawling, and learning to walk. Closing of fontanelles may take longer. In children older aged one year or more, bone growth may be impaired, resulting in an abnormal spine curvature (scoliosis) and varus or valgus knees. Vitamin D supplementation is advised in all infants for the first year with 400 UI/die, whether breast or formula-fed [20, 21•, 22, 26].
Iodine is mainly involved in the synthesis of thyroid hormones. Severe maternal iodine deficiency delays fetal growth and brain development, causing congenital hypothyroidism, including intellectual disability, deaf-mutism, difficulty walking, short stature, and sometimes hypothyroidism (cretinism) [7, 17, 18].
Iron deficiency develops in stages. First, the demand for iron exceeds the amount consumed in the diet, causing the progressive depletion of iron reserves in the bone marrow. When reserves are reduced, the absorption of dietary iron increases to compensate for this deficiency. The deficiency impairs red blood cell synthesis in later stages, ultimately causing anemia. Severe and prolonged iron deficiency can also cause a functional alteration of cellular enzymes that contain iron. Most iron deficiency symptoms are due to anemia. In addition, patients may suffer from pica, a compulsive desire to eat nonfood substances (e.g., ice, dirt, paint, starch, ash). Other severe deficiency symptoms include skin and mucosal damage, such as glossitis and cheilosis [7, 19, 20, 32].
Omega-3 s are polyunsaturated fatty acid (PUFA) that includes eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as the essential precursor alpha-linolenic acid (ALA). Omega-3 deficiency is associated with skin problems, mood disorders, and attention deficits and difficulty to concentrate. In the fetus, they act in neuronal and eye development [30, 33, 34].
Different Diets at Risk of Micronutrient Deficiency
Food of plant origin contains higher amounts of carbohydrates and dietary fiber and lower amounts of saturated fats and proteins than food of animal origin. Due to the lower fat content, a diet containing predominantly plant-based foods provides, on average, lower daily energy intake and reduced meal energy density. The high-fiber intake may interfere with the absorption of some minerals, especially iron, zinc, and calcium, due to phytates present in cereals, seeds, and legumes [28••, 29••]. A lower consumption of fats can have some beneficial effects on BMI and cardiovascular health especially reducing saturated fat but, on the other hand, attention should be paid to the types and sources of fats, particularly regarding the risk of inadequate absorption of omega-3 fatty acids. Among macronutrients, a qualitatively and quantitatively adequate protein intake is essential for the correct growth of children. Moreover, essential amino acids cannot be synthesized from the human organism and are present in adequate concentration prevalently in food of animal origin. Therefore, plant-based foods often lack specific amino acids. It is important to consume plant-based foods of different groups throughout the day in order to compensate for specific amino acid deficiencies. Breastfed infants have a sufficient protein intake even if the mother follows a vegetarian diet, but are exposed to a risk of protein malnutrition and micronutrient deficiency when given plant-based non-dairy milk alternatives, such as cereal, legume or nut “milk”. Soy or plant-based formulas, enriched with necessary proteins and micronutrients, can instead be easily used [30, 31•]. Regarding micronutrients, they appear to be more represented, varied, and bioavailable in animal-source foods than in foods of plant origin. Hence, it can be assumed that vegetarian diets can represent a risk for infants and children’s health in their first 1000 days of life. Data currently available from conducted studies do not formally establish a clear relationship between these dietary patterns and the intake or status of micronutrients [28••].
Vegetarian Diets
The degree of animal-source food restriction defines the different vegetarian patterns, even though a more comprehensive approach also considers the diversity of included foods, as illustrated in Table 2. Historically, vegetarian diets have always been discouraged during the first 1000 days of life due to concerns about nutritional deficiencies and poor growth. On the contrary, position papers from North America state that well-planned vegetarian and vegan diets, when appropriately supplemented, are suitable for all life stages [32]. European statements strongly recommend that vegetarian/vegan diets should not be adopted without medical and dietetic supervision [33, 34]. Micronutrient deficiency risk is present for all vegetarian diets and specifically includes micronutrients of which animal-source food represents a more bioavailable origin: B12, vitamin A, iron, zinc, vitamin D, and essential fatty acids. On the other hand, vegetarian diets do not expose children to copper or selenium deficiency risk. During pregnancy and breastfeeding, vegetarian diets provide sufficient protein and micronutrient amounts, except for B12 and iron, which the mother should supplement. The main nutritional deficit of breast milk of vegetarian mothers is related to vitamin B12, with the risk for micronutrient deficiency depending on how restrictive the diet is. The maternal vegetarian diet represents the main cause of vitamin B12 deficiency in breastfed infants [36]. Recommendations for supplementations of other micronutrients during pregnancy and breastfeeding vary among countries. It seems prudent to suggest personalized supplementation only under medical supervision.
Lacto-ovo-vegetarian Diet
The lacto-ovo vegetarian diet is the most common type of vegetarian diet; it excludes meat and fish but includes dairy products and eggs. A particular category is represented by flexitarians, who occasionally eat meat or fish. Upon the beginning of complementary feeding, a lacto-ovo-vegetarian diet exposes infants to a low risk of micronutrient deficiency if it includes a sufficient intake of dairy products [28••, 31•]. Nonetheless, most plant-based foods have low iron and zinc content and their bioavailability from vegetarian sources is lower than from animal sources. Dietary advice should be given regarding food preparation techniques due to the possible mineral leaching during cooking and regarding limitation of the fiber content of the diet in order to increase zinc and iron absorption [37]. Children are at risk of iron deficiency due to their rapid growth. Parallelly, infants following a vegetarian diet are particularly at risk for zinc deficiency and should be monitored. For the same reason, during the first 1000 days of life, children following a vegetarian diet should have a higher iron intake (1.8 × intake of omnivorous children) [34], which can be guaranteed in most cases only through supplementation. The recommended iron prophylaxis for full-term newborns is 1 mg/kilogram per day (maximum 15 mg/die) from birth; its importance is linked to the evidence that iron deficiency in the early years of life has been related to anxiety, depression, and schizophrenia in adult age. Although the risk of B12 deficiency for infants on a lacto-ovo-vegetarian diet is lower than for infants following more restrictive diets, supplementation is encouraged. Due to the lack of fish in the diet, the risk of insufficient intake of essential fatty acids should be taken into consideration, especially ALA (and α-linolenic acid); supplementation of 100–200 mg of DHA daily is suggested [38••].
Lacto-vegetarian Diet
A lacto-vegetarian diet excludes eggs. As a consequence, children following a lacto-vegetarian diet have a higher risk of vitamin B12 and iron deficiency [35].
Ovo-vegetarian Diet
The ovo-vegetarian diet excludes milk and dairy products. As a consequence, for children following an ovo-vegetarian diet, calcium and vitamin D supplements are required to meet recommended intakes. Furthermore, due to the increasing level of restriction of animal source food, the risk of vitamin B12 deficiency is higher than in lacto-ovo-vegetarian diet [35].
Vegan Diet
The vegan diet is the most restrictive and excludes all animal source food, as well as products containing ingredients derived from animal source foods. The major issue in the vegan diet is the total lack of vitamin B12 in food of plant origin. Severe vitamin B12 deficiency in the first 1000 days of life has been associated with altered neuronal myelination, which causes damage to the auditory and visual systems e interferes with learning and social interaction [36]. Symptoms and long-term prognosis depend on the severity and duration of the deficiency. All mothers and infants who follow vegetarian or vegan diets should take supplements. The dosage for Vitamin B12 in infants is 0.4 μg/day for the first 6 months, increasing up to 5 μg/day until the age of 3 years [33]. Newborns with vitamin B12–deficient mothers are usually asymptomatic at birth and develop clinical signs at 4–6 months of age, ranging from megaloblastic anemia to irreversible neurological damage.
Vitamin A is present in foods of both animal and plant origin; its bioavailability is conditioned by the quantity and quality of lipids consumed, which enable its absorption. The risk of vitamin A deficiency is low for the lacto-ovo-vegetarian diet but increases for pre-school children following vegan diets due to their limited food preferences [28••] On the other hand, dietary intake has a limited influence on individual vitamin D status because few foods are rich in vitamin D, mostly foods of animal origin. Both lacto-ovo-vegetarian and vegan diets poorly guarantee optimal vitamin D intake, even if the risk of deficiency seems higher in children following a vegan diet. Vitamin D supplementation is required in the first year regardless of the diet followed (600 UI/die in pregnancy, 400UI/die in the first year of life, then dose adjusted according to dietary habits and sun exposure). Calcium deficit is infrequent in the lacto-ovo-vegetarian diet because of the consumption of dairy products. On the contrary, children who follow a vegan diet do not have sufficient calcium intake to cover their requirements. The calcium content of breast milk is not influenced by a vegan maternal diet; however, at the beginning of complementary feeding, it is necessary to ensure adequate calcium intake through fortified foods or supplements. The calcium requirement in the first two years of life is 120 mg/day and requires an intake of approximately 300 mg/die [35, 37, 38••].
The vegan diet also poses a higher risk of iron-deficiency compared to other vegetarian diets. Children following an ovo-vegetarian diet get a small quantity of heme–iron from eggs, which is more-bioavailable, while subjects who follow a vegan diet introduce only non-heme iron through their diet. Moreover, iron bioavailability is limited by dietary fiber and phytate but increased by vitamin C. Therefore, it is necessary to consume foods which are fortified with iron [28••].
Regarding long-term consequences on health, it is not possible to establish with certainty at what age the beginning of a vegetarian diet can come without side effects on growth and nutritional status [35]. Benefits of vegetarian and vegan diets include lower BMI and lower risk of obesity and hypertension, with reduced levels of lipids, lipoproteins, glucose, insulin, and C-reactive protein when compared to omnivorous diets [35, 39]. No significant differences were found regarding thyroid function, precocious puberty, premature thelarche, or menstrual irregularities [35]. No conclusive results are available regarding the relationship between dietary patterns and neoplastic risk; the effects of the vegetarian diets are likely due not only to the exclusion of meat but also to the inclusion of a wide range of plant foods containing potentially protective substances [35, 40]. The effects of nutrient deficiencies are strongly related to the stage of development of each specific brain area and tend to be irreversible. Considering the important short and long-term outcomes of nutrient deficiency on neurodevelopment, vegan diets in the first 1000 days without proper supplementation are considered inadequate for optimal psychomotor development [35].
Macrobiotic Diet
Macrobiotic diets are even more restrictive than vegan diet and are based on cereals, seeds, vegetables, seaweed, and soy products and may include fish. Children following macrobiotic diets present a similar profile of risk for micronutrient deficiency similar to that of children on vegan diet, with the main risk being vitamin B12 deficiency. Based on reported cases, restrictive diets such as a macrobiotic diet are inappropriate during a child’s early development because they have a negative impact on growth and micronutrients status [31•].
Medical Conditions Associated with Micronutrient Deficiencies
Genetic Conditions
Inherited Metabolic Diseases
It is known that diet represents a primary form of treatment for many inherited metabolic diseases and is essential to prevent and minimize intellectual disability and epilepsy. Therapeutic strategies can include protein-restricted diet, single or multiple amino acids-restricted diet, ketogenic diet, fat-restricted diet, galactose free diet, fructose-free diet, and targeted supplementation of macro and micronutrients. Moreover, nutrition plays a crucial role in sick-day emergency protocols, especially in younger children with organic acidurias, certain aminoacidopathies, urea cycle disorders, and fatty acid oxidation defects [41]. The restriction of specific diet components potentially reduces micronutrient status; hence, a comprehensive vitamin and mineral supplement should be provided and becomes an essential adjunct to dietary treatment. Moreover, feeding differences are common in children with inherited metabolic disorders, with meals complicated by poor appetite, limited food variety, and lengthy mealtimes [41,42,43]. Phenylketonuria (PKU) is the most common inherited disorder of amino acid metabolism. It results from deficiency in phenylalanine hydroxylase. Newborn screening for PKU facilitates the beginning of treatment right after birth and helps prevent intellectual disability, mental health disorders, and major health problems. The basis of the treatment in PKU patients is through diet: They need to follow a low-phenylalanine diet, although newer medications may allow some people with PKU to eat a diet that has a higher or an unrestricted amount of phenylalanine depending on their residual enzyme activity [44]. Treatment consists of three parts: natural protein or phenylalanine restriction to 25% or less of what would be a regular intake, phenylalanine-free L-amino acid supplements to meet protein and other nutrients requirements, and low protein food to meet energy requirements [45••]. In fact, a source of L-amino acids is required, and supplementation in PKU patients is achieved through L-amino acid supplements with added age-specific vitamin and mineral profiles, including pregnancy. No tailored micronutrient dietary reference values have been established for PKU, so the optimal intake of micronutrients on a low phenylalanine diet is unknown [44, 46]. Guidelines recommend to supplement micronutrients according to the daily requirements for healthy people. Most L-amino acid supplements have added vitamins and minerals; therefore, taking any additional supplements may not be necessary to meet the daily nutrient requirements of PKU patients [47]. Amino acid supplements are not palatable and can cause gastrointestinal symptoms such as abdominal pain, diarrhea, and constipation in young children. These aspects are correlated with low dietary adherence that can expose patients to the risk of micronutrient deficiency. Maternal phenylketonuria (MPKU) poses risks to the developing fetus as elevated phenylalanine (Phe) levels, crossing the placental blood membrane, have well-recognized teratogenic effects [47]. This syndrome is characterized by intrauterine growth retardation, facial dysmorphism, microcephaly, congenital heart disease, low birth weight, developmental delay, intellectual disabilities, and an elevated risk of miscarriage, often linked to inadequate maternal metabolic control.
Maintaining a strict, phenylalanine-restricted diet is vital in maternal PKU to prevent harm to the fetus [45••, 46, 47]. Particular attention should be paid to dietary assessment, anthropometric parameters, and clinical features of micronutrient deficiency during every outpatient evaluation [46]. In addition to PKU, many other inherited metabolic diseases require a daily micronutrient supplementation, including maple syrup urine disease, isovaleric acidemia, methylmalonic aciduria, propionic aciduria, glutaric aciduria type 1, homocystinuria, hypermethioninemia, glycogen storage diseases, urea cycle disorders, fatty acids oxidation defects, and other rare diseases.
Cystic Fibrosis
Cystic fibrosis is the most common autosomal recessive disease reducing life expectancy [48]. The malfunctioning of the CFTR channel causes an accumulation of thickened mucus secretions in organs throughout the body, including the lungs, liver, pancreas, gallbladder, and intestines. Cystic fibrosis patients are prone to malnutrition and micronutrient deficiency primarily because the thickened secretions obstruct the intra-pancreatic ducts, reducing the delivery of digestive enzymes to the intestines and impairing the absorption of key nutrients. Exocrine pancreas insufficiency leads to poor absorption of fat-soluble vitamins. The severity of the CFTR variant correlates with pancreatic insufficiency: patients with severe variants (classes I, II, and III) have an altered exocrine function early in life, often at birth [49••]. Cholestasis and CFLD (cystic fibrosis-related liver disease) reduces available bile salts, and the lack of pancreatic bicarbonate secretions causes intestinal acidification, which further limits fatty acid and vitamin absorption. Moreover, these patients have an intrinsically higher metabolic demand, higher essential fatty acid turnover, and intestinal dysmotility [66]. Survival and lung function in children and adults inversely correlates with the degree of malnutrition [50]. As a consequence, nutritional care is a fundamental part of the multidisciplinary approach for these patients. Due to malabsorption and energy deficit, the standard of care for patients with cystic fibrosis is a high-calorie and high-fat diet with pancreatic enzyme replacement therapy (PERT) and the oral supplementation of vitamins [49••]. A daily supplementation of liposoluble vitamins A, D, K, and E is recommended [51]. Due to the increased sweating, intestinal malabsorption, and chronic inflammation, patients with cystic fibrosis may have higher than normal requirements for electrolytes and minerals: ESPGHAN recommends that sodium, iron, calcium, and zinc status be assessed regularly and supplemented if needed [51]. A strict nutritional assessment through evaluation of weight-for-length curves, longitudinal growth trajectory, and BMI is crucial to determine when to intensify the nutrition intervention. Moreover, it seems that antioxidants can positively impact disease outcome [52••]. The nutritional state of cystic fibrosis patients has significantly improved in recent years and is mainly attributed to early diagnosis through newborn screening.
Developmental Conditions: Preterm and Low-Birth-Weight Infants
Preterm and low-birth-weight infants (SGA, small for gestational age) are intrinsically at risk of micronutrient deficiencies during the first 1000 days of life because of limited body stores, immature regulation systems, and high nutritional demands [53•]. Moreover, the third trimester of pregnancy is a period of rapid fetal brain growth and development in terms of cortical thickening, myelination, axonal development, vascularization, and cerebellar growth [54]. Fetal malnutrition, whether in excess or deficiency, can predispose to the development of chronic pathologies in adulthood. “Fetal programming” comprises the endocrine-metabolic conditions occurring during intrauterine life, which could contribute to long-term diseases through epigenetic mechanisms. Diet is a powerful modulator of epigenetics both in prenatal and post-natal life; it can condition DNA methylation and influence “fetal programming” [1]. IUGR (intra-uterine-growth-restriction) fetuses, due to maternal malnutrition, are at greater risk of developing impairment of metabolic and cardiovascular systems (hypertension, diabetes, glucose intolerance, insulin resistance). Pregnant women should be adequately informed of the importance of fetal nutrition on the health of the unborn child, motivating them to adopt a healthy lifestyle [55]. No evidence supports the improvement of fetal growth after nutrient supplementation by the pregnant mother in this specific circumstance [56]. Other factors influencing complementary food introduction in preterm/low birth weight infants are feeding difficulties and increased rate of acute illnesses. Limited agreement exists on nutritional management aimed at guaranteeing optimal growth and neurodevelopment [57••]. As a consequence, the length and dosage of micronutrient supplementation during the first 1000 days of life differ among centers. Peculiar micronutrient deficiencies in this patient category include iron, vitamin D, zinc, LCPUFAs, calcium, and phosphorus. Prematurity ceases placental iron transfer, and the following rapid catch-up growth typical of premature babies further reduces the body’s iron stores. AAP and ESPGHAN recommend tailored iron supplementation according to birth weight, gestational age, type of feeding, and iron status, with measurements of iron storage upon hospital discharge, during follow-up and at the beginning of complementary feeding [38••, 58]. The suggested dietary iron intake is 3–4 (max 6) mg/kg/die for VLBW infants (< 1500 g), 2–3 mg/kg/die for infants with birth weight < 1500 g, 2 mg/kg/die for weight 1500–2000 g, and 1–2 mg/kg/day for weight > 2500. Formula feeding provides 2.25 mg/kg of iron if consumed at 150 ml/kg/die; exclusive human feeding provides lower amounts [38••]. Although there is a lack of trials with long-term neuro-developmental outcomes, long-term iron supplementation appears to result in a reduction in iron deficiency and anemia in preterm and LBW infants [59]. As a consequence, in order to meet nutritional needs during the first 1000 days of life, iron supplementation should be continued in preterm/low birth weight infants after hospital discharge, with doses tailored according to iron status during complementary feeding and proper avoidance of iron overload [57••]. Because they lack an appropriate storage system, preterm newborns at 40 weeks post-conceptional age have lower serum zinc levels compared to full-term neonates. Even though serial measurement of zinc serum concentration is not recommended unless, in case of evidence of zinc deficiency (e.g., acrodermatitis enterohepatica or poor growth), zinc supplementation during the first year of life may be advisable, especially in children with impaired growth. The risk of zinc overload should not be a concern since it has no pro-oxidant effect (recommended daily intake of 2–2.25/kg, up to 3 mg/kg in extremely preterm neonates) [38••, 57••, 60,61,62]. Premature infants are at risk of osteopenia because most bone mineralization occurs during the third trimester of pregnancy, and postnatal calcium absorption is suboptimal. Fortification of breast milk or use of preterm formulas are recommended in hospitals, but there is no consensus on their use after discharge [38••, 57••, 63•, 64]. Assessment of metabolic bone biomarkers (vitamin D, calcium, phosphorus, alkaline phosphatase (ALP), parathyroid hormone) is advisable in VLBW infants 2–4 weeks after discharge [65]; oral calcium and phosphate supplementation should be started when ALP levels are equal or above 800–1000 UI/ml [65] with a total daily intake of 120–200 mg/kg/die and 70–115 mg/kg/die respectively (ESPHGHAN 2022) [38••]. Neonatal vitamin D storage is directly related to maternal vitamin D status. A sufficient vitamin D supply is difficult to achieve even for full-term newborns because both human and formula milk contain inadequate levels; rapid growth and long hospitalization of preterm infants can further increase the risk of deficiency. Hence, vitamin D prophylaxis should be implemented in all infants for the first year of life at the dosage of 400 UI/die; ESPGHAN suggests a higher dose for preterm infants up to full-term corrected age (daily vitamin D intake of 400–700 IU/kg/die, maximum dosage 1000 IU/die) [21•, 22, 38••, 57••, 66]. Placental transfer of LCPUFA (DHA (docosahexaenoic acid) and AA (arachidonic acid)) occurs mostly during the third trimester, and brain accumulation is considerable. Moreover, preterm infants are unable to convert fatty acids precursors (linoleic acid (LA) and α-linolenic acid (ALA)) to DHA due to high requirements of fatty acids and metabolic immaturity. As conflicting results derive from studies investigating the benefits of LCPUFA supplementation, there is currently no sufficient evidence for consensus recommendation [38••, 57••]. Preterm infants fed orally could be at risk for folate deficiency. Modern preterm formulas have decreased the need for folic acid supplementation, although folic acid supplementation remains common [67•].
Behavioral Conditions: Attention-Deficit/Hyperactivity and Autism Spectrum Disorders
Nutritional deficiencies and quality of the diet are linked to the pathogenesis of various common mental disorders, such as depression, schizophrenia, autism spectrum disorder, and attention-deficit hyperactivity disorder. Specifically, diet can interact with other lifestyle factors, and children with attention-deficit/hyperactivity disorder (ADHD) show less adherence to healthy eating patterns than children without this disorder [68••, 69]. Consequently, low blood levels of zinc, magnesium, and ferritin are detected in children with ADHD [70•]. Feeding problems, such as food selectivity, food refusal, and abnormal dietary patterns, are highly prevalent in children with autistic spectrum disorder [71, 72]. Food selectivity causes a reduced consumption of whole grains, milk and dairy products, beans and soy products, vegetables, and fruits by children with autism spectrum disorder. When comparing the nutritional status of children with autism with that of neurotypical children, differences can be found in biomarkers indicative of vitamin insufficiency and increased oxidative stress, with several biomarkers associated with variations in the severity of autism. Studies report decreased dietary intake and lower serum levels in folate; vitamins B12, D, A, E, and K; iron; calcium; and zinc in children with autism compared to neurotypical controls [73]. Therefore, it is essential to provide detailed nutritional evaluation and individualized nutrition interventions for children with ADHD and autism spectrum disorders.
Acquired Conditions
Epilepsy Requiring a Ketogenic Diet
Ketogenic dietary therapies (KDT) are considered an adjuvant treatment in several medical conditions, included drug-resistant forms of epilepsy but also inherited metabolic disorders like GLUT1 deficiency syndrome and pyruvate dehydrogenase deficiency, Prader-Willi syndrome, and some specific types of cancers. Exploiting a process known as nutritional ketosis, the diet reduction of carbohydrates intake facilitates ketogenesis in order to provide an alternate source of energy. It has proved to reduce the frequency and severity of seizure in some forms of epilepsy [74•]. The ketogenic diet also poses some issues as nausea, constipation, fatigue, dehydration, electrolyte imbalances, and micronutrient deficiency. The limited intake of fruits, vegetables, carbohydrates, and calcium-rich foods can lead to vitamin and mineral deficiencies. In children following KDT, some common deficiencies include vitamin B, vitamin C, vitamin D, calcium, selenium, and magnesium [75] due to limited intake of grains, fruit and vegetables. KDT are individualized treatments carefully planned by multidisciplinary teams. Multivitamin and mineral supplements that are free of carbohydrates are always recommended to prevent micronutrient deficiencies [76, 77]. Constant monitoring is necessary for all patients on KDT in order to assess its impact on child’s growth. Further studies are needed to investigate possible long-term effects of these diets when started in children [74•].
Food Allergies
The diet of expectant mothers can induce tolerance to solid food in the offspring through the influence of fetal immune development [78•], even though, at present, it is not clear whether any one nutrient is more important in terms of prevention [79]. Nonetheless, antigen avoidance by expecting and breastfeeding mothers is not suggested to prevent allergy, even in high-risk children [78•, 79,80,81]. Certain foods are more common triggers of significant acute allergic reactions during the first 1000 days of life, i.e., cow’s milk, hen’s egg, soy, wheat, peanuts, and seafood. Children with a familiar or personal history of atopy are at higher risk of developing other atopic diseases. Currently, a delay in the introduction of complementary foods in the diet of high-risk infants beyond what is generally recommended for all infants (from the 5–6th month) is not recommended. No skin prick or IgE testing screening is necessary before introducing the allergen [82••]. Risk factors for nutritional deficits in patients undergoing elimination diets during the first 1000 days of life are represented by multiple food allergies, altered weaning schedules, elimination of food staples such as cow’s milk or wheat, and picky eating. All allergic patients on elimination diets would benefit from referral to a dietitian to learn how to substitute the eliminated food while minimizing the risk of nutrient deficiencies and poor growth [79, 83, 84••, 85••].Wheat is a major source of iron, thiamine, niacin, riboflavin, folic acid, magnesium, and vitamin B6; choosing whole or enriched available alternative grains improves the nutritional quality of a wheat-restricted diet during the first 1000 days of life [86]. Eggs contain vitamin B12, riboflavin, biotin, and selenium, and they are common ingredients in the Western diet; therefore, allergic patients need to learn to replace eggs in recipes [87••]. Egg replacement products, which can be used in cooking and baking, are available on the market, and although they provide a similar consistency, they are not similar to eggs in terms of nutritional profile. However, the micronutrients contained in eggs can be found in a wide range of other animal products. Soybean, peanuts, tree nuts, and shellfish elimination, if not combined with allergies to previously mentioned food, is unlikely to impact nutritional intake [79] negatively. Most individuals with soy allergy can tolerate highly refined soy oil and soy lecithin, which are present in many manufactured foods. Fish has an important nutritional value; it is rich in vitamins B, D, and A; selenium; calcium and phosphorus; iron; zinc; magnesium; iodine; and omega-3. Since Omega-3 is present in a few other foods, supplementation could be advisable in children affected by fish allergy [88]. However, because individuals with seafood allergy are rarely allergic to all seafood, people with fish allergy may be able to eat shellfish, and those allergic to shellfish may tolerate canned fish [89•]. Supplements may be necessary for individuals with multiple food allergies, especially those excluding other foods in addition to milk. Table 2 summarizes micronutrients provided from excluded foods and possible substitute.
Celiac Disease
Celiac disease is a hereditary disorder caused by sensitivity to the gliadin fraction of gluten, a protein contained in wheat, rye, and barley. In a genetically susceptible individual, gluten-sensitive T-cells are activated upon contact with gluten; this causes an inflammatory response in the small bowel, leading to the mucosal villi’s characteristic atrophy. Symptoms in children include failure to thrive and non-specific gastrointestinal manifestations. Laboratory abnormalities often occur and include anemia due to malabsorption, namely, iron deficiency or folate deficiency anemia. Because the iron absorption process develops mainly in the proximal duodenum, iron deficiency anemia is frequently the presenting symptom of the disease in children. International recommendations underline the importance of periodic screening for essential micronutrient deficiency in celiac patients, focusing on iron, folic acid, and vitamins D and B12. The treatment of celiac disease consists of a gluten-free diet (avoiding foods containing wheat, rye, and barley) aimed at reducing the inflammatory response and restoring the functional anatomy of intestinal villi, along with vitamin and mineral supplements depending on the type of deficiency [90, 91].
Nutrition-Drug Interaction
Prolonged intake of some drugs can cause micronutrient deficiencies due to decreased absorption or increased gastrointestinal loss. Examples of drugs used in the first 1000 days which could interfere with micronutrient absorption are reported in Table 3. The main action of proton pump inhibitors (PPIs) is to reduce the production of gastric acid; therefore, their use can lead to a deficiency in micronutrients whose absorption depends on low gastric pH. Gastric acid also plays a key role in the intestinal absorption of B12 from food proteins. PPI use reduces the absorption of protein-bound B12 and may lead to B12 deficiency in some individuals, although results are conflicting. There is insufficient evidence to recommend routine screening of vitamin B12 status or routine supplementation of patients taking PPIs [92, 93]. Moreover, some evidence indicates that PPI use may harm iron, calcium and vitamin C absorption. Nonetheless, there is no evidence of duration or dose–response effect, and the possibility of existing cofactors cannot be excluded [94]. Antibiotics alter the intestinal bacterial flora, decreasing the synthesis of folic acid and vitamin K. Diuretics cause renal loss of vitamins (group B, especially B1, and vitamin C) and minerals (especially potassium, magnesium and calcium). Bile acids sequestrant resins can reduce the absorption of fat-soluble vitamins. Aspirin can cause a significant reduction in vitamin C in white blood cells and blood platelets, with consequent bleeding risks. Nonsteroidal anti-inflammatory drugs (NSAIDs) and corticosteroids can also reduce the availability of calcium, magnesium, folic acid, potassium, vitamin C, vitamin D, and selenium. Some chemotherapeutic drugs act by inhibiting the transformation of folic acid into its active form, blocking a fundamental process for cell replication. Antitubercular drug isoniazid cause vitamin B6 deficiencies. Antiepileptics, such as phenobarbital and phenytoin, alter the absorption of vitamins and calcium.
Conclusions
Early life malnutrition in terms of both macro and micronutrients can cause metabolic derangements which could impair or delay the physical and cognitive development of the individual. Deficiencies should be promptly identified in order to tailor interventional strategies specifically to the metabolic needs of the individual [95, 96]. When dietary reference intakes are unmet, collaboration between the pediatrician and a pediatric nutritionist or dietitian is advisable. Nutritional biomarkers are useful for assessing nutrient intake, but they have limitations in terms of accuracy. Just combining anamnesis, clinical evaluation, growth, and dietary assessment, pediatricians can effectively identify and address potential micronutrient deficiencies in children during the first 1000 days of life. Overall, most micronutrient deficiencies can be prevented during the first 1000 days of life through nutrition guidance, food fortification, or supplementation. Timely supplementation can provide a lifelong advantageous impact on child development. It is evident that pediatricians have a fundamental task. However, both environmental factors and a cultural worsening of dietary habits make it difficult. On one hand, micronutrient content can be altered by rising temperature and increasing atmospheric carbon dioxide, reducing the overall yield of micronutrient-rich foods (fruits, vegetables, fish, and nuts). On the other hand, consumption of excessive quantities of refined and processed foods and sugar-sweetened beverages is increasing, contributing to inadequate intake of micronutrients [77]. In conclusion, nutrition in the first 1000 days of life through correct, specific, and precise guidance could help prevent the majority of any cardio-vascular risk factors over time.
Data Availability
The data that support the article are available on request from the corresponding author.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Beluska-Turkan K, Korczak R, Hartell B, et al. Nutritional gaps and supplementation in the first 1000 days. Nutrients. 2019;11(12):1–50. https://doi.org/10.3390/nu11122891.
Namazova Baranova L, Pettoello-Mantovani M, Ehrich J. IS-007 European Paediatric Association Archives of Disease in Childhood. 2014;99:A2–A3.
Pietrobelli A, Agosti M. Nutrition in the first 1000 days: ten practices to minimize obesity emerging from Published Science. Int J Environ Res Public Health. 2017. https://doi.org/10.3390/ijerph14121491.
Pietrobelli A, Agosti M, Zuccotti G. Putting the barker theory into the future: time to act on preventing pediatric obesity. Int J Environ Res Public Health. 2016. https://doi.org/10.3390/ijerph13111151.
Larqué E, Labayen I, Flodmark C-E, et al. From conception to infancy - early risk factors for childhood obesity. Nat Rev Endocrinol. 2019;15(8):456–78. https://doi.org/10.1038/s41574-019-0219-1.
Bailey RL, West KP, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33. https://doi.org/10.1159/000371618.
Mattei D, Pietrobelli A. Micronutrients and brain development. Curr Nutr Rep. 2019;8(2):99–107. https://doi.org/10.1007/s13668-019-0268-z.
• Lockyer F, McCann S, Moore SE. Breast milk micronutrients and infant neurodevelopmental outcomes: a systematic review. Nutrients. 2021;13(11):1–16. https://doi.org/10.3390/nu13113848. Further studies are required to inform policy recommendations and practice and maximise the benefits of exclusive breastfeeding for the neurocognitive development of all infants.
• Irvine N, England-Mason G, Field CJ, Dewey D, Aghajafari F. Prenatal folate and choline levels and brain and cognitive development in children: a critical narrative review. Nutrients. 2022;14(2):1–22. https://doi.org/10.3390/nu14020364. Maternal folate and choline concentrations during pregnancy may play a role in children’s cognitive development.
Picone S, Ritieni A, Fabiano A, et al. Lutein levels in arterial cord blood correlate with neuroprotein activin A in healthy preterm and term newborns: A trophic role for lutein? Clin Biochem. 2018;52:80–4. https://doi.org/10.1016/j.clinbiochem.2017.11.017.
Lindbergh CA, Mewborn CM, Hammond BR, Renzi-Hammond LM, Curran-Celentano JM, Miller LS. Relationship of lutein and zeaxanthin levels to neurocognitive functioning: an fMRI study of older adults. J Int Neuropsychol Soc. 2017;23(1):11–22. https://doi.org/10.1017/S1355617716000850.
Gruszecki WI, Strzałka K. Carotenoids as modulators of lipid membrane physical properties. Biochim Biophys Acta. 2005;1740(2):108–15. https://doi.org/10.1016/j.bbadis.2004.11.015.
Wallace TC, Blusztajn JK, Caudill MA, et al. Choline: the underconsumed and underappreciated essential nutrient. Nutr Today. 2018;53(6):240–53. https://doi.org/10.1097/NT.0000000000000302.
Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJL, Nicholson WK. Folic acid supplementation for the prevention of neural tube defects: an updated evidence report and systematic review for the US preventive services task force. JAMA. 2017;317(2):190–203. https://doi.org/10.1001/jama.2016.19193.
Elmadfa I, Meyer AL. Vitamins for the first 1000 days: preparing for life. Int J Vitam Nutr Res Int Zeitschrift fur Vitamin- und Ernahrungsforschung J Int Vitaminol Nutr. 2012;82(5):342–7. https://doi.org/10.1024/0300-9831/a000129.
Rogne T, Tielemans MJ, Chong MF-F, et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol. 2017;185(3):212–23. https://doi.org/10.1093/aje/kww212.
Chittimoju SB, Pearce EN. Iodine deficiency and supplementation in pregnancy. Clin Obstet Gynecol. 2019;62(2):330–8. https://doi.org/10.1097/GRF.0000000000000428.
Levie D, Korevaar TIM, Bath SC, et al. Association of maternal iodine status with child IQ: a meta-analysis of individual participant data. J Clin Endocrinol Metab. 2019;104(12):5957–67. https://doi.org/10.1210/jc.2018-02559.
Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138(12):2534–6. https://doi.org/10.1093/jn/138.12.2534.
Greminger AR, Lee DL, Shrager P, Mayer-Pröschel M. Gestational iron deficiency differentially alters the structure and function of white and gray matter brain regions of developing rats. J Nutr. 2014;144(7):1058–66. https://doi.org/10.3945/jn.113.187732.
• Zittermann A, Pilz S, Berthold HK. Serum 25-hydroxyvitamin D response to vitamin D supplementation in infants: a systematic review and meta-analysis of clinical intervention trials. Eur J Nutr. 2020;59(1):359–69. https://doi.org/10.1007/s00394-019-01912-x. Vitamin D supplementation of 400 IU/day is sufficient for achieving 25OHD concentrations able to prevent nutritional rickets.
Saggese G, Vierucci F, Prodam F, et al. Vitamin D in pediatric age: consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital J Pediatr. 2018;44(1):1–40. https://doi.org/10.1186/s13052-018-0488-7.
Ackland ML, Michalczyk AA. Zinc and infant nutrition. Arch Biochem Biophys. 2016;611:51–7. https://doi.org/10.1016/j.abb.2016.06.011.
Keen CL, Uriu-Hare JY, Hawk SN, et al. Effect of copper deficiency on prenatal development and pregnancy outcome. Am J Clin Nutr. 1998;67(5 Suppl):1003S-1011S. https://doi.org/10.1093/ajcn/67.5.1003S.
Dolk HM, Nau H, Hummler H, Barlow SM. Dietary vitamin A and teratogenic risk: European Teratology Society discussion paper. Eur J Obstet Gynecol Reprod Biol. 1999;83(1):31–6. https://doi.org/10.1016/s0301-2115(98)00228-0.
Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. Geneva: World Health Organization; 2017. PMID: 29565522.
Piccoli GB, Clari R, Vigotti FN, et al. Vegan-vegetarian diets in pregnancy: danger or panacea? A systematic narrative review BJOG. 2015;122(5):623–33. https://doi.org/10.1111/1471-0528.13280.
•• Chouraqui JP. Risk assessment of micronutrients deficiency in vegetarian or vegan children: not so obvious. Nutrients. 2023;15(9):2129. PMID: 37432244; PMCID: PMC10180846. https://doi.org/10.3390/nu15092129. Diets that are more restrictive in animal source foods, such as vegan diets, have a greater likelihood of nutritional deficiencies; vegan and macrobiotic diets should be avoided during pregnancy and childhood.
•• Kiely ME. Risks and benefits of vegan and vegetarian diets in children. Proc Nutr Soc. 2021;80(2):159–64. https://doi.org/10.1017/S002966512100001X. Complete vegan diet for a young child requires substantial commitment, expert guidance, planning, resources an supplementation.
Mariotti F, Gardner CD. Dietary protein and amino acids in vegetarian diets—a review. Nutrients. 2019;11(11):1–19. https://doi.org/10.3390/nu11112661.
• Simeone G, Bergamini M, Verga MC, et al. Do vegetarian diets provide adequate nutrient intake during complementary feeding? A systematic review Nutrients. 2020;14(17):1–23. https://doi.org/10.3390/nu14173591. Based on current evidence, vegetarian and vegan diets during the complementary feeding period have not been shown to be safe; there is a need for education and nutrition guidance and the need for supplementation should be assessed individually.
Melina V, Craig W, Levin S. Position of the academy of nutrition and dietetics: vegetarian diets. J Acad Nutr Diet. 2016;116(12):1970–80. https://doi.org/10.1016/j.jand.2016.09.025.
Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119–32. https://doi.org/10.1097/MPG.0000000000001454.
Redecilla Ferreiro S, Moráis López A, Moreno Villares JM. [Position paper on vegetarian diets in infants and children. Committee on Nutrition and Breastfeeding of the Spanish Paediatric Association]. An Pediatr. 2020;92(5):306.e1–306.e6. https://doi.org/10.1016/j.anpedi.2019.10.013
Simeone G, Bergamini M, Verga MC, Cuomo B, D'Antonio G, Iacono ID, Mauro DD, Mauro FD, Mauro GD, Leonardi L, Miniello VL, Palma F, Scotese I, Tezza G, Vania A, Caroli M. Do vegetarian diets provide adequate nutrient intake during complementary feeding? A systematic review. Nutrients. 2022 Aug 31;14(17):3591. PMID: 36079848; PMCID: PMC9459879. https://doi.org/10.3390/nu14173591.
Kocaoglu C, Akin F, Caksen H, Böke SB, Arslan S, Aygün S. Cerebral atrophy in a vitamin B12-deficient infant of a vegetarian mother. J Health Popul Nutr. 2014;32(2):367–71.
Karcz K, Królak-Olejnik B. Vegan or vegetarian diet and breast milk composition - a systematic review. Crit Rev Food Sci Nutr. 2021;61(7):1081–98. https://doi.org/10.1080/10408398.2020.1753650.
•• Embleton ND, Jennifer Moltu S, Lapillonne A, et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN Committee on Nutrition and Invited Experts. J Pediatr Gastroenterol Nutr. 2023;76(2):248–68. https://doi.org/10.1097/MPG.0000000000003642. Updated ESPGHAN CoN consensus-based conclusions and recommendations on nutrient intakes and nutritional management for preterm infants.
Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011 Dec;128 Suppl 5(5):S213-56. PMID: 22084329; PMCID: PMC4536582. https://doi.org/10.1542/peds.2009-2107C. Epub 2011 Nov 14.
Dinu M, Abbate R, Gensini GF, Casini A, Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–9. https://doi.org/10.1080/10408398.2016.1138447.
Tseng LA, Sowerbutt C, Lee JJY, van Karnebeek CDM. P4 medicine for epilepsy and intellectual disability: nutritional therapy for inherited metabolic disease. Emerg Top life Sci. 2019;3(1):75–95. https://doi.org/10.1042/ETLS20180180.
Evans S, Alroqaiba N, Daly A, Neville C, Davies P, Macdonald A. Feeding difficulties in children with inherited metabolic disorders: a pilot study. J Hum Nutr Diet Off J Br Diet Assoc. 2012;25(3):209–16. https://doi.org/10.1111/j.1365-277X.2012.01229.x.
Daly A, Evans S, Chahal S, et al. The challenges of vitamin and mineral supplementation in children with inherited metabolic disorders: a prospective trial. J Hum Nutr Diet Off J Br Diet Assoc. 2016;29(4):434–40. https://doi.org/10.1111/jhn.12354.
Lammardo AM, Robert M, Rocha JC, et al. Main issues in micronutrient supplementation in phenylketonuria. Mol Genet Metab. 2013;110:S1–5. https://doi.org/10.1016/j.ymgme.2013.08.008.
•• MacDonald A, Van Wegberg AMJ, Ahring K, et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J Rare Dis. 2020;15(1):1–21. https://doi.org/10.1186/s13023-020-01391-y. Practical resource to help health professionals deliver PKU dietary management according to the statements issued by the PKU European Guidelines.
van Spronsen FJ, van Wegberg AM, Ahring K, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. lancet Diabetes Endocrinol. 2017;5(9):743–756. https://doi.org/10.1016/S2213-8587(16)30320-5.
Van Wegberg AMJ, MacDonald A, Ahring K, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12(1):1–56. https://doi.org/10.1186/s13023-017-0685-2.
Van Biervliet S, Hauser B, Verhulst S, et al. Probiotics in cystic fibrosis patients: a double blind crossover placebo controlled study: Pilot study from the ESPGHAN Working Group on Pancreas/CF. Clin Nutr ESPEN. 2018;27:59–65. https://doi.org/10.1016/j.clnesp.2018.06.008.
•• Mariotti Zani E, Grandinetti R, Cunico D, et al. Nutritional care in children with cystic fibrosis. Nutrients. 2023;15(3):1–24. https://doi.org/10.3390/nu15030479. The nutrition support goal in CF care should begin as early as possible after diagnosis and include the achievement of an optimal nutritional status to support the growth stages.
de Chaves CRM, M, Britto JAA de, Oliveira CQ de, Gomes MM, Cunha ALP da. Association between nutritional status measurements and pulmonary function in children and adolescents with cystic fibrosis. J Bras Pneumol publicacao Of da Soc Bras Pneumol e Tisilogia. 2009;35(5):409–14. https://doi.org/10.1590/s1806-37132009000500004.
Turck D, Braegger CP, Colombo C, et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin Nutr. 2016;35(3):557–77. https://doi.org/10.1016/j.clnu.2016.03.004.
•• Sankararaman S, Hendrix SJ, Schindler T. Update on the management of vitamins and minerals in cystic fibrosis. Nutr Clin Pract Off Publ Am Soc Parenter Enter Nutr. 2022;37(5):1074–87. https://doi.org/10.1002/ncp.10899. Thanks to the availability of several nutrition interventions such as oral/enteral nutrition ,enteric-coated pancreatic enzymes, and water-miscible CF-specific vitamin supplements, frank vitamin deficiencies-with the exception of vitamin D-are rarely encountered in current clinical practice.
• Ruys CA, van de Lagemaat M, Rotteveel J, Finken MJJ, Lafeber HN. Improving long-term health outcomes of preterm infants: how to implement the findings of nutritional intervention studies into daily clinical practice. Eur J Pediatr. 2021;180(6):1665–73. https://doi.org/10.1007/s00431-021-03950-2. Preterm infants are at risk for nutritional deficiencies and extrauterine growth restriction.
Cormack BE, Harding JE, Miller SP, Bloomfield FH. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: a narrative review. Nutrients. 2019. https://doi.org/10.3390/nu11092029.
Cetin I, Del Balzo V, Di Renzo GC, et al. Nutrizione in gravidanza e durante l’allattamento, Linee guida. Fond Confalonieri Ragonese su mandato SIGO, AOGOI, AGUI. 2018.
Say L, Gülmezoglu AM, Hofmeyr GJ. Maternal nutrient supplementation for suspected impaired fetal growth. Cochrane Database Syst Rev. 2003;(1):CD000148. PMID: 12535390. https://doi.org/10.1002/14651858.CD000148.
•• Ilardi L, Proto A, Ceroni F, et al. Overview of important micronutrients supplementation in preterm infants after discharge: a call for consensus. Life. 2021;11(4):1–13. https://doi.org/10.3390/life11040331. Summary of indications for iron, zinc, vitamin D, calcium, phosphate and long-chain polyunsaturated fatty acids (LCPUFAs) supplementation in preterm infants during complementary feeding.
Sinha B, Dudeja N, Chowdhury R, et al. Enteral zinc supplementation in preterm or low birth weight infants: a systematic review and meta-analysis. Pediatrics. 2022;150(August):1–7. https://doi.org/10.1542/peds.2022-057092J.
McCarthy EK, Dempsey EM, Kiely ME. Iron supplementation in preterm and low-birth-weight infants: a systematic review of intervention studies. Nutr Rev. 2019;77(12):865–77. https://doi.org/10.1093/nutrit/nuz051.
Bhatia J, Griffin I, Anderson D, Kler NDM. Selected macro/micronutrient needs of the routine preterm infant. J Pediatr. 2013;162(3 Suppl):S48–55. https://doi.org/10.1016/j.jpeds201211053.
Alshaikh B, Abo Zeed M, Yusuf K, Guin M, Fenton T. Effect of enteral zinc supplementation on growth and neurodevelopment of preterm infants: a systematic review and meta-analysis. J Perinatol Off J Calif Perinat Assoc. 2022;42(4):430–9. https://doi.org/10.1038/s41372-021-01094-7.
Staub E, Evers K, Askie LM. Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates. Cochrane database Syst Rev. 2021;3(3):CD012797. https://doi.org/10.1002/14651858.CD012797.pub2
• Mihatsch W, Thome U, de Pipaon MS. Update on calcium and phosphorus requirements of preterm infants and recommendations for enteral mineral intake. Nutrients. 2021;13(5):1–10. https://doi.org/10.3390/nu13051470. Because preterm infants are at risk of not achieving a bone mineral content equivalent to term infants, oral calcium and phosphate supplementation to preterm neonates and toddlers should be individualized according to metabolic bone biomarkers.
O’Reilly P, Saviani M, Tou A, Tarrant A, Capra L, McCallion N. Do preterm bones still break? Incidence of rib fracture and osteopenia of prematurity in very low birth weight infants. J Paediatr Child Health. 2020;56(6):959–63. https://doi.org/10.1111/jpc.14852.
Faienza MF, D’Amato E, Natale MP, et al. Metabolic bone disease of prematurity: diagnosis and management. Front Pediatr. 2019;7(APR):1–8. https://doi.org/10.3389/fped.2019.00143.
Munshi UK, Graziano PD, Meunier K, Ludke J, Rios A. Serum 25 hydroxy vitamin D levels in very low birth weight infants receiving oral vitamin D supplementation. J Pediatr Gastroenterol Nutr. 2018;66(4):676–9. https://doi.org/10.1097/MPG.0000000000001831.
• Martini L, Pecoraro L, Salvottini C, Piacentini G, Atkinson R, Pietrobelli A. Appropriate and inappropriate vitamin supplementation in children. J Nutr Sci. 2020;9:1–8. https://doi.org/10.1017/jns.2020.12. Vitamin supplementation is indicated in all those conditions in which a vitamin deficiency is found.
•• Shareghfarid E, Sangsefidi ZS, Salehi-Abargouei A, Hosseinzadeh M. Empirically derived dietary patterns and food groups intake in relation with attention deficit/hyperactivity disorder (ADHD) a systematic review and meta-analysis. Clin Nutr ESPEN. 2020;36:28–35. https://doi.org/10.1016/j.clnesp.2019.10.013. "healthy" dietary pattern significantly decreased the risk of ADHD.
Woo HD, Kim DW, Hong Y-S, et al. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients. 2014;6(4):1539–53. https://doi.org/10.3390/nu6041539.
• Lange KW, Nakamura Y, Reissmann A. Diet and food in attention-deficit hyperactivity disorder. J Futur Foods. 2022;2(2):112–8. https://doi.org/10.1016/j.jfutfo.2022.03.008. The cumulative benefits of the range of ingredients comprising healthy diets, such as the Mediterranean diet, may result in better outcomes compared to a supplementation of individual nutrients.
Bandini LG, Anderson SE, Curtin C, et al. Food selectivity in children with autism spectrum disorders and typically developing children. J Pediatr. 2010;157(2):259–64. https://doi.org/10.1016/j.jpeds.2010.02.013.
Bicer AH, Alsaffar AA. Body mass index, dietary intake and feeding problems of Turkish children with autism spectrum disorder (ASD). Res Dev Disabil. 2013;34(11):3978–87. https://doi.org/10.1016/j.ridd.2013.08.024.
Zhu J, Guo M, Yang T, et al. Nutritional status and symptoms in preschool children with autism spectrum disorder: a two-center comparative study in Chongqing and Hainan Province. China Front Pediatr. 2020;8(September):1–11. https://doi.org/10.3389/fped.2020.00469.
• Corsello A, Trovato CM, Di Profio E, et al. Ketogenic diet in children and adolescents: the effects on growth and nutritional status. Pharmacol Res. 2023. https://doi.org/10.1016/j.phrs.2023.106780. Due to its unbalanced ratio of lipids, carbohydrates and proteins, a clinical evaluation of possible side effects with a strict evaluation of growth and nutritional status is essential in all patients following ketogenic diet.
Leone A, De Amicis R, Lessa C, et al. Food and food products on the Italian market for ketogenic dietary treatment of neurological diseases. Nutrients. 2019;11(5):1–21. https://doi.org/10.3390/nu11051104.
Kossoff EH, Zupec-Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175–92. https://doi.org/10.1002/epi4.12225.
De Giorgis V, Tagliabue A, Bisulli F, Brambilla I, Camerini A, Cusmai R, Darra F, Dianin A, Domenica E, Lodi MAM, Matricardi S, Messana T, Operto F, Ragona F, Russo E, Varesio C, Volpi L, Zanaboni MP, Pasca L, Veggiotti P. Ketogenic dietary therapies in epilepsy: recommendations of the Italian League against Epilepsy Dietary Therapy Study Group. Front Neurol. 2023 Jul 10;14:1215618. PMID:37497012; PMCID: PMC10368245. https://doi.org/10.3389/fneur.2023.1215618.
• Venter C, Agostoni C, Arshad SH, et al. Dietary factors during pregnancy and atopic outcomes in childhood: a systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2020;31(8):889–912. https://doi.org/10.1111/pai.13303. Prenatal supplementation with vitamin D may have beneficial effects for prevention of asthma, no consistent evidence regarding other dietary factors exists.
Skypala IJ, McKenzie R. Nutritional issues in food allergy. Clin Rev Allergy Immunol. 2019;57(2):166–78. https://doi.org/10.1007/s12016-018-8688-x.
Kramer MS. Breastfeeding and allergy: the evidence. Ann Nutr Metab. 2011;59(Suppl 1):20–6. https://doi.org/10.1159/000334148.
Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, forpreventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2012;2012(9):CD000133. PMID: 22972039; PMCID: PMC7045459. https://doi.org/10.1002/14651858.CD000133.pub3.
•• David M. Fleischer Md, Edmond S. Chan. A consensus approach to the primary prevention of food allergy through nutrition: guidance from the American Academy of Allergy, Asthma, and Immunology; American College of Allergy, Asthma, and Immunology; and the Canadian Society for Allergy and Clinical Immunology. J Allergy Clin Immunol Pract. 2021 Jan;9(1):22–43. PMID: 33250376. https://doi.org/10.1016/j.jaip.2020.11.002. Epub 2020 Nov 26. To prevent peanut and/or egg allergy, both peanut and egg should be introduced around 6 months of life, but not before 4 months. There is no protective benefit from the use of hydrolyzed formula in the first year of life against food allergy or food sensitization.
Boyce JA, Assa’ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 Suppl):S1-58. https://doi.org/10.1016/j.jaci.2010.10.007.
•• Muraro A, de Silva D, Halken S, Worm M, Khaleva E, Arasi S, Dunn-Galvin A, Nwaru BI, De Jong NW, Rodríguez Del Río P, Turner PJ, Smith P, Begin P, Angier E, Arshad H, Ballmer-Weber B, Beyer K, Bindslev-Jensen C, Cianferoni A, Demoulin C, Deschildre A, Ebisawa M, Fernandez-Rivas MM, Fiocchi A, Flokstra-de Blok B, Gerdts J, Gradman J, Grimshaw K, Jones C, Lau S, Loh R, Alvaro Lozano M, Makela M, Marchisotto MJ, Meyer R, Mills C, Nilsson C, Nowak-Wegrzyn A, Nurmatov U, Pajno G, Podestà M, Poulsen LK, Sampson HA, Sanchez A, Schnadt S, Szajewska H, Van Ree R, Venter C, Vlieg-Boerstra B, Warner A, Wong G, Wood R, Zuberbier T, Roberts G; GA2LEN Food Allergy Guideline Group; GALEN Food Allergy Guideline Group. Managing food allergy: GA2LEN guideline 2022. World Allergy Organ J. 2022 Sep7;15(9):100687. https://doi.org/10.1016/j.waojou.2022.100687. PMID: 36119657; PMCID: PMC9467869. 2022-Recommendations for management of food allergy from the Global Allergy and Asthma European Network (GA2LEN). Guidelines were developed using the Appraisal of Guidelines for Research and Evaluation (AGREE) II framework and the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
•• Vandenplas Y, Brough HA, Fiocchi A, et al. Current guidelines and future strategies for the management of cow’s milk allergy. J Asthma Allergy. 2021;14(August):1244–56. https://doi.org/10.2147/JAA.S276992. Although the majority of the plant-based beverages are nutritionally inadequate, some are nutritionally adapted for toddlers.
Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. 2016;9:13–25. https://doi.org/10.2147/JAA.S81550.
•• Leech SC, Ewan PW, Skypala IJ, et al. BSACI 2021 guideline for the management of egg allergy. Clin Exp Allergy. 2021;51(10):1262–78. https://doi.org/10.1111/cea.14009. Following an acute allergic reaction, egg avoidance advice should be provided. Egg allergy usually resolves, and reintroduction can be achieved at home.
Pecoraro L, Dalle Carbonare L, Castagnoli R, Marseglia GL, Piacentini G, Pietrobelli A. IgE-mediated fish allergy in children: is omega-3 supplementation useful? Int J Food Sci Nutr. 2022;73(2):154–7. https://doi.org/10.1080/09637486.2021.1957782.
• Pecoraro L, Infante S, Fuentes-Aparicio V, Cabrera-Freitag P, Antonucci N, Alvarez-Perea A. IgE-mediated fish allergy in pediatric age: does canned tuna have a chance for tolerance? Pediatr allergy Immunol Off Publ Eur Soc Pediatr Allergy Immunol. 2021;32(5):1114–7. https://doi.org/10.1111/pai.13481. Omega-3 supplementation may seem advisable in children affected by fish allergy.
Talarico V, Giancotti L, Mazza GA, Miniero R, Bertini M. Iron deficiency anemia in celiac disease. Nutrients. 2021;13(5):1–11. https://doi.org/10.3390/nu13051695.
Mearin ML, Agardh D, Antunes H, et al. ESPGHAN position paper on management and follow-up of children and adolescents with celiac disease. J Pediatr Gastroenterol Nutr. 2022;75(3):369–86. https://doi.org/10.1097/MPG.0000000000003540.
Chan L-N. Drug-nutrient interactions. JPEN J Parenter Enteral Nutr. 2013;37(4):450–9. https://doi.org/10.1177/0148607113488799.
Koziolek M, Alcaro S, Augustijns P, et al. The mechanisms of pharmacokinetic food-drug interactions - A perspective from the UNGAP group. Eur J Pharm Sci Off J Eur Fed Pharm Sci. 2019;134:31–59. https://doi.org/10.1016/j.ejps.2019.04.003.
Haastrup PF, Thompson W, Søndergaard J, Jarbøl DE. Side effects of long-term proton pump inhibitor use: a review. Basic Clin Pharmacol Toxicol. 2018;123(2):114–21. https://doi.org/10.1111/bcpt.13023.
Mayneris-Perxachs J, Swann JR. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur J Nutr. 2019;58(3):909–30. https://doi.org/10.1007/s00394-018-1679-0.
Società Italiana di Nutrizione Umana. LARN: Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana, IV revision of Dietary Reference Intake for the Italian Population (LARN 2014). Milan: SICS Editore.
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization: LP and AP. Literature search: CP, LP, and AD. Original draft preparation: CP, LP, and AD. Writing-review and editing: AP, GP, OCA, and AD. Supervision: AP and GP. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Panzeri, C., Pecoraro, L., Dianin, A. et al. Potential Micronutrient Deficiencies in the First 1000 Days of Life: The Pediatrician on the Side of the Weakest. Curr Obes Rep 13, 338–351 (2024). https://doi.org/10.1007/s13679-024-00554-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-024-00554-3