Abstract
Obesity is closely associated with a multitude of musculoskeletal conditions. Many musculoskeletal diseases, including knee osteoarthritis and foot pain, are commonly thought to be related to mechanical overload, and the link to obesity intuitively supports such a notion. Walking is significantly changed in the presence of obesity—changes that are similar to those associated with physical disability among individuals in need of personal assistance in daily life. However, although obesity, osteoarthritis, and other painful musculoskeletal conditions exhibit similar changes in locomotion biomechanics, longitudinal data that confidently demonstrate a causal biomechanical link between obesity and osteoarthritis and plantar fasciitis do not exist and are needed to design better and rational treatments of patients with coexistence of obesity and musculoskeletal disorders.
Similar content being viewed by others
Introduction
The global epidemic of obesity is well recognized with an extreme proportion of the adult population being overweight or clinically obese. In 2008 the World Health Organization estimated that 1.5 billion people over the age of 20 years were overweight, and by 2015, more than 700 million individuals are expected to suffer from obesity. Obesity has wide-ranging implications on many aspects of health, including musculoskeletal health. Similar to many musculoskeletal conditions, obesity affects mobility and physical function, leading to disability and loss of independent lifestyle. In this review, we focus on the biomechanical impacts of obesity on gait and locomotion with special reference to knee osteoarthritis (OA) and plantar fasciitis.
Obesity and Walking Biomechanics
Walking may seem a simple and easy task to perform but it is an extraordinarily complex skill that takes years to develop and mature. Biomechanically, walking is a multifaceted bipedal movement produced by accelerating and decelerating body segments in a complex and highly organized manner, resulting in efficient and automated movements of the limbs.
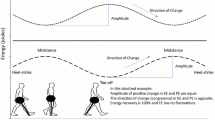
Mechanically, locomotion is significantly affected by higher body mass. According to the laws of Newton movement of a greater mass requires generation of greater forces and moments. Consequently, the metabolic costs of walking are increased with higher bodymass as the forces and moments are generated internally by muscle contractions. Thus, one of the most intuitive gait changes that occur with increasing body mass is reduced walking speed [1] as a means to lower the metabolic costs. Both preferred self-selected walking speed and maximal walking speed have been reported as lower in obese subjects compared to their nonobese counterparts and direct negative associations between body mass index (BMI) and walking performance are reported [1, 2, 3••]. Lower habitual and maximal walking speeds are well recognized signs of physical disablement and the need of assistance in everyday living [4]. Other functional aspects of walking performance are also affected by obesity and overweight. Step lengths, the number of steps taken during a day together with the daily distance covered on a day, are all negatively associated with increasing BMI [2].
Besides being the largest joint in the body, the knee is subjected to enormous forces and moments due to its placement between the two longest bones in the body. The anatomy of the knee is complex, which reflects the multifaceted mechanical demands the knee is exposed to during normal movements and locomotion. Subtle changes in the external environment or demands can cause dramatic changes in the biomechanics of the knee during basic human movements, such as walking. It is therefore no surprise that injuries or disease to the knee have more profound effects on mobility and independent locomotion when compared to the other joints of the lower extremity. Because the articular surfaces of the distal femur and proximal tibia are geometrically incongruent, the dynamic stability of the knee joint is to be provided by forces from surrounding muscles and soft tissues. When taking external forces from the ground into account together with the tight control of body segment movements, the neuromuscular system is certainly challenged during ambulation and with obesity this challenge is increased.
Perturbations (e.g. obesity and/or OA) of the neuromuscular system create susceptibility to joint dysfunction. For example, a slightly more flexed knee when accepting the load from increased body weight would augment the demand on the knee extensors (i.e., the quadriceps muscle) to produce an extensor moment to prevent the knee from collapsing. Being the major determinant of joint stability and force magnitude and distribution across the knee joint [5•], dysfunction of the quadriceps is potentially dangerous [6]. This could create abnormal joint loadings leading to traumatic structural damage or cumulative tissue overloading.
Increasing BMI in adults leads to major spatiotemporal modifications in the gait pattern, such as shorter stride length, lower cadence, increased double support phase, and greater toe-out angle—all associated with a lower walking velocity [3••, 7–9, 10•, 11–13]. Such spatiotemporal modifications allow for longer initial joint rotations, which may reduce the rate of joint and soft tissue loading [3••]. Obese people also adapt functionally to higher body weight by lowering the demands of the quadriceps by walking with less knee flexion during stance [3••]. In obese subjects a higher BMI was associated with more extended legs [14]. By lowering the sagittal moment arm of the knee, such kinematic strategy may be adopted to reduce pain and lessen demands to the antigravity muscles of the legs, while over time it may lead to muscular dysfunction. Walking with reduced demands to the quadriceps is a well-known phenomenon in anterior cruciate ligament–deficient knees (a known risk factor for knee OA) and is termed quadriceps avoidance gait [15]. The reduced demand on the quadriceps renders the hamstrings and the lateral ligaments to provide more dynamic sagittal and frontal knee stability during walking, challenging the homeostasis in the knee.
DeVita et al. [14] demonstrated that morbidly obese subjects (average BMI 42.3 kg/m2) walk with similar demands to their quadriceps muscle compared to lean (average BMI 20.8 kg/m2) subjects. Other data point toward greater knee joint loads in obese compared with normal weight adults [16], and these higher joint loads can be attributed to the larger body mass and ground reaction forces. Patients with knee OA often adopt such quadriceps avoidance gait [17, 18] in an attempt to decrease the forces through the knee joint—presumably to alleviate pain [19]. Muscular activity and the proper coordination hereof have been shown to affect the stability in the frontal plane [20–22] and alter the load distribution between medial and lateral compartments of the knee [23]. Because obesity and knee OA often develop together and lead to very similar changes in the biomechanics of the knee it is difficult to ascertain the causal relationship between obesity-related mechanical overload and development of knee OA.
Obesity and Knee OA
OA, the most common form of arthritis [24], affects a large part of the population, and is a major cause of disability [25–27]. Objectively, OA is defined as a progressive disorder of the joints primarily characterized by gradual loss of cartilage with concurrent development of bony spurs and cysts. The diagnosis is clinical—mainly by the presence of pain—and is most often confirmed by radiography. Symptomatic OA is defined as the presence of the radiographic features of OA in combination with symptoms attributable to OA. Among joints affected by OA, the knee joint is particularly significant, with its importance for ambulation and thereby social function of the individual. The burden of morbidity, primary care visits, and health care costs associated with knee OA are even higher than more high-profile diseases such as diabetes, cancer, and cardiovascular diseases [28]. In addition, the age-related prevalence of knee OA is expected to increase in the future as the large group of baby boomers gets older.
Obesity and knee OA share pathogenetic phenotypes and the development of one disease increases the risk of the other and may trigger the onset of a vicious cycle [29]. Obesity has long been recognized as a risk factor for knee OA and the relationship was documented for the first time in the middle of last century [30]. Now, more than 60 years later we are facing immense and increasing socioeconomic costs due to the complications associated with OA and the obesity pandemic.
Both excess body weight and biomechanics are suggested to play an increasingly important part in both knee OA initiation and progression. In particular, the pathomechanical effects of abnormal joint loadings during walking have been in focus because of the repetitive high joint loadings walking creates daily. Obesity and knee OA are primarily thought to be linked due to excess joint loads that are thought to be pathogenic and leading to knee OA [31–33]. Yet, not all obese patients develop knee OA, nor are all patients with knee OA obese; some obese patients seem to adapt functionally and/or metabolically and avoid development of symptomatic knee OA. Thus, the relationships between knee OA, obesity, and biomechanical factors are complex. Both obesity and knee OA are years in development, encumbering research to identify causal relationships between the two. Obesity significantly affects mobility and walking biomechanics—as does knee OA. Thus, abnormal biomechanics may be both a precursor and a result of obesity in patients with knee OA.
Etiologically, two main pathways are suggested to lead to knee OA; a biochemical pathway and a biomechanical pathway. While this review targets the biomechanical effects of obesity, the biochemical processes are integrated parts of the obesity-related knee OA pathology. Although the pathway by which obesity is primarily thought to cause knee OA is through excess or abnormal joint loading [32, 34–36], a causal relationship between obesity-related joint loads and knee OA development and progression has not been demonstrated. The association between obesity and joint loads during walking seems mechanically intuitive (ie, higher body mass results in higher joint loadings) and it is generally accepted that knee OA is, at least partly, biomechanically driven [36, 37]. However, obesity is also associated with hand OA in spite of the lacking intuitive link between excess joint loads and OA in non-weight–bearing joints [38]. Longitudinal studies that relate knee OA development and progression to mechanical loading (including those of overweight and obesity) are very sparse [35, 39], and it is not possible to confidently link OA to mechanical overload.
Weight Loss in Obese Patients with Knee OA
Theoretically, weight loss could improve dysfunction of the knee related to OA by virtue of the pain reduction reported as a concomitant result of weight loss [40]. Recent studies of weight loss show that the peak knee compressive force can be reduced more than what can be accounted for by the weight loss alone [41••, 42]. The study by Messier et al. [42] showed a 1:4 association between weight loss and reduction in peak joint load even though the weight loss was moderate in magnitude (<5 %). In Aaboe et al. [41••], these results were confirmed reporting a large weight loss (>10 %) and a 1:2 association between weight loss and peak joint load reduction. Thus, biomechanical adaptations that “off-load” the knee occur to a higher extent than what can be accounted for by the mere weight loss, indicating changes in the neuromuscular coordination strategies used during walking.
Aaboe et al. [41] also reported that the axial knee load impulse was significantly reduced by 13 % after weight loss. The axial impulse represents the total or cumulative mechanical load on the knee during one step cycle. These results may have important implications in that not only the peak knee loading but also the total cumulative amount of joint loading exposed to the knee is reduced. However, the clinical relevance of a reduced peak load and axial impulse is currently unknown as no studies have addressed the long-term consequences of weight loss on knee OA progression.
Weight loss significantly reduces knee OA pain [40, 43], supporting previous findings that load-reducing interventions attenuate clinical OA symptoms [44–46]. Conversely, pain relief has been shown to increase knee joint loading during walking and stair climbing [47–50], which raises concerns about a counteraction to the joint load reduction. However, the 2- to 4-unit load reduction with every unit of weight loss [41••, 42] presumably prevail over any increase in joint loads due to reduced pain—in particular because the weight loss studies also encompass changes in symptoms.
Given adequate time, musculoskeletal tissues including cartilage and bone adapt their properties to gradual changes in loads [51]. Likewise, in obese people, body weight generally increases relatively slowly leaving sufficient time for adaptations in walking strategy to accommodate the additional loading. Similarly, during a weight loss intervention body weight decreases gradually over time and the walking strategy adapts to a lower load.
Thorp et al. showed that bone mineral density in the proximal tibia varies as a function of joint loading [52, 53]. Moreover, in areas of increased bone mass, density magnetic resonance imaging reveals bone marrow lesions in support of the hypothesis that the local density of bone reflects loading within the knee [54]. Knee loadings in the medial compartment are also associated with cartilage defects and larger tibial subchondral bone area, supporting a biomechanical explanation of the relationship between morphological changes of bone and knee OA [55]. This is important because increasing the subchondral bone density (as in sclerosis) reduces the viscoelastic properties of the bone and may thereby influence force transmission ability causing aberrant knee joint loadings.
Obesity and Plantar Fasciitis
The plantar aponeurosis (PA) is a fibrous structure of triangular shape that arises from the medial process of the calcaneal tuberosity. From this point it fans out into five slips that attach to the plantar side of the proximal phalanx of each toe [56, 57]. During toe extension the PA wraps around the heads of the metatarsals, thereby pulling the heel and creating a windlass mechanism that is responsible for raising the arch of the foot [56, 57].
Chronic plantar heel pain (CPHP) is a generalized term for a broad spectrum of conditions affecting the heel or the plantar part of the foot. CPHP includes subcalcaneal bursitis, neuritis, subcalcaneal spur, peripheral nerve entrapment, fat pad degeneration, and plantar fasciitis [58–60]. It is estimated that 1 in 10 people will develop CPHP during their lifetime [60]. The most common cause of CPHP is plantar fasciitis, which is generally observed in people over 40 years of age and appears to be equally common in both sexes [61, 62]. The condition is usually diagnosed based on clinical criteria alone, but can also be detected with the use of ultrasonography [62, 63]. The most common clinical feature is pain localized to the medial tubercle of calcaneus, usually worst in the first few steps in the morning or after longer periods of rest [59, 63]. The pain is described as throbbing and often decreases after further ambulatory to return again with continued weight bearing. Up to 50% of the patients diagnosed with plantar fasciitis, also have heel spurs, but these findings seem incidental and does not correlate well with the patients symptoms [59].
The etiology of plantar fasciitis is poorly understood, but it is believed to have a multifactorial origin, primarily due to mechanical overload of the PA [56, 63]. This overload and excessive strain is believed to lead to microscopic tears within the fascia, provoking an inflammatory repair process [63, 64]. Studies of histopathological findings have failed to show evidence of inflammatory infiltrate being present in chronic forms of plantar fasciitis [63].
Several intrinsic and extrinsic factors have been proposed to have an effect on the development of the condition. Age, body weight, BMI, pes planus (low arch), pes cavus (high arch), subtalar joint pronation, unequal limb length, tibial and subtalar varum, femoral/tibial torsion, reduced ankle dorsiflexion/tight Achilles tendon, increased ankle plantar flexion, weak plantar flexors, and heel pad characteristics have all been suggested as intrinsic factors. Foot wear, surface properties, trauma and activity type, level, frequency, intensity, or duration have all been suggested as extrinsic factors [63, 65].
In 2006 Irving et al. [61] conducted a systematic review of the literature that identified associations between intrinsic or extrinsic factors and the development of plantar fasciitis. The strongest association was found with BMI greater than 25 kg/m² or presence of subcalcaneal spur. Increased weight, increased age, decreased ankle dorsiflexion, decreased first metatarsophalangeal joint extension, and prolonged standing all showed weak associations [61]. More recent studies [58, 65, 66, 67•] have found similar results. In a case–control study with 80 participants suffering from CPHP, Irving et al. [58] found significantly higher BMI in the case than in the control group (BMI 29.8 ± 5.4 kg/m2 vs 27.5 ± 4.9 kg/m2), and that individuals with CPHP were more likely to be obese (BMI > 30 kg/m2). In a cross-sectional study of 1411 patients attending an orthopedic foot and ankle specialist, Frey and Zamora [66] demonstrated a 1.4 times increased probability of being diagnosed with plantar fasciitis if patients were overweight (BMI > 25 kg/m2) or obese (BMI > 30 kg/m2).
The evidence should still be interpreted with caution, since the association has only been studied in a limited number of studies thus far, and with a case–control, cross-sectional, or case-series design.
The establishment of causality is not possible due to the fact that it is unclear whether increased BMI was present prior to the development of plantar fasciitis in the studied populations. To be able to determine a causal effect of obesity, studies with prospective designs are warranted. However, it is still plausible that being overweight or obese might be a risk factor for plantar fasciitis, and it is even plausible that this association is mediated by mechanical stress. However, indications of such association have only been found in a few studies [68, 69], and further research in this area is needed.
Conclusions
Obesity causes significant changes in the biomechanics of walking—changes that are very similar to common changes associated with impaired mobility as a consequence of musculoskeletal diseases. There is no doubt that obesity is related to a multitude of musculoskeletal disorders, of which this review has focused on knee OA and plantar fasciitis. While the epidemiological data clearly show associations, the causal mechanisms are very poorly described. Many musculoskeletal diseases (including knee OA and foot pain) are commonly thought to be related to mechanical overload, and the link to obesity intuitively supports such a notion. However, longitudinal data that confidently support such mechanistic causalities do not exist and are needed to design better and rational treatments of patients with coexistence of obesity and musculoskeletal disorders.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Woo J, Leung J, Kwok T. BMI, body composition, and physical functioning in older adults. Obesity (Silver Spring). 2007;15:1886–94.
Yamakawa K, Tsai CK, Haig AJ, et al. Relationship between ambulation and obesity in older persons with and without low back pain. Int J Obes Relat Metab Disord. 2004;28:137–43.
•• Ko S, Stenholm S, Ferrucci L. Characteristic gait patterns in older adults with obesity--results from the Baltimore Longitudinal Study of Aging. J Biomech 2010, 43:1104–1110. This study demonstrates important gait changes with obesity in non-OA individuals that are associated with excessive joint loading.
Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl. 1996;34:1–35.
• Pandy MG, Andriacchi TP. Muscle and joint function in human locomotion. Annu Rev Biomed Eng 2010, 12:401–433. This review describes how computational modeling can be combined with noninvasive gait measurements to describe and explain muscle and joint function in human locomotion. Muscles are a major contributor to the contact force transmitted by that joint and therefore affect its stability.
Henriksen M, Alkjaer T, Lund H, et al. Experimental quadriceps muscle pain impairs knee joint control during walking. J Appl Physiol. 2007;103:132–9.
de Souza SA, Faintuch J, Valezi AC, et al. Gait cinematic analysis in morbidly obese patients. Obes Surg. 2005;15:1238–42.
Lai PP, Leung AK, Li AN, Zhang M. Three-dimensional gait analysis of obese adults. Clin Biomech (Bristol, Avon). 2008;23 Suppl 1:S2–6.
Russell EM, Braun B, Hamill J. Does stride length influence metabolic cost and biomechanical risk factors for knee osteoarthritis in obese women? Clin Biomech (Bristol, Avon). 2010;25:438–43.
• Segal NA, Yack HJ, Kholer S. Weight, rather than obesity distribution, explains peak external knee adduction moment during level gait. Am J Phys Med Rehabil 2009, 88:180–188. This cross-sectional study does not support a significant difference in knee medial compartment loading based on obesity distribution, but does support greater knee frontal torque with higher body weight.
Spyropoulos P, Pisciotta JC, Pavlou KN, et al. Biomechanical gait analysis in obese men. Arch Phys Med Rehabil. 1991;72:1065–70.
Vismara L, Romei M, Galli M, et al. Clinical implications of gait analysis in the rehabilitation of adult patients with "Prader-Willi" Syndrome: a cross-sectional comparative study ("Prader-Willi" Syndrome vs matched obese patients and healthy subjects). J Neuroeng Rehabil. 2007;4:14.
Nantel J, Mathieu ME, Prince F. Physical activity and obesity: biomechanical and physiological key concepts. J Obes. 2011;2011:650230.
DeVita P, Hortobagyi T. Obesity is not associated with increased knee joint torque and power during level walking. Journal of Biomechanics. 2003;36:1355–62.
Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37:1948–56.
Browning RC, Kram R. Effects of obesity on the biomechanics of walking at different speeds. Med Sci Sports Exerc. 2007;39:1632–41.
Kaufman KR, Hughes C, Morrey BF, et al. Gait characteristics of patients with knee osteoarthritis. Journal of Biomechanics 2001, 907–915.
McKean KA, Landry SC, Hubley-Kozey CL, et al. Gender differences exist in osteoarthritic gait. Clin Biomech (Bristol, Avon). 2007;22:400–9.
Henriksen M, Graven-Nielsen T, Aaboe J, et al. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care Res. 2010;62:501–9.
Olmstead TG, Wevers HW, Bryant JT, Gouw GJ. Effect of muscular activity on valgus/varus laxity and stiffness of the knee. J Biomech. 1986;19:565–77.
Pope MH, Johnson RJ, Brown DW, Tighe C. The role of the musculature in injuries to the medial collateral ligament. J Bone Joint Surg Am. 1979;61:398–402.
Markolf KL, Graff-Radford A, Amstutz HC. In vivo knee stability. A quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60:664–74.
Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9.
Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58:26–35.
Prevalence of disabilities and associated health conditions among adults--United States, 1999. MMWR Morb Mortal Wkly Rep 2001, 50:120–125.
Ling SM, Fried LP, Garrett ES, et al. Knee osteoarthritis compromises early mobility function: The Women's Health and Aging Study II. J Rheumatol. 2003;30:114–20.
Felson DT, Naimark A, Anderson J, et al. The prevalence of knee osteoarthritis in the elderly. The framingham osteoarthritis study. Arthritis Rheum. 1987;30:914–8.
Aspden RM. Obesity punches above its weight in osteoarthritis. Rheumatol: Nat Rev; 2010.
Bliddal H, Christensen R. The management of osteoarthritis in the obese patient: practical considerations and guidelines for therapy. Obes Rev. 2006;7:323–31.
Fletcher E, Lewis-Faning E. Chronic rheumatic diseases: statistical study of 1000 cases of chronic rheumatism. Postgrad Med J. 1945;21:137.
Eckstein F, Wirth W, Hudelmaier M, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59:1563–70.
Andriacchi TP, Mundermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006;18:514–8.
Andriacchi TP. Dynamics of knee malalignment. Orthop Clin North Am. 1994;25:395–403.
Hunter DJ, Wilson DR. Imaging the Role of Biomechanics in Osteoarthritis. Rheum Dis Clin N Am. 2009;35:465–83.
Miyazaki T, Wada M, Kawahara H, et al. Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61:617–22.
Andriacchi TP, Mundermann A, Smith RL, et al. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32:447–57.
Dieppe PA. Relationship between symptoms and structural change in osteoarthritis. what are the important targets for osteoarthritis therapy? J Rheumatol Suppl. 2004;70:50–3.
Carman WJ, Sowers M, Hawthorne VM, Weissfeld LA. Obesity as a risk factor for osteoarthritis of the hand and wrist: a prospective study. Am J Epidemiol. 1994;139:119–29.
Bennell KL, Bowles KA, Wang Y, et al. Higher dynamic medial knee load predicts greater cartilage loss over 12 months in medial knee osteoarthritis. Ann Rheum Dis. 2011;70:1770–4.
Christensen R, Astrup A, Bliddal H. Weight loss: the treatment of choice for knee osteoarthritis? A randomized trial. Osteoarthritis Cartilage. 2005;13:20–7.
•• Aaboe J, Bliddal H, Messier SP, et al. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis Cartilage 2011. This longitudinal study shows that changes in biomechanics during walking are closely coupled with a clinical relevant weight loss in obese patients with knee OA. In addition, weight loss leads to adaptations in gait reducing the knee loads more than can be explained by the weight loss magnitude.
Messier SP, Gutekunst DJ, Davis C, Devita P. Weight loss reduces knee-joint loads in overweight and obese older adults with knee osteoarthritis. Arthritis Rheum. 2005;52:2026–32.
Riecke BF, Christensen R, Christensen P, et al. Comparing two low-energy diets for the treatment of knee osteoarthritis symptoms in obese patients: a pragmatic randomized clinical trial. Osteoarthritis Cartilage. 2010;18:746–54.
Kuroyanagi Y, Nagura T, Matsumoto H, et al. The lateral wedged insole with subtalar strapping significantly reduces dynamic knee load in the medial compartment gait analysis on patients with medial knee osteoarthritis. Osteoarthritis Cartilage. 2007;15:932–6.
Sasaki T, Yasuda K. Clinical evaluation of the treatment of osteoarthritic knees using a newly designed wedged insole. Clin Orthop Relat Res 1987, 181–187.
Pollo FE, Otis JC, Backus SI, et al. Reduction of medial compartment loads with valgus bracing of the osteoarthritic knee. Am J Sports Med. 2002;30:414–21.
Henriksen M, Simonsen EB, Alkjaer T, et al. Increased joint loads during walking - A consequence of pain relief in knee osteoarthritis. Knee. 2006;13:445–50.
Hurwitz DE, Ryals AR, Block JA, et al. Knee pain and joint loading in subjects with osteoarthritis of the knee. J Orthop Res. 2000;18:572–9.
Schnitzer TJ, Popovich JM, Andersson GB, Andriacchi TP. Effect of piroxicam on gait in patients with osteoarthritis of the knee. Arthritis Rheum. 1993;36:1207–13.
Shrader MW, Draganich LF, Pottenger LA, Piotrowski GA. Effects of knee pain relief in osteoarthritis on gait and stair-stepping. Clin Orthop 2004, 188–193.
Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:95–101.
Thorp LE, Wimmer MA, Block JA, et al. Bone mineral density in the proximal tibia varies as a function of static alignment and knee adduction angular momentum in individuals with medial knee osteoarthritis. Bone. 2006;39:1116–22.
Hurwitz DE, Sumner DR, Andriacchi TP, Sugar DA. Dynamic knee loads during gait predict proximal tibial bone distribution. J Biomech. 1998;31:423–30.
Lo GH, Hunter DJ, Zhang Y, et al. Bone marrow lesions in the knee are associated with increased local bone density. Arthritis Rheum. 2005;52:2814–21.
Creaby MW, Wang Y, Bennell KL, et al. Dynamic knee loading is related to cartilage defects and tibial plateau bone area in medial knee osteoarthritis. Osteoarthritis Cartilage 2010.
Erdemir A, Hamel AJ, Fauth AR, et al. Dynamic loading of the plantar aponeurosis in walking. J Bone Joint Surg Am. 2004;86-A:546–52.
Caravaggi P, Pataky T, Goulermas JY, et al. A dynamic model of the windlass mechanism of the foot: evidence for early stance phase preloading of the plantar aponeurosis. J Exp Biol. 2009;212:2491–9.
Irving DB, Cook JL, Young MA, Menz HB. Obesity and pronated foot type may increase the risk of chronic plantar heel pain: a matched case–control study. BMC Musculoskelet Disord. 2007;8:41.
Tu P, Bytomski JR. Diagnosis of heel pain. Am Fam Physician. 2011;84:909–16.
Hossain M, Makwana N. Not Plantar Fasciitis: the differential diagnosis and management of heel pain syndrome. Orthopaedics and Trauma. 2011;25:198–206.
Irving DB, Cook JL, Menz HB. Factors associated with chronic plantar heel pain: a systematic review. J Sci Med Sport. 2006;9:11–22.
Ozdemir H, Yilmaz E, Murat A, et al. Sonographic evaluation of plantar fasciitis and relation to body mass index. Eur J Radiol. 2005;54:443–7.
Wearing SC, Smeathers JE, Urry SR, et al. The pathomechanics of plantar fasciitis. Sports Med. 2006;36:585–611.
Wearing SC, Hennig EM, Byrne NM, et al. Musculoskeletal disorders associated with obesity: a biomechanical perspective. Obes Rev. 2006;7:239–50.
Werner RA, Gell N, Hartigan A, et al. Risk factors for plantar fasciitis among assembly plant workers. PM R. 2010;2:110–6.
Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28:996–9.
• Tanamas SK, Wluka AE, Berry P, et al. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res (Hoboken) 2012, 64:262–268. This study shows that increasing BMI, specifically android fat mass, is strongly associated with foot pain and disability mediated by both biomechanical and metabolic mechanisms.
Prichasuk S, Subhadrabandhu T. The relationship of pes planus and calcaneal spur to plantar heel pain. Clin Orthop Relat Res 1994, 192–196.
Riddle DL, Pulisic M, Pidcoe P, Johnson RE. Risk factors for Plantar fasciitis: a matched case–control study. J Bone Joint Surg Am. 2003;85-A:872–7.
Disclosure
Conflicts of interest: M. Henriksen: has received grant support, and expenses to travel to scientific meetings have been covered by Cambridge weight plan, UK; L.B. Jørgensen: none; J. Aaboe: has received grant support, and expenses to travel to scientific meetings have been covered by Cambridge weight plan, UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henriksen, M., Jørgensen, L.B. & Aaboe, J. Obesity and Walking: Implications for Knee Osteoarthritis and Plantar Heel Pain. Curr Obes Rep 1, 160–165 (2012). https://doi.org/10.1007/s13679-012-0017-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13679-012-0017-8