Abstract
Body weight significantly impacts health and quality of life, and is a leading risk factor for the development of knee osteoarthritis (OA). Weight cycling may have more negative health consequences compared to steady high or low weight. Using the Osteoarthritis Initiative dataset, we investigated the effects of weight cycling on physical function, quality of life, and depression over 72-months compared to stable or unidirectional body weight trajectories. Participants (n = 731) had knee OA and were classified as: (1) stable-low (BMI < 25), (2) stable-overweight (BMI = 25–29.9), and (3) stable-obese (BMI ≥ 30); (4) steady-weight-loss; (5) steady-weight-gain (weight loss/gain ≥ 2.2 kg every 2-years); (6) gain–loss–gain weight cycling, and (7) loss–gain–loss weight cycling (weight loss/gain with return to baseline), based on bi-annual assessments. We compared Knee Injury and Osteoarthritis Outcome Knee-Related Quality of Life, Function in Sports and Recreation, Physical Activity in the Elderly, Short Form SF-12, repeated chair rise, 20-m gait speed, and Center for Epidemiological Studies Depression using repeated-measures ANOVA. The steady weight loss group demonstrated the worst pain, physical function, and depressive symptoms over time (p’s < 0.05). More research is needed to confirm these findings, and elucidate the mechanisms by which steady weight loss is associated with functional decline in knee OA.
Similar content being viewed by others
Introduction
Long-term knee joint health, knee-related quality of life and overall wellbeing is dependent on maintenance of healthy body weight. Obesity is an independent risk factor for onset and progression of knee osteoarthritis (OA), impairment of knee function, and deterioration of physical and emotional wellbeing1,2,3. Functional biomarkers, such as timed stair climb and walking performance, are worse in individuals with higher obesity classes4. Cross-sectional data reveal worse self-efficacy with mobility4 and worse knee OA symptoms with higher body mass index (BMI) values than lower BMIs5. Perceived physical function and physical performance (e.g., walking and stair navigation) are both inversely related to depressive symptomology in obese persons with knee OA6. Machine learning models show that the five most important predictors of future mobility limitation were gait speed, chair rise performance, self-reported health status, BMI, and depression7.
Population trends show that people are now living longer with heavier body weight across the lifespan, and U.S. adults are now reporting progressively more attempts with weight loss than in years prior8. Weight loss occurring over months to years can improve knee pain severity, self-reported physical function, and mobility among individuals with knee OA9. Weight change trajectories are dynamic however, and can vary from stable, increasing, decreasing and cycling. Weight regain occurs in over 30% of individuals one year after weight loss and up to 95% by year five10. Weight cycling consists of variable magnitudes of weight gain and loss over time and places individuals at a greater risk for skeletal muscle mass loss, muscle strength decline, comorbid burden, onset of various cancers, and depressive symptoms11,12,13,14,15,16. While the role of body weight on knee OA symptoms is recognized, the effects of weight cycling on mobility loss, quality of life, and emotional wellbeing compared to stable weight or linear changes in body weight in this population, have not been previously examined. We conducted systematic literature searches and were not able to find investigations of these collective relationships up through May 2023. For patients with knee OA, disability risk prediction may become more precise with a better understanding of the potential impact of weight change patterns.
The purposes of this study were to determine among patients with knee OA: (1) the effect of body weight cycling within the context of BMI, on pain, physical function, function-related quality of life compared to stable and unidirectional weight/ BMI change, and (2) second, determine how the specific patterns of weight/ BMI change impacted depressive symptom scores over 72 months compared to stable or unidirectional patterns of body weight change. We hypothesized that progressive weight/BMI gain and weight/BMI cycling involving weight loss and regain, would both be associated with worse pain, physical function and quality of life outcomes, and higher depressive symptom scores compared to other weight and BMI patterns.
Methods
Study design
Data were obtained from the Osteoarthritis Initiative (OAI) clinical dataset version 0.2.3, release created 9.0401M4. These data are available for public access (https://nda.nih.gov/oai/) and permission was obtained by the team from NIMH Data Archive (NDA) to access the data. The OAI is a multicenter, observational population-based study of knee OA and is comprised of three subgroups (N = 4796): the Incidence Cohort, the Progression Cohort and the Control group. As originally designed, age-eligible women and men were recruited from the community and enrolled at four recruitment centers (University of Maryland Baltimore, Johns Hopkins University; Ohio State University; University of Pittsburgh; Memorial Hospital of Rhode Island/Brown University). The OAI was designed to better understand the progression of knee OA and treatment impact in people at-risk-for or with the disease. All participants underwent comprehensive clinical screenings including radiographic imaging and surveys. Kellgren Lawrence (KL) osteoarthritis severity scores were applied by radiologists at the participating sites.
Participants and grouping
Participants were first classified into seven groups based on temporal change patterns of body weight assessed biannually, over a 72-month period (N = 731). The definition of weight cycling was applied from the Diabetes Prevention Program Research Group17. Cycling was defined as body weight change by ≥ 2.2 kg with return to at least that same body weight two years later. The groups were then converted to body mass index (BMI) categories for clinical context. We acknowledge that there are many additional weight/BMI change patterns that existed. Given the sheer volume of data, we present here two common weight cycling patterns in addition to stable and unidirectional weight change. Therefore, the seven groups were as follows:
-
(1)
Stable-low-weight (BMI < 25 kg/m2) weight at each time point 63.0 ± 9.6 kg
-
(2)
Stable-overweight (BMI = 25–29.9 kg/m2) weight at each time point 77.2 ± 9.8 kg
-
(3)
Stable-obese (BMI ≥ 30 kg/m2) weight at each time point 92.0 ± 12.4 kg
-
(4)
Steady-weight-loss (loss ≥ 2.2 kg every 2-years) weight at each time point: 90.1 ± 11.8 kg, 85.7 ± 11.6 kg, 81.2 ± 12.2 kg and 76.2 ± 13.2 kg,
-
(5)
Steady-weight-gain (gain ≥ 2.2 kg every 2-years) weight at each time point: 89.1 ± 14.4 kg, 95.2 ± 15.6 kg, 99.9 ± 15.6 kg and 104.7 ± 17.1 kg
-
(6)
Gain–loss–gain weight cycling (gain ≥ 2.2 kg, loss ≥ 2.2 kg, gain ≥ 2.2 kg) weight at each time point: 84.7 ± 16.2 kg, 90.9 ± 16.3, 83.1 ± 15.2 kg and 88.8 ± 16.4 kg
-
(7)
Loss–gain–loss weight cycling (loss ≥ 2.2 kg, gain ≥ 2.2 kg, loss ≥ 2.2 kg) weight at each time point: 91.0 ± 13.9 kg, 84.8 ± 12.9, 90.4 ± 13.6 kg and 85.1 ± 13.6 kg.
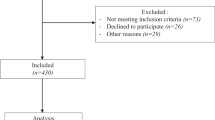
To be included in the analyses, participants had to have body weight and BMI values at each of the four time points with the minimum threshold for change defined above. Participants who underwent a total knee or total hip replacement were also removed from the dataset to eliminate the confounding effect of surgery-induced improvement in function and pain over six years. Data from the remaining 731 participants were included in these analyses. Figure 1 provides the BMI patterns among the seven participant groups. These data demonstrate the successful separation of participants into the weight change strata outlined here.
Health status and covariates at baseline
Anthropometrics, demographics, and cigarette smoking status were extracted from the dataset. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). The age-adjusted Charlson Comorbidity Index (CCI)18 was calculated as a comorbid burden estimate, where age adjustment consisted of assigning each decade of life a comorbidity score of one point in addition to presence or not of 19 conditions. Several characteristics were extracted from the datasets that could influence study outcomes and were covariates. These included: (1) insurance status (some form of health insurance or not), (2) marital status (married or not), (3) educational status (stratified into less than high school or not), (4) employment (currently working for pay or not), (5) household income (< or ≥ $50,000), and (6) home living situation (living alone or not). Kellgren Lawrence (KL) osteoarthritis severity scores19 for both knees were extracted from the dataset from available patients. The use of pain medications was extracted from the question did you take pain medications today (yes/no).
Knee pain severity and total painful joints
Presence of knee pain was considered a secondary outcome and was defined as the participant report of pain, aching, or stiffness on more than half days a month, past 12 months20, Pain severity was reported in the dataset using an 11-point numerical pain rating scale, where 0 = no pain and 10-worst possible pain. Pain presence and severity were collected at baseline and month 72. We determined the prevalence of knee joints that experienced no pain (pain-free, with scores of 0) at baseline and developed pain at month 72. The prevalence of participants who had knee pain at baseline (score ≥ 1 point) who did not report knee pain at month 72 (pain-free) was also determined. The total number of lower body painful joints was summed from right and left foot, ankle, knee, hip and the back at baseline and month 72.
Subjective outcome measures
Five self-reported and performance-based functional metrics were selected to represent functional capacity (in the home, work, community and social domains), and quality of life. These included the Knee Injury and Osteoarthritis Outcome Score (KOOS), Short Form SF-12, Physical Activity in the Elderly (PASE), repeated chair rise test and the 20-m gait speed test.
Knee injury and osteoarthritis outcome score (KOOS)
The KOOS evaluates the short and long-term symptoms in individuals with knee osteoarthritis or injury21. This is a valid and reliable instrument that consists of five different subscales, two of which were included: Function in Sports and Recreation (KOOS-SFR; involves questions about difficulty with tasks such as jumping, running, squatting or kneeling), and the knee-related quality of life (KOOS-QOL; involves questions about awareness of knee problems, modifications to lifestyle). From Likert scale responses on SFR and QOL items, scores are transformed to a 0–100 scale with 0 representing extreme knee problems and 100 representing no knee problems22. The KOOS has good internal consistency and test–retest reliability23, correlates with other self-reported measures of physical function and is sensitive to change24,25.
Short form SF-12
The SF-12 is a generic QOL instrument that captures self-reported impact of health on everyday life and is a shorter version of the SF-3626. Eight health-related domains with one or two questions per domain are included (limitations in physical activities, social activities and usual role activities, bodily pain, general mental health, vitality and general health perceptions). A Physical Composite Score (PCS) was determined using scores from the 12 questions. The scores are norm-based, with a mean of 50 in the general population, and higher scores indicate better health26. The SF-12 is validated for use in osteoarthritis and has strong correlations with clinical variables27.
Physical activity in the elderly (PASE)
The PASE is a valid, 12-item self-administered questionnaire developed for the use in adults over 65 years of age to estimate the amount of physical activity performed over the last seven days28. Physical activities include walking outside, household chores (light or heavy), sports and recreational activities (light, moderate and strenuous, muscle strengthening/endurance activity) and work hours. The intensity level, duration and frequency are used to create a score ranging from 0 to 793 points, with higher scores representing higher activity levels28. This instrument has good test–retest reliability (r = 0.75), and has recently been recommended as the assessment to measure total physical activity in older adults29. Subscales for muscle strengthening/endurance activity and walking activity in hours per day were used.
Physical function tests
Performance-based, valid measures of physical function extracted from the dataset included the repeated chair rise test (timed completion of five chair stands), and the 20-m gait speed test. Gait speeds and gait speed loss are related to loss of independence and quality of life30. Gait speed was determined by dividing the time to complete the test by the walk test distance, and are expressed in m/s. Loss of mobility was estimated using gait speed thresholds and reduction in gait speed over time. Gait speed of less than 1 m/s is associated with elevated risk for self-reported mobility disability among older adults31. A loss in gait speed of > 0.2 m/s is associated with an elevated risk for mortality among individuals with knee OA32. Mobility loss in this study was operationalized as the change in gait speed from baseline to month 72. The proportion of individuals in each BMI group with mobility loss was calculated for analysis.
Center for epidemiological studies depression score (CES-D)
The CES-D is a 20-item instrument that asks respondents to rate how often over the past seven days they experienced symptoms associated with depression, such as trouble focusing, poor appetite, and feelings of loneliness, sadness, failure in life self-dislike33. Response options range from 0 to 3 for each item (0 = Rarely or None of the Time, 1 = Some or Little of the Time, 2 = Moderately or Much of the time, 3 = Most or Almost All the Time). Scores range from 0 to 60, with high scores indicating greater depressive symptoms. The CES-D has high reliability in older adults (Cronbach’s alpha 0.84)34, and can effectively discriminate between persons with and without depression who have chronic pain (sensitivity 81.8%, specificity 72.7%)35.
Statistical analyses
Data analysis was conducted in IBM SPSS version 26.0 (Armonk, NY). Distributional form was examined for each outcome variable. For outcomes where skewness and kurtosis existed (i.e., 20-m gait speed, repeated chair rise time, and SF-12 Physical subscore), Log10 transformations were performed. Given that the analyses of the transformed variables yielded the same results as the raw data, we present the raw data here for ease of interpretation. Kruskal–Wallis tests were performed on baseline categorical variables, presence of joint pain at baseline and month 72, and changes in mobility (yes/no values for walking speed of < 1 m/s and a reduction in gait speed over time by > 0.2 m/s). Bonferroni corrections at an alpha level of 0.05 were used to adjust for Type 1 errors in the Kruskal–Wallis tests. One-way analyses of variance (ANOVAs) were used to determine if BMI group differences existed in continuous baseline variables. Repeated measures ANOVAs were used to determine BMI group differences on clinical outcomes (pain, function, quality of life, depressive symptoms), and KL scores. Based on baseline BMI group differences sex, age, race, marital status, employment, education level, CCI, number of painful joint pain sites and pain medication use were included as covariates in the analyses. Univariate ANOVA was performed on change in gait speed from baseline to month 72, adjusted for baseline speed. Participants with complete datasets for each outcome variable were included in each analysis. Statistical significance was set at p < 0.05.
Results
Participant characteristics
Characteristics at baseline are presented in Table 1. BMI group differences were found for weight and waist (p’s < 0.001). The mean percent fluctuation ranges in body weight over two-year intervals were less than 0.5% for all stable BMI groups (i.e., BMI < 25 kg/m2, BMI 25–29.9 kg/m2, BMI ≥ 30 kg/m2), − 4.9% to − 6.4% in the steady-weight, 5.0% to 6.9% in the steady-weight-gain group, − 5.9% to 6.6% in the loss–gain–loss group, and 7.6% to − 8.4% in the gain–loss–gain group. Several other BMI group differences existed in age, sex, marital status, employment, education, CCI, KL score, total number of painful joints and pain medication use. The steady-weight-loss group had the highest percentage of participants who were female, unmarried, not employed and had the highest CCI scores, KL scores, and number of painful joints (all p < 0.05).
Supplemental Table 1 provides the severity of knee pain for each group at baseline and month 72. Group differences existed among the pain severity for both limbs at baseline and at month 72; the highest left and right knee pain ratings in the steady weight loss and loss–gain–loss groups (p < 0.001). The prevalence of participants who became pain free by month 72 was lowest in the steady weight gain and steady weight loss groups (0–11%), and highest in the stable weight > 30 kg/m2 (left limb) and stable weight 25–29.9 kg/m2 (right limb). The highest prevalence of pain free joints that became painful by month 72 were in the gain–loss–gain group (left limb) and steady weight gain group (80.0%; right limb). Supplemental Fig. 1 shows the change in KL scores from baseline to month 72 using available data from each BMI group. A group X time interaction was found for KL score in the right knee (p = 0.038), but not the left.
Physical function outcomes
Performance-based measures of function are presented in Figs. 2, 3, and 4. The interaction for group X time was statistically significant for gait speed (F(4,18) = 1.55, p = 0.002), and for the main effect of group on gait speed (p < 0.001). For changes in mobility, a greater percentage of participants in the steady-weight-loss group experienced mobility loss (habitual gait speeds < 1 m/s) over the study period compared to the stable-low BMI group (p = 0.008). As seen in Fig. 4, there was no statitistically significant group X time interaction for repeated chair stand time (p = 0.929), but a main effects for BMI group were statistically significant (p < 0.001). For both gait speed and chair rise tests, the steady-weight-loss group performed the worst over time.
The self-reported measures of physical function, KOOS and PASE scores are provided in Table 2 and Fig. 5a, b. The group X time interaction for KOOS-QOL was found to be statistically significant (F(4,18) = 3.045, p < 0.001), with significant main effects of BMI group and time (p’s < 0.001). Group X time interaction effect was not statistically significant for KOOS SFR, but there was a main effect for BMI group (p < 0.001), such that the steady-weight-loss group reported the lowest SFR scores over time. The group X time interaction was statistically significant for SF-12 PCS scores (F(4,18) = 1.855 p = 0.016), with main effects for group and time (p’s < 0.05). There was no statistically significant group X time interaction for PASE muscle strengthening activity (p = 0.361), but there was a main effect for group (p < 0.001). There was no statistically significant interaction or main effects of group or time found for PASE daily hours of walking (p = 0.562).
Depressive symptomatology
CES-D scores are provided in Table 3. The group X time interaction did not achieve statistical significance (F(4,18) = 1.453, p = 0.098), but there was main effect of group (p < 0.001). The steady-weight-loss group reported the highest scores at each time point.
Discussion
Our purpose was to determine the effect of body weight cycling on self-reported and performance-based measures of pain, physical function, quality of life (QOL), and depressive symptomatology compared to stable or unidirectional trajectories of body weight in persons with knee osteoarthritis (OA). In contrast to our expectations, patients with steady weight loss over 72 months emerged as the group with the worst pain and physical function, greater mobility loss, and worst QOL. Patients with stable weight patterns (i.e., BMI < 25 kg/m2 and 25–30 kg/m2) reported consistent daily participation in muscle strengthening/endurance activity and low depressive symptoms over time, commensurate with preservation of gait speed and chair stand time, as well as more positive self-reported outcomes (i.e., pain, KOOS, and SF-12 scores). Weight cycling patterns of ± ≥ 2.2 kg did not have a significant effect on any study outcome.
The main findings were in contrast to our hypotheses. We did demonstrate the expected patterns of generally worse outcomes with stable, but BMI > 30 kg/m2 trajectories and with linear weight/ BMI gain over time compared to stable BMI < 25 kg/m2 at each timepoint. In this study, we grouped participants in weight/BMI cycling groups if they demonstrated a minimum weight change of 2.2 kg over two years. It is possible that larger group differences in subjective and objective outcomes may have emerged with a larger weight change threshold. Another possibility is that weight cycling may change physical function reserves, depending on the initial body weight. For example, weight loss in a person with high BMI may improve functional outcomes, but have limited or worsening effect among individuals with healthy BMI values. Over time, potential effects of the same weight change may wash out functional effects when averaged across one weight/BMI trajectory. This area warrants additional study to determine the precise impact of weight gain or loss among different BMI strata. The reason for the worse outcomes in the steady weight loss group are not clear. The data show that this group experienced the highest depressive symptom burden even at baseline (Table 3) and a high prevalence of living alone, not married and the highest prevalence of low income compared to most groups. The Charlson comorbid index on average was also highest among all groups. This ‘profile’ may represent a group who might not want to, or cannot, be as functionally capable if they do not have the support or mental wellbeing to do so. Our upcoming research is working to understand the prospective relationship between psychological stress, BMI and physical function.
Comparative studies of weight cycling effects on clinical outcomes in this population are very limited, and research has focused primarily on the structural progression of knee OA with linear changes in body weight and subjective function. Compared to stable weight, progressive weight gain is related to worsening cartilage defects using magnetic resonance imaging data and worsening synovitis36,37, whereas weight loss reduces medial tibial cartilage loss and improves knee symptoms and subjective function38. Prior studies using OAI data showed that participants who lost > 10% body weight compared to 5–10% body weight had less progression of cartilage deterioration over four years than participants with stable weight39. There appears to be a dose–response relationship of body weight change on pain and function outcomes. For example, weight gain (> 10%) is associated with increased self-reported pain and decreased physical function scores compared to smaller body weight changes (< 5%)40. Evidence from studies in older adult cohorts have reported relationships between weight cycling and mobility disability, such that the risk for self-reported mobility disability was elevated in patients who had lost weight or cycled their body weight by 5% compared to patients with stable weight (hazard ratios ranged from 1.39 to 1.67)41. In the Look AHEAD Study, individuals with type II diabetes were examined over 8–9 years. Female weight cyclers demonstrated worse physical performance (9–10% worse Physical Performance Battery scores, 6–8% slower gait speed) than weight losers and maintainers. Males produced 8–19% lower handgrip strength scores than weight re-gainers or weight losers and maintainers42.
Our primary study finding, that steady weight loss was associated with worse outcomes, highlights an important consideration for older adults. Clinical relevance of the differences among BMI groups should be with caution. Some objective differences in gait speed (ranging from 2 to 13%; 0.03–0.18 m/sec) and chair rise time (ranging from 4 to 18%; 0.4–2.7 s) were small but statistically significant. Similarly, subjective group differences among KOOS (ranging from 4 to 28%; 3.3–24.1 points) and SF-12 scores (ranging from 4 to 15%; 1.5–7.9 points) were also statistically significant. Minimal clinically important differences (MCID) the repeated chair rise time is 2.3 s and in gait speed 0.2 m/s. MCID for SF-12 PCS are 1.8 points, and for the KOOS 19.6–21.1 points among persons with severe OA and knee replacement. Thus, our data generally fall close to these values. Muscle loss may accompany overall body weight loss and thereby impede physical function. The impact of steady weight loss in middle-to-older age individuals with knee OA may be counteracted with consistent participation in muscle strengthening and endurance activities. Unfortunately, we were not able to ascertain the loss of muscle mass versus adipose tissue in our groups thus limiting the conclusions that can be drawn from these findings. Future work in this area is needed to better understand the factors contributing to functional decrements in this population.
Interestingly, we found that those who perceived greater difficultly with completing daily activities and considered themselves more functionally limited walked and rose from a chair more slowly than participants who self-rated higher function. Participants who perceived less disability and functional impairment demonstrated better physical performance scores over time. This differential response among groups is likely influenced by factors in addition to body weight. These factors could include variations in confidence in knee function43, knee pain severity44, pain-related cognitions such as fear, self-efficacy45 and catastrophizing46, other psychological factors47,48, and comorbid disease burden. Low levels of knee confidence, negative emotional states (depression), and unfavorable psychological profiles (‘severe’, ‘extremely troubled’) are related to higher self-rated disability, worse walk speeds, chair stand time and stair navigation, and elevated pain6,43,47. Depressive symptoms are closely interrelated with physical disability49, and depression accelerates OA progression50. Elevation of depressive symptomatology over time correspond with higher incidence of mobility disability among older adults49. This is also supported by our own findings where self-reported depressive symptomatology was highest and lowest among the steady weight loss and the stable weight groups, respectively. Among patients with knee OA and obesity, depression is related to perceived impairment of function and less to measured physical task performance6. Thus, self-reported difficulties in function may have more to do with emotional burden rather than physical difficulty alone. The interrelationships of these factors are complex, affecting patient beliefs and subsequently, functional outcomes and quality of life48. Comorbid obesity and depression are independently associated with functional limitations, but have not been shown to independently modify the relationship between knee OA and physical function51. Pain severity and patient beliefs also influence physical function in patients with OA51. Fear of movement is common in patients with knee OA and commonly results in avoidance of physical activities that could hurt while performing them52, which further contributes to loss of muscle strength and endurance51. Additional work is needed to determine the relationships between dynamic changes in body weight and composition, physical activity, psychological characteristics, and pain and disability in this population.
Among adults with OA and obesity, lower limb strength predicts walking and weight-bearing task performance44, while 60% of the variance in self-reported physical function can be explained by pain severity, BMI, anxiety and leg muscle weakness53. Muscle strengthening and endurance benefits for clinical management of knee OA have been systematically reviewed and meta-analyzed, and indicate improvements in muscle strength, pain and disability occur54. Inclusion of regular exercise also reduces depression symptoms55, manages numerous comorbidities56, mitigates muscle loss57 and reduces activity limitations58. Individuals with knee OA may derive significant physical and psychological long-term benefit from participation in some type of muscle strengthening/ endurance activity, especially when coping with depressive symptoms59.
Limitations and future directions
We had the opportunity to leverage the robust OAI dataset with clinically valuable measures, but there are some limitations to this study that deserve comment. A significant challenge in the study of weight cycling overall is the inconsistency on the agreement of a definition of what weight cycling is11. Clear definitions and discrete categories of weight change in both absolute and relative terms will improve interpretability of findings in future work. While we examined the impact of weight and BMI trajectories on our outcomes, detailed information of body composition (adipose vs. skeletal muscle), specific leg strength changes, in addition to information on intentional weight loss strategies could have clarified some of our observations on performance-based metrics. A limitation of many large population studies is the heavy use of self-report measures. In the OAI, several validated, reliable, but self-report measures related to pain effects on quality of life and physical function were used. These measures likely comprise elements of recall, social desirability or habituation bias. As the PASE instrument was designed for the elderly, but the OAI included adults as young as 55 years, there may be some items that are less relevant for the middle-aged participants. We acknowledge that depression is only one aspect of mental health and future research focused on disentangling the unique impact of various combinations of psychological and cognitive factors relevant to OA-related pain severity, specific comorbid conditions, and weight trajectories is needed. Expansion of psychological factors such as measures of fear, confidence, self-efficacy, and multivariable psychological profiles in future prospective studies of OA would greatly improve our interpretation of pain and functional changes over time, and guide the development of precise interventions to prevent disability in this population. We acknowledge that a couple of our study groups had small samples, and that multiple comparisons were made across several outcomes, both of which may have affected our results and produced some findings that may have been due to chance. Finally, the lack of complete data for all participants limited our sample sizes in some trajectory groups for six-year follow-ups; it is possible that there may be additional weight trajectory effects that were masked due to small samples. In light of these limitations, the generalizability to the OA population as a whole may not yet be possible. Prospective datasets that provide annual or biannual assessment of body weight, BMI in conjunction with physical function and mental health over 30–40 years could provide more insight on the small to large weight cycling impact that is more generalizable.
Conclusions
In this cohort of middle to older-aged adults with knee OA, we did not detect significantly worse effects of weight cycling (gain–loss–gain or loss–gain–loss) on functional outcomes, quality of life or depressive symptoms compared to stable or unidirectional weight change trajectories. Steady weight loss was associated with worse outcomes and highest depressive symptomatology over a 72-month follow-up compared to stable weight, unidirectional or the two weight cycling patterns studied here. Consistent participation in muscle strengthening activities, especially among individuals with steady weight loss alone or in combination with strategies to improve psychological wellbeing may help counteract functional decline.
Data availability
Data were obtained from the Osteoarthritis Initiative (OAI) clinical dataset version 0.2.3, release created 9.0401M4. These data are available for public access (https://nda.nih.gov/oai/) and permission was obtained by the team from NIMH Data Archive (NDA) to access the data.
Change history
19 January 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41598-024-52366-z
References
Arranz, L. I., Rafecas, M. & Alegre, C. Effects of obesity on function and quality of life in chronic pain conditions. Curr. Rheumatol. Rep. 16(1), 390. https://doi.org/10.1007/s11926-013-0390-7 (2014).
Blagojevic, M., Jinks, C., Jeffery, A. & Jordan, K. P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 18(1), 24–33. https://doi.org/10.1016/j.joca.2009.08.010 (2010).
Vincent, H. K., Vincent, K. R. & Lamb, K. M. Obesity and mobility disability in the older adult. Obes. Rev. 11(8), 568–579. https://doi.org/10.1111/j.1467-789X.2009.00703.x (2010).
Garver, M. J. et al. Weight status and differences in mobility performance, pain symptoms, and physical activity in older, knee osteoarthritis patients. Arthritis 2014, 375909. https://doi.org/10.1155/2014/375909 (2014).
Rogers, M. W. & Wilder, F. V. The association of BMI and knee pain among persons with radiographic knee osteoarthritis: A cross-sectional study. BMC Musculoskelet. Disord. 9, 163. https://doi.org/10.1186/1471-2474-9-163 (2008).
Possley, D., Budiman-Mak, E., O’Connell, S., Jelinek, C. & Collins, E. G. Relationship between depression and functional measures in overweight and obese persons with osteoarthritis of the knee. J. Rehabil. Res. Dev. 46(9), 1091–1098. https://doi.org/10.1682/jrrd.2009.03.0024 (2009).
Speiser, J. L. et al. Predicting future mobility limitation in older adults: A machine learning analysis of health ABC Study Data. J. Gerontol. Ser. A. https://doi.org/10.1093/gerona/glab269 (2021).
Han, L. et al. Trends in self-perceived weight status, weight loss attempts, and weight loss strategies among adults in the United States, 1999–2016. JAMA Netw. Open. 2(11), e1915219. https://doi.org/10.1001/jamanetworkopen.2019.15219 (2019).
Chu, I. J. H., Lim, A. Y. T. & Ng, C. L. W. Effects of meaningful weight loss beyond symptomatic relief in adults with knee osteoarthritis and obesity: A systematic review and meta-analysis. Obes Rev. 19(11), 1597–1607. https://doi.org/10.1111/obr.12726 (2018).
Methods for voluntary weight loss and control. NIH technology assessment conference panel consensus development conference, 30 March to 1 April 1992. Ann. Intern. Med. 119(72), 764–770 (1993).
Rossi, A. P. et al. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity 27(7), 1068–1075. https://doi.org/10.1002/oby.22493 (2019).
Madigan, C. D., Pavey, T., Daley, A. J., Jolly, K. & Brown, W. J. Is weight cycling associated with adverse health outcomes? A cohort study. Prev. Med. 108, 47–52. https://doi.org/10.1016/j.ypmed.2017.12.010 (2018).
Quinn, T. J., Manley, M. J., Aziz, J., Padham, J. L. & MacKenzie, A. M. Aging and factors related to running economy. J. Strength Cond. Res. 25(11), 2971–2979. https://doi.org/10.1519/JSC.0b013e318212dd0e (2011).
Zou, H. et al. Association between weight cycling and risk of developing diabetes in adults: A systematic review and meta-analysis. J. Diabetes Investig. 12(4), 625–632. https://doi.org/10.1111/jdi.13380 (2021).
Zou, H. et al. Body-weight fluctuation was associated with increased risk for cardiovascular disease, all-cause and cardiovascular mortality: A systematic review and meta-analysis. Front. Endocrinol. 10, 728. https://doi.org/10.3389/fendo.2019.00728 (2019).
Welti, L. M. et al. Weight Fluctuation and cancer risk in postmenopausal women: The Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 26(5), 779–786. https://doi.org/10.1158/1055-9965.EPI-16-0611 (2017).
Delahanty, L. M. et al. Effects of weight loss, weight cycling, and weight loss maintenance on diabetes incidence and change in cardiometabolic traits in the Diabetes Prevention Program. Diabetes Care. 37(10), 2738–2745. https://doi.org/10.2337/dc14-0018 (2014).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40(5), 373–383 (1987).
Kellgren, J. H. & Lawrence, J. S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 16(4), 494–502 (1957).
Suri, P. et al. Low back pain and other musculoskeletal pain comorbidities in individuals with symptomatic osteoarthritis of the knee: Data from the osteoarthritis initiative. Arthritis Care Res. 62(12), 1715–1723. https://doi.org/10.1002/acr.20324 (2010).
Roos, E. M., Roos, H. P., Lohmander, L. S., Ekdahl, C. & Beynnon, B. D. Knee Injury and Osteoarthritis Outcome Score (KOOS)–development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 28(2), 88–96. https://doi.org/10.2519/jospt.1998.28.2.88 (1998).
Roos, E. M. & Lohmander, L. S. The Knee injury and Osteoarthritis Outcome Score (KOOS): From joint injury to osteoarthritis. Health Qual. Life Outcomes. 1, 64. https://doi.org/10.1186/1477-7525-1-64 (2003).
White, D. K. & Master, H. Patient-reported measures of physical function in knee osteoarthritis. Rheum. Dis. Clin. N. Am. 42(2), 239–252. https://doi.org/10.1016/j.rdc.2016.01.005 (2016).
Ornetti, P. et al. Cross-cultural adaptation and validation of the French version of the Knee injury and Osteoarthritis Outcome Score (KOOS) in knee osteoarthritis patients. Osteoarthr. Cartil. 16(4), 423–428. https://doi.org/10.1016/j.joca.2007.08.007 (2008).
Gonçalves, R. S., Cabri, J., Pinheiro, J. P., Ferreira, P. L. & Gil, J. Reliability, validity and responsiveness of the Portuguese version of the Knee injury and Osteoarthritis Outcome Score-Physical Function Short-form (KOOS-PS). Osteoarthr. Cartil. 18(3), 372–376. https://doi.org/10.1016/j.joca.2009.10.012 (2010).
Ware, J., Kosinski, M. & Keller, S. D. A 12-Item Short-Form Health Survey: Construction of scales and preliminary tests of reliability and validity. Med Care. 34(3), 220–233 (1996).
Gandhi, S. K. et al. Psychometric evaluation of the 12-item short-form health survey (SF-12) in osteoarthritis and rheumatoid arthritis clinical trials. Clin. Ther. 23(7), 1080–1098. https://doi.org/10.1016/s0149-2918(01)80093-x (2001).
Washburn, R. A., Smith, K. W., Jette, A. M. & Janney, C. A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 46(2), 153–162. https://doi.org/10.1016/0895-4356(93)90053-4 (1993).
Sattler, M. C. et al. Current evidence of measurement properties of physical activity questionnaires for older adults: An updated systematic review. Sports Med. 50(7), 1271–1315. https://doi.org/10.1007/s40279-020-01268-x (2020).
Makris, U. E. et al. The relationship among neuromuscular impairments, chronic back pain, and mobility in older adults. PMR 8(8), 738–747. https://doi.org/10.1016/j.pmrj.2016.01.007 (2016).
Miller, M. E. et al. Gait speed and mobility disability: Revisiting meaningful levels in diverse clinical populations. J. Am. Geriatr. Soc. 66(5), 954–961. https://doi.org/10.1111/jgs.15331 (2018).
Master, H. et al. The association between walking speed from short- and standard-distance tests with the risk of all-cause mortality among adults with radiographic knee osteoarthritis: Data from three large United States cohort studies. Osteoarthr. Cartil. 28(12), 1551–1558. https://doi.org/10.1016/j.joca.2020.08.009 (2020).
Radloff, L. S. A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401 (1977).
van de Rest, O., van der Zwaluw, N., Beekman, A. T. F., de Groot, L. C. P. G. M. & Geleijnse, J. M. The reliability of three depression rating scales in a general population of Dutch older persons. Int. J. Geriatr. Psychiatry 25(10), 998–1005. https://doi.org/10.1002/gps.2449 (2010).
Geisser, M. E., Roth, R. S. & Robinson, M. E. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: A comparative analysis. Clin. J. Pain. 13(2), 163–170. https://doi.org/10.1097/00002508-199706000-00011 (1997).
Bucknor, M. D. et al. Association of cartilage degeneration with four year weight gain–3T MRI data from the Osteoarthritis Initiative. Osteoarthr Cartil. 23(4), 525–531. https://doi.org/10.1016/j.joca.2014.10.013 (2015).
Landsmeer, M. L. A. et al. Effect of weight change on progression of knee OA structural features assessed by MRI in overweight and obese women. Osteoarthr. Cartil. 26(12), 1666–1674. https://doi.org/10.1016/j.joca.2018.08.006 (2018).
Teichtahl, A. J. et al. Weight change and change in tibial cartilage volume and symptoms in obese adults. Ann. Rheum. Dis. 74(6), 1024–1029. https://doi.org/10.1136/annrheumdis-2013-204488 (2015).
Gersing, A. S. et al. Is weight loss associated with less progression of changes in knee articular cartilage among obese and overweight patients as assessed with MR imaging over 48 months? Data from the Osteoarthritis Initiative. Radiology 284(2), 508–520. https://doi.org/10.1148/radiol.2017161005 (2017).
Riddle, D. L. & Stratford, P. W. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: A cohort study. Arthritis Care Res. 65(1), 15–22. https://doi.org/10.1002/acr.21692 (2013).
Murphy, R. A. et al. Weight change, body composition, and risk of mobility disability and mortality in older adults: A population-based cohort study. J. Am. Geriatr. Soc. 62(8), 1476–1483. https://doi.org/10.1111/jgs.12954 (2014).
Beavers, K. M. et al. Body weight dynamics following intentional weight loss and physical performance: The Look AHEAD Movement and Memory Study. Obes. Sci. Pract. 1(1), 12–22. https://doi.org/10.1002/osp4.3 (2015).
Colbert, C. J. et al. Knee confidence as it relates to physical function outcome in persons with or at high risk of knee osteoarthritis in the osteoarthritis initiative. Arthritis Rheum. 64(5), 1437–1446. https://doi.org/10.1002/art.33505 (2012).
Yázigi, F., Espanha, M., Marques, A., Teles, J. & Teixeira, P. Predictors of walking capacity in obese adults with knee osteoarthritis. Acta Reumatol. Port. 43(4), 256–263 (2018).
Marks, R. Physical and psychological correlates of disability among a cohort of individuals with knee osteoarthritis. Can. J. Aging. 26(4), 367–377. https://doi.org/10.3138/cja.26.4.367 (2007).
Nebel, M. B. et al. The relationship of self-reported pain and functional impairment to gait mechanics in overweight and obese persons with knee osteoarthritis. Arch. Phys. Med. Rehabil. 90(11), 1874–1879. https://doi.org/10.1016/j.apmr.2009.07.010 (2009).
Cruz-Almeida, Y. et al. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res. 65(11), 1786–1794. https://doi.org/10.1002/acr.22070 (2013).
Helminen, E. E., Arokoski, J. P., Selander, T. A. & Sinikallio, S. H. Multiple psychological factors predict pain and disability among community-dwelling knee osteoarthritis patients: A five-year prospective study. Clin. Rehabil. 34(3), 404–415. https://doi.org/10.1177/0269215519900533 (2020).
Murphy, R. A. et al. Depressive trajectories and risk of disability and mortality in older adults: Longitudinal findings from the Health, Aging, and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci. 71(2), 228–235. https://doi.org/10.1093/gerona/glv139 (2016).
Jacobs, C. A., Vranceanu, A. M., Thompson, K. L. & Lattermann, C. Rapid progression of knee pain and osteoarthritis biomarkers greatest for patients with combined obesity and depression: Data from the Osteoarthritis Initiative. Cartilage. 11(1), 38–46. https://doi.org/10.1177/1947603518777577 (2020).
Zambon, S. et al. Role of osteoarthritis, comorbidity, and pain in determining functional limitations in older populations: European Project on Osteoarthritis. Arthritis Care Res. 68(6), 801–810. https://doi.org/10.1002/acr.22755 (2016).
Gunn, A. H. et al. Fear of movement and associated factors among adults with symptomatic knee osteoarthritis. Arthritis Care Res. 69(12), 1826–1833. https://doi.org/10.1002/acr.23226 (2017).
Nur, H., Sertkaya, B. S. & Tuncer, T. Determinants of physical functioning in women with knee osteoarthritis. Aging Clin. Exp. Res. 30(4), 299–306. https://doi.org/10.1007/s40520-017-0784-x (2018).
Bartholdy, C. et al. The role of muscle strengthening in exercise therapy for knee osteoarthritis: A systematic review and meta-regression analysis of randomized trials. Semin. Arthritis Rheum. https://doi.org/10.1016/j.semarthrit.2017.03.007 (2017).
Hall, M. et al. Comparative effectiveness of exercise programs for psychological well-being in knee osteoarthritis: A systematic review and network meta-analysis. Semin. Arthritis Rheum. 51(5), 1023–1032. https://doi.org/10.1016/j.semarthrit.2021.07.007 (2021).
Pedersen, B. K. & Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25(Suppl 3), 1–72. https://doi.org/10.1111/sms.12581 (2015).
Liao, C. D. et al. Effects of muscle strength training on muscle mass gain and hypertrophy in older adults with osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res. 72(12), 1703–1718. https://doi.org/10.1002/acr.24097 (2020).
Daste, C. et al. Physical activity for osteoarthritis: Efficiency and review of recommandations. Jt. Bone Spine 88(6), 105207. https://doi.org/10.1016/j.jbspin.2021.105207 (2021).
Tsutsumi, T., Don, B. M., Zaichkowsky, L. D. & Delizonna, L. L. Physical fitness and psychological benefits of strength training in community dwelling older adults. Appl. Hum. Sci. 16(6), 257–266. https://doi.org/10.2114/jpa.16.257 (1997).
Acknowledgements
Data and/or research tools used in the preparation of this manuscript were obtained and analyzed from the controlled access datasets distributed from the Osteoarthritis Initiative (OAI), a data repository housed within the NIMH Data Archive (NDA). OAI is a collaborative informatics system created by the National Institute of Mental Health and the National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS) to provide a worldwide resource to quicken the pace of biomarker identification, scientific investigation and OA drug development. Dataset identifier(s): http://dx.doi.org/10.15154/1524463.
Author information
Authors and Affiliations
Contributions
All authors meet the requirements for authorship. H.K.V., A.J.J., K.V.R., and Y.C.A. designed and conceptualized the research; H.K.V. and K.V.R. acquired the data; H.K.V. analyzed the data; H.K.V., A.J.J., and Y.C.A. interpreted the data; all authors wrote the first draft of the manuscript and contributed critically to revising the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the name of Yenisel Cruz-Almeida, which was incorrectly given as Yenisel Almeida-Cruz.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vincent, H.K., Johnson, A.J., Sibille, K.T. et al. Weight-cycling over 6 years is associated with pain, physical function and depression in the Osteoarthritis Initiative cohort. Sci Rep 13, 17045 (2023). https://doi.org/10.1038/s41598-023-44052-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-44052-3
- Springer Nature Limited