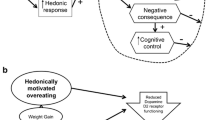
Abstract
Purpose of Review
The current article discusses five neural vulnerability theories for weight gain and reviews evidence from prospective studies using imaging and behavioral measures reflecting neural function, as well as randomized experiments with humans and animals that are consistent or inconsistent with these theories.
Recent Findings
Recent prospective imaging studies examining predictors of weight gain and response to obesity treatment, and repeated-measures imaging studies before and after weight gain and loss have advanced knowledge of etiologic processes and neural plasticity resulting from weight change.
Summary
Overall, data provide strong support for the incentive sensitization theory of obesity and moderate support for the reward surfeit theory, inhibitory control deficit theory, and dynamic vulnerability model of obesity, which attempted to synthesize the former theories into a single etiologic model. Data provide little support for the reward deficit theory. Important directions for future studies are delineated.

Similar content being viewed by others
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
References
World Health Organization. Obesity and overweight. Fact Sheet. Retrieved 5.1.2021 from https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. 2020.
Hartmann-Boyce J, Johns DJ, Jebb SA, Summerbell C, Aveyard P, Behavioural Weight Management Review G. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obes Rev. 2014;15(11):920–32. doi: https://doi.org/10.1111/obr.12220.
Davis C, Strachan S, Berkson M. Sensitivity to reward: implications for overeating and overweight. Appetite. 2004;42(2):131–8. https://doi.org/10.1016/j.appet.2003.07.004.
Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–71. https://doi.org/10.1093/cercor/13.10.1064.
Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124(Pt 9):1720–33. https://doi.org/10.1093/brain/124.9.1720.
Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr. 2013;98(6):1377–84. https://doi.org/10.3945/ajcn.113.069443.
Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–15. https://doi.org/10.1016/s1053-8119(03)00253-2.
Ferreira JG, Tellez LA, Ren X, Yeckel CW, de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J Physiol. 2012;590(4):953–72. https://doi.org/10.1113/jphysiol.2011.218289.
Shearrer GE, Stice E, Burger KS. Adolescents at high risk of obesity show greater striatal response to increased sugar content in milkshakes. Am J Clin Nutr. 2018;107(6):859–66. https://doi.org/10.1093/ajcn/nqy050.
Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci. 2011;31(12):4360–6. https://doi.org/10.1523/JNEUROSCI.6604-10.2011.
Nolan-Poupart S, Veldhuizen MG, Geha P, Small DM. Midbrain response to milkshake correlates with ad libitum milkshake intake in the absence of hunger. Appetite. 2013;60(1):168–74. https://doi.org/10.1016/j.appet.2012.09.032.
Salbe AD, DelParigi A, Pratley RE, Drewnowski A, Tataranni PA. Taste preferences and body weight changes in an obesity-prone population. Am J Clin Nutr. 2004;79(3):372–8. https://doi.org/10.1093/ajcn/79.3.372.
Geha PY, Aschenbrenner K, Felsted J, O’Malley SS, Small DM. Altered hypothalamic response to food in smokers. Am J Clin Nutr. 2013;97(1):15–22. https://doi.org/10.3945/ajcn.112.043307.
Dong D, Jackson T, Wang Y, Chen H. Spontaneous regional brain activity links restrained eating to later weight gain among young women. Biol Psychol. 2015;109:176–83. https://doi.org/10.1016/j.biopsycho.2015.05.003.
Rapuano KM, Laurent JS, Hagler DJ, Jr., Hatton SN, Thompson WK, Jernigan TL, et al. Nucleus accumbens cytoarchitecture predicts weight gain in children. Proc Natl Acad Sci U S A. 2020;117(43):26977–84. doi: https://doi.org/10.1073/pnas.2007918117. This prospective fMRI study shows that cellular density in the nucleus accumbens is related to weight gain at 1-year follow-up in a large sample (N = 2,133) of 9- and 10-year-olds. These findings suggest that differences in the microstructure of reward-related structures may underlie excessive weight gain in childhood.
Winter SR, Yokum S, Stice E, Osipowicz K, Lowe MR. Elevated reward response to receipt of palatable food predicts future weight variability in healthy-weight adolescents. Am J Clin Nutr. 2017;105(4):781–9. https://doi.org/10.3945/ajcn.116.141143.
Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci. 2015;35(28):10316–24. https://doi.org/10.1523/JNEUROSCI.3607-14.2015.
Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–52. https://doi.org/10.1126/science.1161550.
Stice E, Yokum S. Relation of neural response to palatable food tastes and images to future weight gain: Using bootstrap sampling to examine replicability of neuroimaging findings. Neuroimage. 2018;183:522–31. https://doi.org/10.1016/j.neuroimage.2018.08.035.
Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biol Psychiatry. 2013;73(9):869–76. https://doi.org/10.1016/j.biopsych.2012.11.019.
Sun X, Kroemer NB, Veldhuizen MG, Babbs AE, de Araujo IE, Gitelman DR, et al. Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci. 2015;35(20):7964–76. https://doi.org/10.1523/JNEUROSCI.3884-14.2015.
Nelson TD, Brock, R.L., Yokum, S., Tomaso, C.C., Savage, C.R., Stice, E. Much ado about missingness: a demonstration of full information maximum likelihood versus listwise deletion to address missingness in fMRI data. Neuroimage. submitted. This study found that elevated activation in the striatum in response to milkshake tastes predicted BMI gain over 1-year follow-up in a large sample (N = 383).
Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047–58. doi: https://doi.org/10.1016/j.neubiorev.2012.12.001.
Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, et al. Brain dopamine and obesity. Lancet. 2001;357(9253):354–7. https://doi.org/10.1016/s0140-6736(00)03643-6.
de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, et al. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI Res. 2011;1(1):37. https://doi.org/10.1186/2191-219X-1-37.
Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–43. https://doi.org/10.1016/j.neuroimage.2008.06.002.
Eisenstein SA, Antenor-Dorsey JA, Gredysa DM, Koller JM, Bihun EC, Ranck SA, et al. A comparison of D2 receptor specific binding in obese and normal-weight individuals using PET with (N-[(11)C]methyl)benperidol. Synapse. 2013;67(11):748–56. https://doi.org/10.1002/syn.21680.
Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, et al. Obesity is associated with decreased mu-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci. 2015;35(9):3959–65. https://doi.org/10.1523/JNEUROSCI.4744-14.2015.
Frank GK, Reynolds JR, Shott ME, Jappe L, Yang TT, Tregellas JR, et al. Anorexia nervosa and obesity are associated with opposite brain reward response. Neuropsychopharmacology. 2012;37(9):2031–46. https://doi.org/10.1038/npp.2012.51.
Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol. 2008;117(4):924–35. https://doi.org/10.1037/a0013600.
Thanos PK, Michaelides M, Piyis YK, Wang GJ, Volkow ND. Food restriction markedly increases dopamine D2 receptor (D2R) in a rat model of obesity as assessed with in-vivo muPET imaging ([11C] raclopride) and in-vitro ([3H] spiperone) autoradiography. Synapse. 2008;62(1):50–61. https://doi.org/10.1002/syn.20468.
Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci. 2010;30(39):13105–9. https://doi.org/10.1523/JNEUROSCI.2105-10.2010.
Yokum S, Stice E. Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: a repeated-measures fMRI study. Am J Clin Nutr. 2019;110(6):1275–86. doi: https://doi.org/10.1093/ajcn/nqz204. This repeated-measures fMRI study found that weight gain in adolescents is associated with a decrease in responsivity of regions associated with taste and reward processing to palatable high-fat- and high-fat/high-sugar food tastes.
Burger KS. Frontostriatal and behavioral adaptations to daily sugar-sweetened beverage intake: a randomized controlled trial. Am J Clin Nutr. 2017;105(3):555–63. https://doi.org/10.3945/ajcn.116.140145.
Temple JL, Bulkley AM, Badawy RL, Krause N, McCann S, Epstein LH. Differential effects of daily snack food intake on the reinforcing value of food in obese and nonobese women. Am J Clin Nutr. 2009;90(2):304–13. https://doi.org/10.3945/ajcn.2008.27283.
Tey SL, Brown RC, Gray AR, Chisholm AW, Delahunty CM. Long-term consumption of high energy-dense snack foods on sensory-specific satiety and intake. Am J Clin Nutr. 2012;95(5):1038–47. https://doi.org/10.3945/ajcn.111.030882.
Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. NeuroReport. 2002;13(12):1575–8. https://doi.org/10.1097/00001756-200208270-00017.
Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, et al. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122(6):1257–63. https://doi.org/10.1037/a0013111.
Geiger BM, Haburcak M, Avena NM, Moyer MC, Hoebel BG, Pothos EN. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience. 2009;159(4):1193–9. https://doi.org/10.1016/j.neuroscience.2009.02.007.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13(5):635–41. https://doi.org/10.1038/nn.2519.
Thompson JL, Drysdale M, Baimel C, Kaur M, MacGowan T, Pitman KA, et al. Obesity-induced structural and neuronal plasticity in the lateral orbitofrontal cortex. Neuropsychopharmacology. 2017;42(7):1480–90. https://doi.org/10.1038/npp.2016.284.
Val-Laillet D, Layec S, Guerin S, Meurice P, Malbert CH. Changes in brain activity after a diet-induced obesity. Obesity (Silver Spring). 2011;19(4):749–56. https://doi.org/10.1038/oby.2010.292.
Alsio J, Olszewski PK, Norback AH, Gunnarsson ZE, Levine AS, Pickering C, et al. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171(3):779–87. https://doi.org/10.1016/j.neuroscience.2010.09.046.
Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341(6147):800–2. https://doi.org/10.1126/science.1239275.
Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105(4):1028–34. https://doi.org/10.1016/j.physbeh.2011.11.023.
Deckersbach T, Das SK, Urban LE, Salinardi T, Batra P, Rodman AM, et al. Pilot randomized trial demonstrating reversal of obesity-related abnormalities in reward system responsivity to food cues with a behavioral intervention. Nutr Diabetes. 2014;4: e129. https://doi.org/10.1038/nutd.2014.26.
Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, et al. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg. 2010;20(3):369–74. https://doi.org/10.1007/s11695-009-0015-4.
Ranaldi R, Pocock D, Zereik R, Wise RA. Dopamine fluctuations in the nucleus accumbens during maintenance, extinction, and reinstatement of intravenous D-amphetamine self-administration. J Neurosci. 1999;19(10):4102–9.
Hardman CA, Herbert VM, Brunstrom JM, Munafo MR, Rogers PJ. Dopamine and food reward: effects of acute tyrosine/phenylalanine depletion on appetite. Physiol Behav. 2012;105(5):1202–7. https://doi.org/10.1016/j.physbeh.2011.12.022.
Sotak BN, Hnasko TS, Robinson S, Kremer EJ, Palmiter RD. Dysregulation of dopamine signaling in the dorsal striatum inhibits feeding. Brain Res. 2005;1061(2):88–96. https://doi.org/10.1016/j.brainres.2005.08.053.
Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83(7):1197–209. https://doi.org/10.1016/0092-8674(95)90145-0.
Robbins T, Everitt, B. Motivation and reward. In: M. Zigmond FB, S. Landis, J. Roberts, & L. Squire, editor. Fundamental Neuroscience. San Diego: Academic Press; 1999. p. 1245–60.
Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–52. https://doi.org/10.1523/JNEUROSCI.5958-11.2012.
Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring). 2014;22(12):2544–51. https://doi.org/10.1002/oby.20882.
Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring). 2011;19(9):1775–83. https://doi.org/10.1038/oby.2011.168.
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010;1350:43–64. https://doi.org/10.1016/j.brainres.2010.04.003.
Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–9. https://doi.org/10.1126/science.275.5306.1593.
Tindell AJ, Berridge KC, Aldridge JW. Ventral pallidal representation of pavlovian cues and reward: population and rate codes. J Neurosci. 2004;24(5):1058–69. https://doi.org/10.1523/JNEUROSCI.1437-03.2004.
Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. https://doi.org/10.1126/science.1105370.
Balleine BW, Daw ND, O’Doherty JP Multiple forms of value learning and the function of dopamine. In: P. W. Glimcher EF, C. Camerer, & R. A. Poldrack editor. Neuroeconomics: decision making and the brain Academic Press; 2008. p. 367–85.
Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes (Lond). 2010;34(10):1494–500. https://doi.org/10.1038/ijo.2010.84.
Rapuano KM, Zieselman AL, Kelley WM, Sargent JD, Heatherton TF, Gilbert-Diamond D. Genetic risk for obesity predicts nucleus accumbens size and responsivity to real-world food cues. Proc Natl Acad Sci U S A. 2017;114(1):160–5. https://doi.org/10.1073/pnas.1605548113.
Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage. 2010;50(4):1618–25. https://doi.org/10.1016/j.neuroimage.2010.01.081.
Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–47. https://doi.org/10.1016/j.neuroimage.2008.02.031.
Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond). 2009;33(9):1063–73. https://doi.org/10.1038/ijo.2009.138.
Gearhardt AN, Yokum S, Harris JL, Epstein LH, Lumeng JC. Neural response to fast food commercials in adolescents predicts intake. Am J Clin Nutr. 2020;111(3):493–502. https://doi.org/10.1093/ajcn/nqz305. This study found that elevated response in the striatum in response to fast-food commercials is implicated in greater ad lib fast food intake.
Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63(1):415–22. https://doi.org/10.1016/j.neuroimage.2012.06.070.
Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96(5):989–99. https://doi.org/10.3945/ajcn.112.042341.
Adise S, Geier CF, Roberts NJ, White CN, Keller KL. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite. 2018;128:167–79. https://doi.org/10.1016/j.appet.2018.06.014.
Evans GW, Fuller-Rowell TE, Doan SN. Childhood cumulative risk and obesity: the mediating role of self-regulatory ability. Pediatrics. 2012;129(1):e68-73. https://doi.org/10.1542/peds.2010-3647.
Francis LA, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Arch Pediatr Adolesc Med. 2009;163(4):297–302. https://doi.org/10.1001/archpediatrics.2008.579.
Yokum S, Gearhardt AN, Stice E. In search of the most reproducible neural vulnerability factors that predict future weight gain: analyses of data from six prospective studies. Soc Cogn Affect Neurosci. 2021. https://doi.org/10.1093/scan/nsab013.
Yokum S, Bohon C, Berkman E, Stice E. Test-retest reliability of functional MRI food receipt, anticipated receipt, and picture tasks. Am J Clin Nutr. 2021. https://doi.org/10.1093/ajcn/nqab096.
Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–21. https://doi.org/10.1016/j.neuroimage.2011.10.071.
Werthmann J, Field M, Roefs A, Nederkoorn C, Jansen A. Attention bias for chocolate increases chocolate consumption–an attention bias modification study. J Behav Ther Exp Psychiatry. 2014;45(1):136–43. https://doi.org/10.1016/j.jbtep.2013.09.009.
Calitri R, Pothos EM, Tapper K, Brunstrom JM, Rogers PJ. Cognitive biases to healthy and unhealthy food words predict change in BMI. Obesity (Silver Spring). 2010;18(12):2282–7. https://doi.org/10.1038/oby.2010.78.
Clark EN, Dewey AM, Temple JL. Effects of daily snack food intake on food reinforcement depend on body mass index and energy density. Am J Clin Nutr. 2010;91(2):300–8. https://doi.org/10.3945/ajcn.2009.28632.
Teegarden SL, Scott AN, Bale TL. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience. 2009;162(4):924–32. https://doi.org/10.1016/j.neuroscience.2009.05.029.
Burger KS, Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage. 2014;99:122–8. https://doi.org/10.1016/j.neuroimage.2014.05.066.
Zellner MR, Ranaldi R. How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning. Neurosci Biobehav Rev. 2010;34(5):769–80. https://doi.org/10.1016/j.neubiorev.2009.11.011.
Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52(4):1696–703. https://doi.org/10.1016/j.neuroimage.2010.05.059.
Diergaarde L, Pattij T, Nawijn L, Schoffelmeer AN, De Vries TJ. Trait impulsivity predicts escalation of sucrose seeking and hypersensitivity to sucrose-associated stimuli. Behav Neurosci. 2009;123(4):794–803. https://doi.org/10.1037/a0016504.
Han JE, Boachie N, Garcia-Garcia I, Michaud A, Dagher A. Neural correlates of dietary self-control in healthy adults: a meta-analysis of functional brain imaging studies. Physiol Behav. 2018;192:98–108. https://doi.org/10.1016/j.physbeh.2018.02.037.
Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW 3rd, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58(2):582–92. https://doi.org/10.1016/j.appet.2011.11.029.
Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, et al. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–78. https://doi.org/10.1016/j.neuroimage.2013.07.028.
Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage. 2015;109:318–27. https://doi.org/10.1016/j.neuroimage.2014.12.073.
Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes (Lond). 2012;36(5):656–64. https://doi.org/10.1038/ijo.2011.175.
Silvers JA, Insel C, Powers A, Franz P, Weber J, Mischel W, et al. Curbing craving: behavioral and brain evidence that children regulate craving when instructed to do so but have higher baseline craving than adults. Psychol Sci. 2014;25(10):1932–42. https://doi.org/10.1177/0956797614546001.
Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci. 2014;9(7):932–8. https://doi.org/10.1093/scan/nst059.
Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99(4):538–43. https://doi.org/10.1016/j.physbeh.2010.01.008.
Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14(1):21–7. https://doi.org/10.1016/0166-2236(91)90179-x.
Anzman SL, Birch LL. Low inhibitory control and restrictive feeding practices predict weight outcomes. J Pediatr. 2009;155(5):651–6. https://doi.org/10.1016/j.jpeds.2009.04.052.
Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol. 2011;101(3):579–92. https://doi.org/10.1037/a0024286.
Nederkoorn C, Jansen E, Mulkens S, Jansen A. Impulsivity predicts treatment outcome in obese children. Behav Res Ther. 2007;45(5):1071–5. https://doi.org/10.1016/j.brat.2006.05.009.
Jonsson B, Bjorvell H, Levander S, Rossner S. Personality traits predicting weight loss outcome in obese patients. Acta Psychiatr Scand. 1986;74(4):384–7. https://doi.org/10.1111/j.1600-0447.1986.tb06258.x.
Lawrence NS, O’Sullivan J, Parslow D, Javaid M, Adams RC, Chambers CD, et al. Training response inhibition to food is associated with weight loss and reduced energy intake. Appetite. 2015;95:17–28. https://doi.org/10.1016/j.appet.2015.06.009.
Stice E, Rohde P, Gau J, Burtryn M, Shaw, H., Cloud, K., & D'Adamo, L. Enhancing efficacy of a dissonance-based obesity and eating disorder prevention program. Experimental therapeutics. Under review.
Stice E, Yokum S, Veling H, Kemps E, Lawrence NS. Pilot test of a novel food response and attention training treatment for obesity: brain imaging data suggest actions shape valuation. Behav Res Ther. 2017;94:60–70. https://doi.org/10.1016/j.brat.2017.04.007.
Veling H, van Koningsbruggen GM, Aarts H, Stroebe W. Targeting impulsive processes of eating behavior via the internet. Effects on body weight Appetite. 2014;78:102–9. https://doi.org/10.1016/j.appet.2014.03.014.
Martijn C, Vanderlinden M, Roefs A, Huijding J, Jansen A. Increasing body satisfaction of body concerned women through evaluative conditioning using social stimuli. Health Psychol. 2010;29(5):514–20. https://doi.org/10.1037/a0020770.
Stice E, Yokum S. Effects of gymnemic acids lozenge on reward region response to receipt and anticipated receipt of high-sugar food. Physiol Behav. 2018;194:568–76. https://doi.org/10.1016/j.physbeh.2018.07.012.
Funding
Support for this work was provided by National Institutes of Health grants DK112762 & MH111782.
Author information
Authors and Affiliations
Contributions
ES and SY contributed to drafting and revising the manuscript. All the authors approved the final version of the manuscript to be published.
Corresponding author
Ethics declarations
Conflict of Interest
The authors do not have any conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
All the reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Etiology of Obesity
Rights and permissions
About this article
Cite this article
Stice, E., Yokum, S. Neural Vulnerability Factors That Predict Future Weight Gain. Curr Obes Rep 10, 435–443 (2021). https://doi.org/10.1007/s13679-021-00455-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-021-00455-9