Abstract
Purpose of Review
Office hysteroscopy (OH) is safe and effective for diagnosing and managing intrauterine pathology. Newer technology like smaller cameras, improved tissue removal devices, and recommendations for pain control now make OH comfortable for both the physician and patient, with OH having a very high level of patient satisfaction. Despite these benefits, OH remains significantly underutilized in the United States. This review aims to outline the current capabilities, technology, and patient and physician factors associated with successful OH.
Recent Findings
OH aids in the diagnosis of abnormal uterine bleeding, the most common reason for visits to the gynecologist, and can treat some causes in the same visit. Pathology most conducive to treatment with OH includes endometrial polyps, uterine septa, retained products of pregnancy, adhesions, and retained intrauterine devices (IUDs). When performing OH, equipment selection should be based on the type of procedure planned. Care should be taken to attempt to reduce pain and anxiety during OH, with recommended methods including preprocedural NSAIDs, vaginoscopy, and appropriate counseling and anxiety reduction.
Summary
Appropriate patient selection is essential for both patient and physician comfort when performing OH. Further research and technology improvement can continue to increase comfort and performance in the office.
Similar content being viewed by others
Introduction
Hysteroscopy is used to visually examine the uterine cavity for structural defects, lesions, and other pathology. Pantaleoni conducted the first hysteroscopy in 1869, and subsequent advancements in technology have significantly expanded the capabilities of this procedure [1]. Advancements in hysteroscopic equipment, including scopes, optics, distention media, and analgesics, have enhanced the practicality of office hysteroscopy (OH) and improved comfort for patients [2]. The benefits of OH include the ability to forgo general anesthesia, increased convenience for both the patient and physician, quicker recovery time, and increased cost-effectiveness compared to the operating room (OR) [3•]. Additionally, the office setup requires less draping and gowning for the procedure, leading to enhanced sustainability and decreased waste.
Despite these benefits, OH continues to be underutilized. According to a study by Shields et al., out of 305 hysteroscopies, only 25% were performed in the office [4]. The discordance could stem from inadequate physician training, barriers to investing in capital equipment, and physician anxieties about patient discomfort.
This article reviews the evidence for the benefits of OH and provides strategies for practicing obstetrician-gynecologists to incorporate OH into their practice.
Uses and Efficacy of Office Hysteroscopy
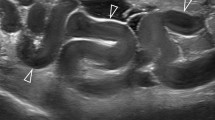
Hysteroscopy has a broad range of applications for both diagnosis and treatment (Fig. 1). Typical uses include diagnosis and management of abnormal uterine bleeding and postmenopausal bleeding, including polyps, submucosal fibroids, and thickened endometrium. OH can also be used to diagnose and remove intrauterine lesions or foreign bodies like intrauterine devices (IUDs), adhesions, retained products of conception (POCs), or uterine septums. Most importantly, OH can be used to rule out abnormalities to prevent unnecessary trips to the operating room. In one study of 130 patients who underwent OH for abnormal uterine bleeding (AUB), 75 did not need to undergo any further testing or procedures in the operating room [5]. OH has also been found to be both safe and effective, with a complication rate of 0–1.5% [5,6,7]. Studies have revealed high success rates for diagnostic OH, as high as 94.8% [8].
Hysteroscopic images of various intrauterine pathology taken in the office. A Grasping a small intrauterine polyp. B Endometrial cancer missed by endometrial biopsy. C Medium uterine septum removed in the office. D Lysis of adhesions in the lower uterine segment to pass the hysteroscope. E A retained IUD with previously avulsed strings. F A grasper removing an embedded IUD arm
Abnormal uterine bleeding constitutes 30% of all visits to the obstetrician-gynecologist [9]. Traditionally, the diagnosis of intracavitary pathology has been based on a combination of tissue collection and ultrasound, even though these methods have low diagnostic capability for intracavitary pathology. Transvaginal ultrasound helps assess the myometrium, but its accuracy in detecting intracavitary pathology is low, with a sensitivity of 56% and specificity of 73% [10]. Hysterosalpingography, which is a procedure in which serial X-rays are taken while the dye is infused into the uterine cavity, has a sensitivity of only 50% and a positive predictive value (PPV) of 30% for the diagnosis of uterine polyps and submucosal fibroids in the asymptomatic infertile population. Sonohysterography (saline infusion sonohysterography or SIS), a technique in which saline is infused into the uterine cavity under transvaginal ultrasound guidance, has a high sensitivity of 87–100% and PPV of > 90% for structural uterine pathologies [11]. SIS has the added ability to assess the entire uterus including the myometrium. However, hysterosalpingography and sonohysterography only serve as diagnostic tests and do not offer the ability to treat pathology.
A systematic review demonstrated that endometrial biopsy is highly accurate in evaluating a global intrauterine process such as endometrial cancer or hyperplasia. However, if cancer occupies less than 50% of the surface area of the endometrial cavity, it is likely to go undetected [12]. A meta-analysis evaluating the effectiveness of diagnostic hysteroscopy compared to hysteroscopy with biopsy, operative hysteroscopy, or hysterectomy showed that diagnostic hysteroscopy achieved an overall success rate of 96.6% in making the proper diagnosis [13]. Additionally, abnormalities were detected in 46.6% of premenopausal and postmenopausal women with AUB [13].
Cost-Effectiveness of Office Hysteroscopy
Due to the considerable cost burden of taking someone to the operating room, the cost-effectiveness of OH has been explored. In the same study looking at OH in patients with AUB, the authors noted a cost savings of $1498 per patient [10], and a systematic review confirmed substantially lower costs for office hysteroscopy than hysteroscopy in the operating room [12].
A highly referenced decision analysis examined the cost-effectiveness of office hysteroscopy screening prior to in vitro fertilization (IVF) [6]. The costs were estimated to be €126 per office hysteroscopy and €2550 per IVF cycle. Office hysteroscopy was always more cost-effective than no screening, even when only patients with intrauterine pathology benefited from the intervention.
Additionally, a cost analysis aimed to estimate the overall cost per pregnancy for hysteroscopic polypectomy performed prior to any assisted reproductive therapy (ART) [14]. Using published data and a healthcare cost reporting website in the United States in 2016 dollars, the authors estimated the charges of office polypectomy at $823 and OR polypectomy at $4507, compared to a single cycle of IVF at $17,360. For patients seeking pregnancy, hysteroscopic polypectomy was found to be cost-effective compared to expectant management when the procedural cost was less than $7164, both of which the average office and OR polypectomy charges fell under.
Hysteroscopic metroplasty can be performed in an office and OR setting, allowing the physician and patient to choose a more cost-effective approach if appropriate. The authors prefer to only perform small to medium-sized septum removals in the office (compared to complete septum, which extends through the cervix or into the vagina) for patient comfort and procedure length. Data is conflicting on whether hysteroscopic metroplasty affects the live birth rate [15]. However, hysteroscopic metroplasty is associated with a reduction in preterm labor and obstetric complications [16]. The costs associated with a preterm neonate are high. In the United States, the preterm birth rate hovers between 9 and 10%, accounting for over $16.9 billion in direct medical care [17]. This equates to a cost of nearly $45,000 (2005 dollars, unadjusted for inflation) per patient per preterm birth. Though not wholly preventative, the cost differential between office hysteroscopy and care of a preterm neonate is high. Therefore, we consider the benefits to greatly outweigh the risks.
Office Hysteroscopy Equipment
Hysteroscopes are now available in various sizes, lens angles, and functionalities. When initiating an office hysteroscopy program, it is essential to consider the specific procedures that will be performed, which can include diagnostic evaluations, directed biopsy, polypectomy, IUD removal, lysis of adhesions, removal of products of conception, metroplasty, or even myomectomy in certain instances. Diagnostic hysteroscopes have a smaller outside diameter but do not have attachments for anything other than inflow. On the other hand, operating hysteroscopes have a wider outer sheath and allow for the passage of tools such as scissors, graspers, tenaculums, tissue extractors, or biopsy forceps. Diagnostic and surgical hysteroscopes are now available in sizes as small as 2.8 and 3.8 mm, respectively.
Additionally, the smallest bipolar resectoscope now has an outer diameter of 5 mm. Compared to other common gynecologic procedures in the office, these hysteroscopes provide additional comfort for both the physician and the patient. For example, a standard endometrial biopsy pipelle has a diameter of 3 mm, whereas an intrauterine device insertion sheath ranges from 4–5 mm.
Lenses are available in various angles. An ideal choice for a diagnostic hysteroscope is a 30° lens, which offers excellent visibility around sharp angles with minimal adjustment required, providing increased comfort for the patient. A 12° lens is preferable for an operative hysteroscope as it allows for a clearer view of the operating equipment positioned directly in front of the lens.
Finally, one must consider whether they want to use rigid or flexible hysteroscopes. Rigid hysteroscopes enable vaginoscopy (described further under “pain control”) and more durable operative procedures. These systems sometimes necessitate a substantial initial investment but are more economical than disposable alternatives when used with any frequency. Flexible hysteroscopes offer the ability to visually navigate sharp angles without contacting sensitive cervical walls. Nevertheless, flexible hysteroscopes commonly incorporate costly fiber-optic cables for which maintenance can be expensive, and these devices are frequently restricted to diagnostic functions alone. Flexible scopes often lack the capability to perform vaginoscopy, which is a recommended method for pain management.
Disposable hysteroscopes were created to provide an option to avoid the initial capital expenses and sterilization requirements associated with reusable hysteroscopes. However, the cost incurred for each usage often outweighs the reimbursement, and the generation of plastic medical waste poses a significant environmental concern. Most of these instruments possess a 3 to 5 mm outer sheath and have limited capacity to perform operative procedures outside of directed biopsy. These hysteroscopes may be ergonomically challenging to use and have flimsy sheaths that do not allow for vaginoscopy. Given the larger size, overall expense, and waste creation, the authors cannot recommend disposable hysteroscopes at this time for office hysteroscopy practices. The technology must advance to smaller outer diameters before they can be compared to reusable hysteroscopes.
Operative Tools
In setting up an office operative hysteroscopy program, assessing which tools will be required is essential. Most office treatments, such as polypectomy, lysis of adhesions, and metroplasty, can be performed using scissors. Additionally, a grasper can be used for procedures including guided biopsy, IUD removal, and removal of products of conception. Although a biopsy forceps is beneficial for targeted biopsies, a grasper can offer the same capability while also having the ability to remove retained IUDs.
More recently, mechanical tissue extraction devices that fit down a 5-French operating channel have expanded the ability to remove large sections of tissue in the office, either by manual operation by hand or with the use of power. A tabletop suction apparatus or fluid management system accompanies some of these. A single-blinded non-inferiority trial that randomized 140 patients to an operative hysteroscopic procedure using a manual morcellation device that can be used in-office versus a standard electromechanical morcellation device (typically when the patient is under anesthesia) concluded that the manual morcellation device was non-inferior. While geometric mean resection times were, on average, 1 min longer with manual morcellation, the mean instrument setup time and total procedure time fell below pre-defined non-inferiority limits [18••]. Additionally, the physician may opt to utilize a generator with the 15-Fr bipolar resectoscope and 5-Fr bipolar twizzle for surgeries such as metroplasty or myomectomy if they desire to employ energy.
Procedure Room Setup and Supplies
Office hysteroscopy can be performed in a small space, such as a repurposed examination room. No procedure rooms are required. A height-adjustable examination table is preferable. Aside from the hysteroscope, a visualization system is necessary, which includes a camera, light source, and monitor. Additionally, the system should have the capability to take images for the electronic health record. Essential components include tubing for both inflow and outflow, seals for the operative port, and a means of measuring the inflow and outflow.
The authors use a 1-L normal saline bag, either to gravity or contained within a pressure bag, for the inflow while allowing the outflow to hang freely, either into a basin or into a drape positioned under the buttocks. This manual system uses gravity and a difference in elevation between the bag and the level of the uterus to create intrauterine pressures ranging from 70–100 mg Hg [19]. A Mayo stand or cart can be helpful for setting the equipment on and easily reaching it during the procedure. Furthermore, it is essential to have a speculum, tenaculum, dilators, and local anesthetic readily accessible in case they are required, though the authors prefer to avoid the use of these supplies if possible. A cleaning process is necessary for all reusable devices, including high-level disinfection or autoclaving in-office or through a centralized sterile processing facility.
Normal saline is the most utilized distention media in office settings due to its widespread availability and various bag sizes. Most office hysteroscopy settings do not require an automated fluid management device, as these take up a significant amount of space and have expensive tubing, canisters, and waste requirements. Manually computing the fluid deficit can be accomplished by measuring the amount remaining in the inflow bag and using a graded under-buttocks drape or a receptacle at the foot of the bed. If the hysteroscope lacks an outflow channel, the inflow volume is usually negligible, making it unnecessary to determine an actual deficiency. The suggested maximum deficit threshold for normal saline is 2500 mL [13]. If the physician expects to do office hysteroscopy procedures that exceed this limit, it is advisable to use a fluid management device. Additionally, if the surgeon chooses to use a hypotonic solution such as glycine with a monopolar energy system, a fluid management machine is required. Early studies show that a deficit of 1 L of a hypotonic solution resulted in a serum sodium drop of 10 mmol/L, which can drop a patient from a normal sodium range into hyponatremia [20]. The authors recommend that monopolar systems should no longer be used due to patient risk. However, these tools are still ubiquitous in hospital systems.
Patient Selection
Selecting suitable patients is crucial for ensuring the comfort of both the patient and the physician when initiating an office hysteroscopy program. A prospective cohort study found that pain was most common in nulliparous patients, those with cervical pathology, and procedures lasting more than 2 min [21]. Physicians may consider initiating diagnostic hysteroscopy in multiparous patients without cervical pathology as a means of developing confidence before proceeding to operative hysteroscopy. The option of undergoing office hysteroscopy is particularly beneficial for patients undergoing ART, particularly IVF, with a plan for embryo transfer. The standard of care is to perform a uterine cavity evaluation on all patients undergoing embryo transfer to optimize implantation rates [14]. Most practices perform saline-infused sonography for initial assessment and then refer patients with equivocal or abnormal exams for hysteroscopy. Timely evaluation is essential in this population because embryo transfer is the last step in the process and, if unsuccessful, may mean that patients will have to undergo additional rounds of IVF to create more embryos. Success rates of IVF decrease in an age-dependent fashion. Therefore, office hysteroscopy is a mechanism to bypass the limited availability of OR space.
Pain Control
Studies suggest that office hysteroscopy is well tolerated by patients overall without any pre-medication. A retrospective cohort of 3000 consecutive women undergoing office hysteroscopy procedures in India between 2012 and 2018 showed good tolerance of the procedure, with only one patient with poor tolerance of the procedure due to a vasovagal syncopal event, and > 98% of patients receiving definitive management by the initial procedure. Additionally, approximately 80% of individuals undergoing the procedure reported a pain score of 0, while the remainder reported mild (VAS scores of 1–3) or moderate (VAS scores 4–7) discomfort [22]. This study and others are proponents of nonpharmacologic methods to reduce patient anxiety and pain, such as “vocal local,” where a nurse or provider verbally engages the patient and shows them operative findings on the monitor [22, 23].
The administration of anesthesia and analgesia during office hysteroscopy has also been extensively researched. A systematic review showed that the use of non-steroidal anti-inflammatory drugs (NSAIDs) led to a significant reduction in pain, both during and after office hysteroscopy. The administration of opioids and antispasmodics resulted in a decrease in perceived discomfort during the procedure, but it was also linked to an increased likelihood of experiencing adverse side effects. Therefore, it is recommended that patients without contraindications should be instructed to take oral NSAIDs before undergoing office hysteroscopy [24••].
A further meta-analysis of more than 1300 patients showed that all types of anesthetics, such as topical, transcervical, intracervical, paracervical, and intracornual, had some effect on pain control. Still, none was better than any other, and local anesthetics did not reduce procedure failure [25••]. Several randomized controlled trials have documented discomfort, bradycardia, and hypotension following cervical injection, and therefore, these interventions are not without risk [26,27,28]. Perhaps, the lowest risk-to-reward intervention involving local anesthesia is to add 10 mL of 2% lidocaine to the 1000 mL saline distention medium [29••]. Prior studies using this approach did not show a significant effect on pain; however, these studies did not utilize a “no-touch” vaginoscopic technique. Newer studies using “no-touch” vaginoscopy (described below) in conjunction with intrauterine instillation of lidocaine showed a statistical decrease in VAS pain scores of 1 point and a trend toward decreased procedure length, without any complications reported in either group [29••].
Vaginoscopy is a procedure that does not require a speculum, tenaculum, or dilators. Instead, the hysteroscope is placed directly into the patient’s vagina to create distention using saline. No speculum, tenaculum, or paracervical block is provided. The hysteroscope is then guided through the cervical canal and into the uterus. Hydrodistension of the cervical canal with saline facilitates passage of the angled scope, especially in cases with challenging angles or stenosis. Without the presence of other instruments in the vagina which can induce a pain response, the vaginoscopic technique allows for greater maneuverability without patient pain.
Extensive studies over a span of 25 years have revealed that using a vaginoscopic technique reduces pain when compared to traditional hysteroscopy using a speculum. Additionally, there is no significant difference in the success rates of the procedures [28, 30, 31]. Vaginoscopy can be started with a 2.8-mm diagnostic hysteroscope for evaluating the cervical canal and uterus and then switched to a larger operative camera if pathology is found. This serial use of cameras allows for hydrodilatation of the cervical canal, which seems to create less pain than mechanical dilation [28, 31].
A meta-analysis examining the effectiveness of cervical preparations in reducing pain during outpatient hysteroscopy found no pain reduction in premenopausal or postmenopausal women who needed cervical dilation of 5 mm or less, which is the largest size of our operative hysteroscope [32]. Prior administration of misoprostol might lead to adverse effects, including abdominal pain, fever, and gastrointestinal disturbance.
Finally, it is essential to implement measures to decrease preprocedural anxiety. A separate meta-analysis revealed that preprocedural anxiety exacerbates the discomfort felt during hysteroscopy. The duration of waiting before the procedure can heighten feelings of anxiety, whereas the presence of music during the procedure has the potential to alleviate anxiety [33]. Providing patients with counseling regarding expectations and informing them about the option to stop the procedure at any point are crucial. Patients who have a preference for undergoing a procedure while under sedation should be provided with the necessary arrangements in either an operating room or a surgery center. It is imperative that all patients participate in a comprehensive informed consent procedure and are scheduled during the optimal time for visualizing the endometrial cavity. Patients are usually scheduled for appointments right after their menstrual period ends, ideally between cycle days 4 and 14.
Surgical Approach
Office hysteroscopy can be employed in several of the following scenarios: (1) to evaluate for structural uterine causes underlying patient symptoms or conditions (e.g., miscarriage or recurrent pregnancy loss) and (2) to further evaluate and treat an abnormal finding on TVUS or saline-infused sonography. In both cases, the office hysteroscopy procedure can be used as a “see and treat” modality, whereby diagnosis and treatment occur simultaneously. In cases where the intrauterine pathology is not amenable for treatment in-office (e.g., type 3 uterine fibroids or large type 1 and 2 submucosal fibroids), the procedure serves as a diagnostic tool with the added benefit of providing vital information for pre-operative planning to optimize the time spent in the operating room [34].
In cases where patient discomfort is suspected or there is low suspicion of needing operative intervention, the authors recommend initiating the procedure with a 30° 2.8-mm diagnostic hysteroscope. The small size of the scope minimizes patient discomfort by allowing for hydrodilatation and passage through the internal cervical os with a device smaller than an endometrial biopsy pipelle. After advancing the scope through the cervical os into the uterine cavity, the presence, location, and degree of uterine pathology can be assessed. If an operative intervention is required, the scope can be removed, and a continuous-flow operating sheath or additional hysteroscope can easily be added, which allows for the passage of semi-flexible 5-Fr instruments. If necessary, discomfort is minimized via serial hydrodilatation of the cervix by upsizing it to the larger operative scope.
In cases where there is a high suspicion of uterine pathology requiring operative intervention or in which cervical stenosis is not suspected (e.g., multiparous patients), the authors recommend starting with a rigid operative hysteroscope, which will allow for a quick introduction of operative instruments.
While provider comfort, technique, and skill level vary, type 1 and 2 submucosal fibroids are admittedly more challenging to treat in the office. These may require staged procedures to achieve complete resection. The time interval between procedures allows the uterus to contract and push the fibroid further into the cavity, making it more like a type 1 or type 0 fibroid, which avoids touching more sensitive myometrium.
Patient Satisfaction
OH has demonstrated both efficacy and excellent patient satisfaction. In a survey of patients who underwent hysteroscopic polypectomy in the office and the OR, 95% of women who underwent OH expressed a preference for OH in the future. Additionally, patients undergoing OH experienced less need for pain medication after surgery, spent less time away from home, and had shorter recovery periods [35].
Financial Considerations
When initiating an OH program, key factors include initial investment, reimbursement, coding, and establishing suitable sterilizing protocols. In 2017, the relative value units (RVUs) for office operative hysteroscopy (CPT code 58558) increased by 237%, whereas the RVUs for operating room (OR) hysteroscopy cases decreased by 11% [36••]. These modifications indicate that payors acknowledge the advantages of OH and are dedicated to promoting and encouraging its utilization. Equipment capital costs range from $15,000 to $35,000, depending on whether the equipment is new or used, the number of operative trays, and the design of the camera and monitor system [37]. Due to the higher reimbursement, physicians and practices can achieve a breakeven point with fewer than 50 office hysteroscopies in 1 year. The Medicare Physician Fee Schedule Search [38] can be referenced for expected reimbursement based on region and location.
Hysteroscopy Training
The lack of training in residency and physician discomfort with performing office hysteroscopy may be mitigated by simulation. Various models have been created to simulate hysteroscopy, but more research is needed to determine their usefulness in clinical practice [39]. Many models exist, using various animal and vegetable components, synthetic uteri, and virtual reality. The American College of Obstetricians and Gynecologists (ACOG) has developed a training program for residents in hysteroscopy using a synthetic uterine model and a vegetable model. This program aims to enhance trainee skills and knowledge in various aspects, such as hysteroscope assembly, maneuvering the hysteroscope, and using operative tools [40]. Virtual models, such as the HystSim (VirtaMed, Zurich, Switzerland), have advanced in realism and now offer various treatments. However, these models still need to undergo additional validation before they can be included in standardized courses, and they may be cost-prohibitive to obtain outside of simulation centers.
OBGYN specialist providers skilled in performing hysteroscopic procedures in an operative suite under anesthesia can train to transition to an office setting by using vaginoscopy under anesthesia to increase proficiency in entry into the uterine cavity. Once in the uterine cavity, the operator can focus on completing the surgery with swiftness and economy of motion. Additionally, physicians can use the same equipment in the OR as they do in the office to become familiar and comfortable with the usage. Physicians can start by selecting patients who are at low risk for discomfort and are motivated to have a procedure in the office. Early successes will translate into increasing comfort with more advanced procedures.
Conclusion
Even though hysteroscopy can be performed safely in the office and has high efficacy and patient satisfaction, there are still low rates of OH in the United States. Additionally, OH is cost-effective, and physician reimbursement rates have increased for OH compared to hysteroscopy in the OR. Considering the benefits to physicians and patients, OH should be considered a first-line option for diagnosing and treating various uterine pathologies. OH can also serve to triage cases, prevent negative cases from going to the OR, and ensure that more complex pathologies can be done in the appropriate setting.
Data Availability
No datasets were generated or analyzed during the current study.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tarneja P, Duggal BS. Hysteroscopy: past, present and future. Med J Armed Forces India. 2002;58(4):293–4.
Campo R, Santangelo F, Gordts S, et al. Outpatient hysteroscopy. Facts Views Vis Obgyn. 2018;10(3):115–22.
• American College of Obstetricians and Gynecologists. The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee Opinion, Number 800. Obstet Gynecol. 2020;135(3):e138-48. An important overview of best practices for office hysteroscopy, reaffirmed 2023.
Shields J, Dilday E, Chang S, Kho K. Moving hysteroscopy from the office to the operating room: a comparison of clinical outcomes and resource utilization. High Value Practice Academic Alliance National Conference 2018. https://hvpaa.org/moving-hysteroscopy-from-the-office-to-the-operating-room-a-comparison-of-clinical-outcomes-and-resource-utilization/. Accessed 5 Jul 2021.
Moawad NS, Santamaria E, Johnson M, Shuster J. Cost-effectiveness of office hysteroscopy for abnormal uterine bleeding. JSLS. 2014;18(3):e2014.00393. https://doi.org/10.4293/JSLS.2014.003935.
Kasius JC, Eijkemans RJ, Mol BW, Fauser BC, Fatemi HM, Broekmans FJ. Cost-effectiveness of hysteroscopy screening for infertile women. Reprod Biomed Online. 2013;26(6):619–26. https://doi.org/10.1016/j.rbmo.2013.02.015.
Ghaly S, de Abreu LR, Abbott JA. Audit of endometrial biopsy at outpatient hysteroscopy. Aust N Z J Obstet Gynaecol. 2008;48(2):202–6.
Marsh FA, Rogerson LJ, Duffy SR. A randomised controlled trial comparing outpatient versus daycase endometrial polypectomy. BJOG. 2006;113(8):896–901. https://doi.org/10.1111/j.1471-0528.2006.00967.
American College of Obstetricians and Gynecologists. Diagnosis of abnormal uterine bleeding in reproductive-aged women: Practice Bulletin No. 128. Obstet Gynecol. 2012;120:197–206.
Guido RS, Kanbour-Shakir A, Rulin MC, Christopherson WA. Pipelle endometrial sampling. Sensitivity in the detection of endometrial cancer. J Reprod Med. 1995;40:553–5.
Bingol B, Gunenc Z, Gedikbasi A, Guner H, Tasdemir S, Tiras B. Comparison of diagnostic accuracy of saline infusion sonohysterography, transvaginal sonography and hysteroscopy. J Obstet Gynaecol. 2011;31(1):54–8. https://doi.org/10.3109/01443615.2010.532246. PMID: 21280995.
van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG. 2007;114:664–75.
AAGL Advancing Minimally Invasive Gynecology Worldwide, Munro MG, Storz K, et al. AAGL practice report: practice guidelines for the management of hysteroscopic distending media: (replaces hysteroscopic fluid monitoring guidelines. J Am Assoc Gynecol Laparosc. 2000;7:167–168.). J Minim Invasive Gynecol. 2013;20(2):137–48.
Mouhayar Y, Yin O, Mumford SL, Segars JH. Hysteroscopic polypectomy prior to infertility treatment: a cost analysis and systematic review. Eur J Obstet Gynecol Reprod Biol. 2017;213:107–15. https://doi.org/10.1016/j.ejogrb.2017.04.025. Epub 2017 Apr 13. PMID: 28445799; PMCID: PMC5505524.
Noventa M, Spagnol G, Marchetti M, Saccardi C, Bonaldo G, Laganà AS, Cavallin F, Andrisani A, Ambrosini G, Vitale SG, Pacheco LA, Haimovich S, Di Spiezio SA, Carugno J, Scioscia M, Garzon S, Bettocchi S, Buzzaccarini G, Tozzi R, Vitagliano A. Uterine septum with or without hysteroscopic metroplasty: impact on fertility and obstetrical outcomes-a systematic review and meta-analysis of observational research. J Clin Med. 2022;11(12):3290. https://doi.org/10.3390/jcm11123290.PMID:35743362;PMCID:PMC9224595.
Spanish Infertility SWOT Group (SISG), Checa MA, Bellver J, Bosch E, Espinós JJ, Fabregues F, Fontes J, García-Velasco J, Requena A. Hysteroscopic septum resection and reproductive medicine: a SWOT analysis. Reprod Biomed Online. 2018;37(6):709–15. https://doi.org/10.1016/j.rbmo.2018.09.013. Epub 2018 Oct 22. PMID: 30527061.
Meltzer R, Markus AR. An analysis of payment mix patterns of preterm births in a post-affordable care act insurance market: implications for the Medicaid program. WHI. 2020;30(4):248–59. https://doi.org/10.1016/j.whi.2020.04.003. Epub 2020 Jun 4. PMID: 32505430.
•• van Wessel S, Hamerlynck T, van Vliet H, Schoot B, Weyers S. Manual morcellation (Resectr™ 9Fr) vs electromechanical morcellation (TruClear™) for hysteroscopic polypectomy: a randomized controlled non-inferiority trial. Acta Obstet Gynecol Scand. 2023;102(2):209–17. https://doi.org/10.1111/aogs.14493. Epub 2023 Jan 20. PMID: 36680382; PMCID: PMC9889322. One of the only studies on new technology for in-office tissue extractors.
Munro MG, Christianson LA. Complications of hysteroscopic and uterine resectoscopic surgery. Clin Obstet Gynecol. 2015;58(4):765–97. https://doi.org/10.1097/GRF.0000000000000146. PMID: 26457853.
Istre O, Skajaa K, Schjoensby AP, Forman A. Changes in serum electrolytes after transcervical resection of endometrium and submucous fibroids with use of glycine 1.5% for uterine irrigation. Obstet Gynecol. 1992;80(2):218–22. PMID: 1635735.
Zayed SM, Elsetohy KA, Zayed M, Fouda UM. Factors affecting pain experienced during office hysteroscopy. Middle East Fertil Soc J. 2015;20:154–8.
Telang M, Shetty TS, Puntambekar SS, Telang PM, Panchal S, Alnure Y. Three thousand cases of office hysteroscopy: see and treat an Indian experience. J Obstet Gynaecol India. 2020;70(5):384–9. https://doi.org/10.1007/s13224-020-01334-4. Epub 2020 Jul 17. PMID: 33041557; PMCID: PMC7516003.
Sardo ADS, Giampaolino P, Manzi A, De Angelis MC, Zizolfi B, Alonso L, Carugno J. The invisible external cervical os. Tips and tricks to overcome this challenge during in-office hysteroscopy. J Minim Invasive Gynecol. 2021;28(2):172–3. https://doi.org/10.1016/j.jmig.2020.05.027. Epub 2020 Jun 9 PMID: 32526381.
•• De Silva PM, Mahmud A, Smith PP, Clark TJ. Analgesia for office hysteroscopy: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2020;27(5):1034–47. Important systematic review on pain management for office hysteroscopy showing that NSAIDs were best.
•• De Silva PM, Carnegy A, Smith PP, Clark TJ. Local anaesthesia for office hysteroscopy: a systematic review & meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:70–81. Important systematic review on local anesthetic showing that everything had some effect but nothing was perfect.
Lau WC, Lo WK, Tam WH, Yuen PM. Paracervical anaesthesia in outpatient hysteroscopy: a randomised double-blind placebo-controlled trial. Br J Obstet Gynaecol. 1999;106(4):356–9.
Giorda G, Scarabelli C, Franceschi S, Campagnutta E. Feasibility and pain control in outpatient hysteroscopy in postmenopausal women: a randomized trial. Acta Obstet Gynecol Scand. 2000;79(7):593–7.
Bettocchi S, Selvaggi L. A vaginoscopic approach to reduce the pain of office hysteroscopy. J Am Assoc Gynecol Laparoscop. 1997;4(2):255–8.
•• Barel O, Preuss E, Stolovitch N, Weinberg S, Barzilay E, Pansky M. Addition of lidocaine to the distension medium in hysteroscopy decreases pain during the procedure-a randomized double-blind, placebo-controlled trial. J Minim Invasive Gynecol. 2021;28(4):865–71. https://doi.org/10.1016/j.jmig.2020.08.003. Epub 2020 Aug 14. PMID: 32798723. A new technique for local anesthetic that avoids additional needle sticks and can be used with vaginoscopy.
Sagiv R, Sadan O, Boaz M, Dishi M, Schechter E, Golan A. A new approach to office hysteroscopy compared with traditional hysteroscopy: a randomized controlled trial. Obstet Gynecol. 2006;108(2):387–92.
Cooper NA, Smith P, Khan KS, Clark TJ. Vaginoscopic approach to outpatient hysteroscopy: a systematic review of the effect on pain. BJOG. 2010;117(5):532–9.
Cooper N, Smith P, Khan K, Clark T. Does cervical preparation before outpatient hysteroscopy reduce women’s pain experience? A systematic review BJOG. 2011;118:1292–301.
Vitale SG, Caruso S, Ciebiera M, et al. Management of anxiety and pain perception in women undergoing office hysteroscopy: a systematic review. Arch Gynecol Obstet. 2020;301(4):885–94.
Fielden AD, Braden JM, Brooks D, Dunlow SG, Lockrow EG, Endicott S. Evaluating the impact of office hysteroscopy in a military treatment facility. Mil Med. 2020;185(9–10):e1686–92.
Kremer C, Duffy S, Moroney M. Patient satisfaction with outpatient hysteroscopy versus day case hysteroscopy: randomised controlled trial. BMJ. 2000;320(7230):279–82.
•• Cholkeri-Singh, A. Payment changes drive hysteroscopy to the office. Ob Gyn News Available at: https://www.mdedge.com/obgyn/article/152152/practice-management/payment-changes-drivehysteroscopy-office. Accessed 5 Jul 2021. Payment changes have made office hysteroscopy with more complex equipment more feasible for the physician.
Tam T, Archill V, Lizon C. Cost analysis of in-office versus hospital hysteroscopy. Middle East Fertil Soc J. 2016;23(7):S194.
Centers for Medicare and Medicaid Services Physician Fee Schedule Search: https://www.cms.gov/medicare/physician-fee-schedule/search. Accessed 15 Jan 2024.
Gambadauro P, Milenkovic M, Hadlaczky G. Simulation for training and assessment in hysteroscopy: a systematic review. J Minim Invasive Gynecol. 2018;25(6):963–73. https://doi.org/10.1016/j.jmig.2018.03.024.
Simulation: basic hysteroscopy. The American College of Obstetricians and Gynecologists. https://www.acog.org/education-and-events/simulations/scog001/simulation. Accessed 5 Jul 2021.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Author information
Authors and Affiliations
Contributions
All authors - lit review, writing, editing KW - Fig. 1 prep.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wright, K.N., Hamilton, K. & Kosturakis, A. An Overview of Office Hysteroscopy. Curr Obstet Gynecol Rep (2024). https://doi.org/10.1007/s13669-024-00377-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s13669-024-00377-y