Abstract
Although often asymptomatic and detected incidentally, varicocele is a relatively common problem in patients who seek medical attention for infertility problems. Ultrasound (US) is the imaging modality of choice for evaluation, but there is no consensus on the diagnostic criteria, classification, and examination technique. In view of this uncertainty, the Scrotal and Penile Imaging Working Group of the European Society of Urogenital Radiology (ESUR-SPIWG) undertook a systematic review of the available literature on this topic, to use as the basis for evidence-based guidelines and recommendations. This paper provides the results of the systematic review on which guidelines were constructed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Varicocele is defined as dilation of the pampiniform venous plexus draining the testicle, with reflux of venous blood [1, 2]. Although it can be asymptomatic and detected incidentally, it is a relatively common problem in patients who seek medical attention for infertility problems, or complain of chronic scrotal pain or discomfort [3].
Varicocele is detected and graded clinically using the criteria introduced by Dubin and Amelar in 1970, a subjective evaluation which is highly dependent on the expertise of the physician [4]. Colour Doppler ultrasound (US) is the imaging modality of choice [5], but the need for imaging itself is debated. In Europe, the use of US is recommended to confirm clinically suspected varicoceles, whilst in the USA and in Asia, routine use of imaging is not recommended [5]. Moreover, there is no agreement on how to perform the US examination. A variety of classifications is used based on different sonographic parameters, even within the same country, depending on the practice of the individual sonologist and of the referring clinician [2].
There is a large, but extremely heterogeneous body of published investigations on US imaging of varicoceles. Even though variability makes it impossible to perform a metanalysis, a systematic literature review is possible.
The Scrotal and Penile Imaging Working Group of the European Society of Urogenital Radiology (ESUR-SPIWG) attempted this task. The group performed a systematic review of the available literature and released a guideline and recommendation paper with the aim to standardize US imaging for varicoceles in Europe. After having formulated 15 relevant clinical questions (Table 1), 23 evidence-based recommendations are provided (Table 2) [6].
The systematic literature review at the root of these recommendations is presented in its complete form in this work.
The ESUR-SPIWG believes that standardization was necessary not only to improve clinical practice, but also to define the methodological basis for future studies and metanalyses, with the hope of a better understanding of the relationship between varicoceles and infertility.
Methods
Data for this systematic review were collected according to the PRISMA statement 2009 guidelines [7]. Eligibility criteria and methods of analysis were specified in advance. Both controlled and non-controlled studies were included, as well as case–control and cohort studies. Search strategy selection and methods for data extraction and data analysis have been previously reported in detail [6].
Limitations of the literature
ESUR-SPIWG proceeded with full awareness of the limitations of the varicocele literature. These limitations include heterogeneous patient groups, small sample sizes, lack of studies with diagnostic accuracy data, lack of randomized controlled trials or controlled studies with patient outcome data, and use of a variety of outcome measures. Overall, these difficulties precluded use of meta-analytical procedures. Instead, narrative syntheses were used to summarize the evidence for the questions of interest.
When review of the literature revealed insufficient publications to address a recommendation from an evidence basis, the recommendation was obtained from expert opinion based on clinical practice, experience, knowledge and judgment. If differences of opinion among the expert emerged, consensus was achieved using the modified Delphi technique [8].
Systematic review to address question 1
Understanding whether the alleged correlation between impaired spermatogenesis, infertility and varicocele is evidence based is fundamental in making a recommendation for US as a valuable investigation in infertile patients. Infertility is defined as “failure to achieve a clinical pregnancy after 12 months or more of regular unprotected sexual intercourse” [9]. Approximately 15% of couples do not achieve a pregnancy within the first year, although almost half of them will do so in the second year. Up to 12% of men have fertility problems, and when a specific cause for infertility can be identified, a male contributing factor is found in approximately 45–50% of cases [5, 10]. Varicocele is the most commonly identifiable and treatable potential cause of male subfertility, with an estimated prevalence of 15% in the general population, 40% in sub-fertile men, and 75–81% in men with secondary infertility [11,12,13]. There is evidence that clinically palpable, rather than non-palpable, varicoceles are associated with infertility but a correlation between the degree of testicular tissue damage and the size of a varicocele is not proven [14].
Both clinical and laboratory studies show a detrimental effect of varicoceles on spermatogenesis [15, 16]. Testicular function is usually normal at the age of 18–20, but declines progressively depending on the duration of the varicocele [17]. A clinical varicocele is associated with ipsilateral testicular atrophy which may improve following varicocele repair [18,19,20].
Several mechanisms have been proposed to explain the link between varicoceles and impaired spermatogenesis. A multi-factorial aetiology is likely, involving both heat, oxidative stresses and androgen deprivation [21]. The temperature of the testes is usually 2 ℃ below core body temperature; if increased by a varicocele, this can lead to a reduction in sperm quality and increased sperm apoptosis. Testicular temperature reduces following varicocele repair [22,23,24,25]. Increased venous hydrostatic pressure may also result in testicular hypoxia by causing oxidative stress through the creation of reactive oxygen species which may reduce sperm quality through several different mechanisms including DNA damage and fragmentation [26, 27]. Varicocele repair has been shown to reduce the seminal oxidative stress in men with infertility [28, 29]. Adverse effects on Leydig cell function and decreased intra-testicular testosterone levels may also be additional contributing factors [21, 30]. Adrenal catecholamines reflux has also been considered among the factors responsible of fertility impairment in patients with varicoceles [31].
Although sperm quality will frequently improve following treatment, there is conflicting evidence regarding the value of varicocele repair in male infertility [32,33,34]. A systematic review in 2015 found that there was insufficient evidence to support a beneficial effect of varicocele repair on spontaneous pregnancy and only limited evidence that treatment might be beneficial in men with clinical varicocele and abnormal semen parameters [35]. Other studies reported potential beneficial effects in pregnancy outcomes following varicocele repair in sub-fertile men [32, 36, 37]. In the setting of assisted reproduction, another meta-analysis found that varicocele repair resulted in improved live birth and pregnancy rates with in vitro fertilization or intracytoplasmic sperm injection treatment [38]. Many of these studies have concluded that the current level of evidence is insufficient for providing a definitive opinion.
Guidelines for varicocele repair in subfertility are inconsistent. In the UK, the National Institute for Health and Clinical Excellence guidelines advises that surgery for varicocele should not be offered for fertility treatment as there is no definite evidence that it improves pregnancy rates. Opinion from the American Society for Reproductive Medicine and the Society for Male Reproduction and Urology concludes that varicocele repair may be offered to the male partner of an infertile couple where there is evidence of abnormal semen parameters and no/minimal identified female factor [39].
Systematic review to address question 2
Evaluation of the diagnostic potential of US imaging in patients with varicoceles is markedly hampered by difficulties in comparing studies obtained with different US classifications and different imaging parameters. A consensus should play a key role for obtaining more reliable results in future studies.
There is general agreement in Europe that valuable information about varicocele can be obtained using colour flow Doppler, and that evaluation of the presence and characteristics of venous reflux is useful when physical examination alone is insufficient to determine whether treatment is required. Additionally, colour Doppler evaluation plays a key role in the assessment of persistence or recurrence of varicoceles after surgical treatment; veins often remain dilated and clinical examination alone cannot fully assess the presence or absence of persistent venous reflux.
Patients with reflux have significantly impaired semen analysis parameters compared to patients without reflux [40]. A prospective study shows that the main predictive factor of a better seminal response after varicocele correction is evidence of venous reflux at preoperative colour Doppler US [41].
Consequently, a number of sonographic classifications have been proposed to establish firm criteria for the diagnosis, treatment and prognosis of varicocele [2, 42–51]. Unfortunately, examination techniques differ for each of these classifications, and different parameters are used to categorize the severity of the disease. A strict comparison between data from different studies is, therefore, not applicable and pooling the available data to construct a metanalysis is problematic.
Some classifications grade varicoceles according to the characteristics of venous reflux only. The patient is evaluated either supine, upright, or in both positions. Hirsh et al. classify varicocele in three grades according to reflux duration while standing during the Valsalva manoeuvre. Cornud et al. classify varicoceles in three categories according to the duration of reflux during the Valsalva manoeuvre measured with spectral Doppler interrogation; there is no standardisation as to whether the examination should be performed with the patient supine or standing [47]. Oyen and Dabuwala et al. classify reflux into three grades based on the characteristics and length of reflux measured with the patient supine [43, 48]. Iosa and Lazzarini score varicoceles in five grades based on a qualitative evaluation of venous reflux estimated with patient both standing and supine [50]. Patil et al. redefined the criteria used to grade varicoceles with four scores based on reflux times measured while standing [51].
Other classifications use both morphological and Doppler parameters. Hoekstra and Witt score varicoceles by measuring the size of the dilated veins and the presence of reflux during the Valsalva manoeuvre in the upright position. Chiou et al. developed a system which assigns a maximum score of 9 points incorporating the maximum venous diameter (scores 0 to 3), the presence of venous plexuses (scores 0–3) and changes in flow direction during the Valsalva manoeuvre (scores 0–3). Diagnosis of varicocele requires a total score of at least 4 points [46]. All parameters are evaluated with the patient lying supine.
The Sarteschi’s classification, first published in Italian in 1993 [44] and published in English with minor changes by Liguori et al. in 2004 [52], distinguishes five degrees of varicocele, depending on the presence of dilated veins while supine and/or standing, anatomical relationships of the dilated veins with the testis, characteristics of reflux, and testicular size. Pauroso et al. introduced a simplified version of the Sarteschi’s classification based on both varicocele extent and the magnitude of the reflux; the two parameters were assessed only with the patient lying supine [49].
The purpose of every classification system is to provide useful morphological and hemodynamic information for treating varicoceles and predicting treatment outcomes. Unfortunately, a clear consensus has not been reached, and there is no universally recognized system to classify varicocele severity. The thresholds used to differentiate between normal and pathological findings are often different and even conflicting, resulting in US examinations that can be interpreted as positive or negative for varicocele diagnosis and severity depending on the classification system used. The result is that all grading systems have a low predictive value in terms of the effect of varicoceles on impairment of spermatogenesis as well as on sperm quality improvement after varicocele correction.
Since no classification system for varicoceles is universally recognized, the ESUR-SPIWG recommends recording all the parameters of the different classifications, to allow comparison among the results of different studies, regardless of which classification system is used (Table 2, recommendations 1).
Systematic review to address question 3
It is uncertain which venous diameter should be regarded as abnormal for varicocele diagnosis. Additionally, the utility of measuring the dilated vein is debated, as well as where in the scrotum measurement should be obtained, in which patient’s position (standing or supine), and how (at rest or during the Valsalva manoeuvre). A standardised method to measure the veins is necessary to compare results obtained in different studies.
The size threshold of the veins that meets the definition for varicoceles varies in different studies, as does the examination technique employed. In particular, venous diameter is evaluated in the supine position or while standing, either at rest or during the Valsalva manoeuvre in different papers [45, 53–62]. Measurement sites of venous diameter in the scrotum are also variable. This is clinically relevant, because the size of the dilated veins changes with the patient’s position, with the Valsalva manoeuvre, and with the measurement site [57, 60, 62]. The result is a wide variation of measurements both in normal subjects, and in patients with clinical and subclinical varicocele.
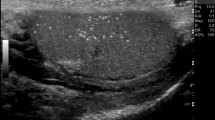
There is no consensus on the threshold values used to define varicocele by the maximum venous diameter. A diameter of 3 mm or more is commonly considered diagnostic for varicocele (Fig. 1), but lower and higher cut-off values have been reported. Cina et al. found an upper value, defined as the 97th percentile, of 3.7–3.8 mm, and showed that the mean diameter is different depending on the measurement site: 2.62 ± 0.53 mm in the spermatic cord, and 2.33 ± 0.56 mm in the peritesticular veins [63]. Karami et al. measured the diameter of the largest testicular vein at four different sites in both upright and supine positions, with or without the Valsalva manoeuvre [61]. They concluded that the best technique for examining patients with suspected varicocele is in the upright position during the Valsalva manoeuvre, and the best site for venous diameter measurement is at the level of epididymal head. Using these parameters, the best size threshold for differentiating normal from clinical varicoceles was 2.65 mm, with sensitivity and specificity of 91% and 89%, respectively. Metin et al. reported that a venous diameter of > 2.95 mm during Valsalva was associated with a sensitivity and specificity of 84% for the diagnosis of clinical varicocele [64]. Half of the patients with spermatic vein diameters of 3–4 mm, and all patients with spermatic vein diameters of 5–6 mm had palpable varicoceles. Eskew et al. reported an accuracy of 63% using cut-off values of 3.6 mm and 2.7 mm for clinical and subclinical varicoceles, respectively [57]. The optimal cut-off value for Pilatz et al. was 2.45 mm, with sensitivity and specificity of 84% and 81%, respectively [58]. Chiou et al. developed a more complex scoring system for varicocele which considers the maximum vein diameter and the sum of the diameters of up to six veins of the dilated plexus [46].
Given the existing wide methodological variability, it is evident that standardization is needed to obtain reproducible results in future studies. For measurement of all quantitative parameters, including venous diameter, it is critically important to document the patient’s position and define the sampling site unequivocally. Non-evidence-based recommendations can be offered advising how the study should be performed. Reviewing the literature, the majority of investigators examine the patient in both the supine and upright positions during the Valsalva manoeuvre, and accept a size threshold of 3 mm during the Valsalva manoeuvre as diagnostic for varicocele [45, 56–59]. The largest vein is measured in the majority of cases, irrespective of its location [53–55, 57, 58, 60, 62]. It is, therefore, advisable to perform US in this way.
When measuring venous diameter, it is critically important to indicate the patient’s position, measurement site (relative to the testis or to the cord), and whether the measure was obtained during the Valsalva manoeuvre or at rest. The ESUR-SPIWG recommends measuring the largest vein, irrespective of location, while standing and during the Valsalva manoeuvre. When the venous diameter is measured in this way, 3 mm or more is considered diagnostic for a varicocele (Table 2, recommendations 2–4).
Systematic review to address question 4
Another debated point is whether measurement of testicular volume is needed and, if so, how it should be performed. Over 80% of the testicular volume is made up of seminiferous tubules and germ cells [65]. Therefore, reduction in germ-cell number results in a smaller volume. Unfortunately, the pooled data for normal testicular volume measured at US vary widely, ranging between 8 and 18 ml in the adult population. In part, this may be due to variation in measurements between US and different imaging modalities [66] and variation in individual practice but, more likely, it is explained by inaccuracies in US measurement and differences in how measurements are obtained. Evaluation of testicular diameters should be as accurate as possible to obtain a good estimate of the testicular volume at US, and the technique needs to be standardized. Compression should be avoided,the testis is very sensitive to probe compression, which significantly influences diameter measurements.
US assessed testicular volume is obtained from the three linear diameters of the testes—length (L), width (W) and height (H), and varies according to the mathematical formula applied for calculation. Three different formulas are used in the literature. The most common is the ellipsoid formula, which is also the one most widely used for automated volume calculation by US systems. Using this formula, the volume is obtained by multiplying the product of the three testicular diameters by 0.52 (V = L × W × H × 0.52) [67]. In another commonly used formula, width and height of the testis are considered to be equal, and testis volume is calculated as a prolate ellipsoid (V = L × 2W × 0.52). A third formula, introduced by Lambert et al., multiplies the product of the three diameters by 0.71 (V = L × W × H × 0.71) [68]. Mbaeri et al. compared the measurements of testicular volume using the three formulas in 62 patients undergoing therapeutic bilateral orchidectomy [69]. The gold-standard volume was calculated by water displacement of the orchidectomy specimen. The results showed that all three methods underestimated testicular volume,the best of the three was Lambert’s formula. A better performance of Lambert’s formula was also demonstrated by Sakamoto et al. [70].
There are no uniform reference values for the normal testicular volume in different populations, probably because measurements obtained in different studies are biased by a variety of circumstances such as the geographic areas and other environmental factors, nutritional status, and ethnicity [71–73]. At US, a total testicular volume of 20–24 ml or more is indicative of normal testicular function for Caucasians and Africans [65, 66, 74–76], while Asians are reported by some investigators as having slightly smaller testes [65, 77]. Bahk et al., however, did not confirm this difference [73].
Studies in infertile men have shown a direct correlation between semen parameters, sex hormone levels and testicular volume measured at US [76]. An average US-detected testicular volume in infertile patients ranges from 10 ml [78] to 13 ± 5 ml [70]. Comparing 136 infertile men and 100 fertile controls, Tijani et al. reported significantly smaller single testis volumes in infertile patients (15.32 ± 3.1 ml in infertile vs. 19.89 ± 3.8 ml in the control group) [74].
In patients with idiopathic hypogonadotropic hypogonadism and micropenis, Sun-Ouck et al. and Rajendra et al. found a significant increase of the testicular volume after medical treatment [79, 80].
A relationship between the testicular volume, measured at US, and varicocele has been documented. Zampieri et al. reported that venous reflux is associated with testicular atrophy and varicocele repair is associated with testicular volume increase [81–83].
The ESUR-SPIWG recommends measuring testicular volume in all patients with varicoceles as a surrogate evaluation of testicular function. The formula used to calculate the volume from the three diameters should be reported. Lambert’s formula is preferred (Table 2, recommendations 5–6).
Systematic review to address question 5
The ESUR-SPIWG acknowledges that a standardized US examination is required for varicocele evaluation in which grey scale, colour Doppler US and spectral Doppler analysis are performed bilaterally with and without Valsalva, while standing and supine (Table 2, recommendation 7).
The ESUR-SPIWG recommends a complete grey scale and colour Doppler investigation with spectral analysis of flow signals in the dilated veins. Furthermore, all parameters should be assessed bilaterally with the patient in both the supine and upright positions. There is no evidence that this approach is necessary in all cases in clinical practice, but it helps to improve standardization and assists comparison between different studies. It also provides harmonization of technique among different centres and improves reproducibility of results. In general, the upright position is more informative and, for standardization purposes, is preferred for measurement of vein diameters and Doppler analysis.
The first part of the examination is grey-scale US. High-resolution images are obtained. The testes are measured with the patient lying supine, and the presence of enlarged (> 3 mm) venous structures demonstrated. The patient is then placed in the upright position and the largest vein (irrespective of the location) is identified and measured during the Valsalva manoeuvre. The second part of the study is evaluation of reflux. The equipment is set to detect slow flow. The pulse repetition frequency is minimized, wall filter set to minimum and gain to the maximum below noise threshold; this can be obtained by increasing the gain up to the level at which artefacts are visible and then decreasing it to a level at which they just disappear.
Colour Doppler and spectral analysis should be performed and interrogation should be obtained at the level of the inguinal canal, in the supratesticular region and in vessels at the level of the testis.
Systematic review to address question 6
The ESUR-SPIWG recommendations emphasise that reflux evaluation is the most important component in the Doppler US examination of varicoceles. Therefore, spectral analysis should supplement colour Doppler interrogation. The essential parameter to measure is the duration of reflux; measuring the reflux peak velocity is not necessary (Table 2, recommendation 8–9). In patients with varicoceles, reflux is considered to be the primary pathologic process that causes testicular damage [84]. Although the precise mechanism through which varicoceles can affect spermatogenesis remains elusive [1], it is thought that if reflux is eliminated, the negative effect on spermatogenesis could reverse [85]. This is at the basis of therapeutic strategies for varicocele correction, whose goal is to improve sperm characteristics by removing retrograde flow within the internal spermatic veins [39, 86–88].
Several investigations support this scenario, showing that Doppler evaluation of reflux is critical for the diagnosis of varicoceles, helps predict the probability of postoperative semen improvement, and has a role in determining the therapeutic approach. It has been shown, in particular, that after varicocele repair, the best postoperative improvement of semen quality is obtained for patients with preoperative evidence of significant reflux at Doppler interrogation. Continuous basal reflux or reversal of flow with Valsalva manoeuvre before varicocele repair is strongly associated with improvement in sperm count and motility on postoperative semen analysis [41, 60, 84].
Diagnosis of reflux is obtained by combining colour Doppler interrogation and spectral analysis. The first analysis is with the colour Doppler mode, which provides a panoramic view of the spermatic vessels, their relationship with testis, information in real time about direction of flow and on how it changes in different positions and during the Valsalva manoeuvre. Use of high-end equipment with good colour Doppler sensitivity is recommended to avoid false-negative results for varicocele detection. Using high-specification equipment, reflux can often also be appreciated at grey-scale US. The examination technique is crucial. Reflux can be missed with the patient supine and it is best detected in the standing position during the Valsalva manoeuvre [62]. Colour Doppler interrogation is subjective, and findings must be substantiated with spectral Doppler analysis which allows measurement of the duration and characteristics of reflux (Fig. 2; this should be quantified [51]. Measurement of reflux peak velocity is optional and can be problematic because it requires a careful angle correction to be obtained in vessels with tortuous courses.
Systematic review to address question 7
When imaging varicoceles, a key point to be clarified is how long the reflux should last to make the diagnosis. Different classification systems use different duration thresholds for diagnosis and grading of reflux [43, 47–51, 63, 89, 90].
In their pioneer study in 1989, Dhabuwala et al. investigated the duration of reflux with dual-frequency bidirectional Doppler [43]. Varicocele was classified as mild if reflux lasted less than 2 s, and severe if it lasted more than 2 s. Cornud et al. also classified varicoceles according to the duration of reflux: described as brief (less than 1 s), intermediate (1–2 s) and permanent (> 2 s) [47]. Brief reflux is considered as physiological in this study and by other investigators [50, 51]. Permanent reflux (> 2 s) was not palpable in 40% of the patients but was always evident at venography. After varicocele repair, reflux disappeared in 70% of cases and changed from continuous to intermediate type in 20% [47]. Oyen et al. also considered a cut-off of 2 s diagnostic for the diagnosis of a varicocele [48]. Only one study on 145 healthy, asymptomatic subjects with normal clinical examination and semen analysis fixed the upper limit for physiological reflux to 3 s, with a velocity of 0.1 m/s [63]. The ESUR-SPIWG suggests a threshold of > 2 s for diagnosis of varicoceles, measured while standing and during Valsalva (Table 2, recommendation 10).
Systematic review to address question 8
Several investigations suggest measuring the reflux peak velocity as a potentially useful Doppler parameter to predict the need for varicocele repair [63, 91–98]. Unfortunately, there is a lack of consensus on where and how to perform the measurement. In most of studies, the peak velocity is measured with the patient supine during the Valsalva manoeuvre [63, 91, 96, 98, 99]. In one the peak, velocity was measured upright with the patient breathing normally [95]. In another, the position of the patient is not reported [93]. Measurement were performed either in the largest vein [91, 98], in the spermatic cord just cephalad to testis [99] or, in general terms, where reflux was identified [63]. Three investigations do not report the measurement site [93, 95]. In most studies, angle correction was not performed and, therefore, the velocity values obtained cannot be regarded as being accurate [91, 95–99].
These differences are relevant, as flow velocity changes depend on the site of measurement [63], patient position and Valsalva [92]. Also, angle correction is essential in all Doppler velocity measurements.
Since the pooled data are inconsistent and biased by relevant methodological shortcomings, the ESUR-SPIWG does not recommended evaluation of reflux peak velocity in routine clinical practice (Tab.2, recommendation 11).
Systematic review to address question 9
One of the problems encountered when comparing different US studies for varicoceles concerns the variability of reporting. A standard report should be useful, in which all the relevant data are collected. The variable clinical practice in reporting patients with varicoceles reflects the differences in examination techniques. The ESUR-SPIWG recommends describing the examination technique and reporting all the US parameters used to evaluate the patient. It also suggests grading varicoceles according to the Sarteschi’s classification (Figs. 3, 4, 5, 6, Table 2, recommendations 12–13).
The following information should be included in the US report:
-
Testicular volume, echogenicity and echotexture
-
Presence of incidental testicular or extratesticular abnormalities other than varicoceles
-
Presence of varices at grey-scale and colour Doppler US, and relationships to the testis (inguinal canal, supra-testicular, around the testis, intratesticular)
-
Diameter of the largest vein (irrespective of the location) measured while standing and during the Valsalva manoeuvre
-
Changes of flow at colour Doppler interrogation and spectral analysis in the spermatic veins according to the patient’s position and before and during Valsalva (length of reflux and changes in waveform characteristics while standing and during the Valsalva manoeuvre).
Systematic review to address question 10
Several studies have investigated intratesticular Doppler waveforms to predict parenchymal damage in infertile patients with varicoceles [100–105]. As discussed in previous paragraphs, the mechanism underlying testicular damage in patients with varicoceles remains uncertain. Defective energy metabolism at the mitochondrial level following decrease of arterial inflow was proposed as one of the factors responsible for impaired spermatogenesis [106]. Pathophysiologically, it is hypothesized that impaired venous drainage causes an increase in venous pressure within the spermatic veins [100]. Venous stasis may also decrease the arterial blood supply and microperfusion of the testes resulting in hypoxia and, in turn, impaired testicular microcirculation [100].
Several studies have investigated whether changes in testicular vascularization were appreciable, since they could represent (indirect) confirmation of this theory. The purpose of these investigations was to provide insight into the pathophysiology of varicoceles, not to identify new parameters for clinical use in patient management. Earlier studies failed to demonstrate changes in testicular blood flow in varicocele patients [101, 102], a negative result confirmed also by a more recent investigation [107]. Other conflicting studies, however, did identify changes in flow Tarhan et al. measured arterial blood flow in the testicular artery of 62 infertile patients with varicoceles and 42 normal fertile subjects used as control group. They found ipsilateral decreased testicular arterial volume blood flow in patients with varicoceles compared to control patients reflecting, in the author’s opinion, impaired microcirculation [103].
Calculation of testicular volume blood flow, however, requires estimating the cross-sectional area of the testicular artery, a difficult evaluation in a millimetric vessel. Other authors used peak systolic velocity (PSV) and resistive index (RI) or pulsatility index (PI) as more reproducible and easily obtained parameters to estimate indirectly perfusion abnormalities. Biagiotti et al. found higher PSV and RI in the testicular arteries of patients with varicoceles [104]. Ünsal et al. reported similar results—interrogating the capsular arteries, they found significantly higher RI and PI values in patients with varicoceles, compared to controls [105]. These research studies are useful to provide an insight into the mechanisms that create testicular parenchymal damage in varicoceles. At present, however, there is insufficient evidence to recommend evaluation of intratesticular arterial Doppler waveforms for clinical use (Table 2, recommendation 14).
Systematic review to address question 11
There is a widely held belief that a unilateral right-sided varicocele should prompt investigation for a retroperitoneal process causing obstruction of the right internal spermatic vein. While this may be justified if a varicocele appears suddenly, be it right left, or bilateral, in the majority of cases the literature does not support this approach.
With an incidence of about 10% of varicoceles, bilateral clinical varicocele is relatively uncommon [108]. However, the incidence of subclinical right-sided varicocele is much more frequent. The World Health Organization reported that approximately 90% of right-sided varicoceles are undiagnosed at palpation [109], a fact confirmed by other investigations [110–113].
As clinical diagnosis is neither sensitive nor accurate, a right-sided varicocele is, in the majority of cases, identified at colour Doppler US when a study is requested to evaluate a clinically evident left-sided one [110–113].
Several authors consider varicocele a bilateral disease, although on the right side, the condition is often subclinical. This explains, in their opinion, bilateral testicular dysfunction in patients with unilateral left clinical varicocele [39, 112, 114]. Patients with left-sided varicocele, either clinical or subclinical, should, therefore, be examined carefully for bilateral varicoceles, and bilateral repair should be considered if a right subclinical varicocele is found [112, 114–117].
In adults, there is some evidence that simultaneous repair of left clinical and right subclinical varicocele is beneficial, as even a small subclinical unrepaired varicocele continues to have a detrimental effect on testis function [117–120]. Contrary opinions, however, exist [121]. In children and adolescents, the value of bilateral repair is even more debatable [94].
Isolated right-sided varicocele is found in less than 1% of patients [108]. In the majority of cases, it is subclinical, identified only during the Valsalva manoeuvre [111, 113]. Literature is scarce and is rarely focused on this topic [122].
A variety of causes can result in right-sided clinical varicocele, including impaired venous drainage from the testis by venous thrombosis, tumour invasion or compression. Isolated and/or palpable right-sided varicocele may occur in otherwise healthy patients with venous anatomical variations [110], such as in situs viscerum inversus [123].
Based on these considerations, the ESUR-SPIWG recommends undertaking colour Doppler US on both sides in patients with left-sided varicoceles, as a subclinical right-sided varicocele will be often revealed. In patients with an isolated clinical right-sided varicocele, it also recommends extending the US examination to the abdomen, to look for congenital anomalies and abdominal/retroperitoneal pathology (Table 2, recommendation 15–16).
Systematic review to address question 12
The need for follow-up in patients with subclinical varicoceles is debated. Current literature supports the thesis that a varicocele has a detrimental effect on testicular size and function from childhood (prepuberal boys) and adolescence (Tanner stage V males from puberty to the age of majority) onwards and that this adverse effect increases with the grade of the varicocele [122]. The need for diagnosing, treating and/or following-up subclinical varicoceles, however, still remains controversial, especially in prepuberal boys. Current evidence shows that varicocele repair significantly improves semen parameters in men with clinical varicoceles [32, 34], but not when the varicocele is subclinical [124]. Also, there is no proven benefit in treating men with a varicocele and normal semen parameters [125].
In adolescents with varicoceles, there is a significant risk of over-treatment since most of them will have no problem achieving pregnancy later in life [126].
There is further evidence that subclinical varicoceles can progress to clinically evident disease. In a paediatric population, Cervellione et al. found progression in 28% of patients during a 4-year period [127]. Zampieri et al. showed a significantly higher progression rate in adolescent athletes, compared to the general population [128].
Testicular atrophy associated with varicoceles is currently the most common indication for prophylactic varicocele repair in adolescents [19, 129], but a study suggests a 6–12 month observation period prior to surgery, or prolonged follow-up if patients present with normal semen analysis at diagnosis and agree to annual semen analysis and scrotal US [130].
As surgical repair is not indicated for all patients with varicoceles, and disease progression may occur, follow-up is advised for non-operated patients. In view of the increased risk for progressive testicular dysfunction in adolescent males compared to older patients, there are two groups of patients with varicoceles in which follow-up is specifically recommended: non-operated adolescents with testicular atrophy, and adolescents and young adults (young males who have attained the age of majority) presenting with subclinical varicoceles and both normal testicle volume and semen analysis. In these patients, annual follow-up is advised by means of physical examination, scrotal US including measurements of testis size and, if patients are willing to comply, semen analysis [130, 131].
Based on the currently available literature, the ESUR-SPIWG recommends follow-up of subclinical varicoceles for untreated adolescents and for young adults with normal testicular volume and normal semen analysis (Table 2, recommendation 17).
Systematic review to address question 13
Another debated point is whether to undertake US follow-up after varicocele repair. The ESUR-SPIWG recommends US follow-up to identify early postoperative complications, and later if semen analysis remains unsatisfactory (Table 2, recommendation 18–20).
In addition to the risks of infection, bleeding, and delayed wound healing, as for all surgical procedures, recurrence of the varicocele and hydrocele formation are the most common complications following varicocele surgical repair.
When a venous embolization technique is employed, the main reported complications are coil migration, vascular trauma, thrombophlebitis of the pampiniform plexus and contrast agent reactions. Technical success rate is reported as being equivalent to that of surgical techniques [132].
With the exception of an early study in which colour Doppler US was not found effective in assessing the outcome of varicocele repair [133], there is agreement that US can be used early after treatment in case of postoperative complications, and later, when needed, to evaluate morphology of the pampiniform plexus, testicular volume and signs of persistent or recurrent disease [133–147]. Clinical evaluation after surgery, especially in high-grade varicocele, usually still detects enlarged veins, but only colour Doppler with spectral analysis can discriminate if there is persisting venous reflux. Hence, colour Doppler US is important in detecting persistent reflux or recurrence.
The clinical workup of patients differs as regards indications for imaging following varicocele repair, particularly timing, and length of sonographic follow-up. No clinically and scientifically based recommendations are available on this specific clinical problem.
In several centres, US is considered after varicocele repair only if early or late complications appear (such as hydrocele, pain, epididymitis), if there is evidence of recurrence on clinical examination or if postoperative sperm analysis is unsatisfactory. In other centres both sperm analysis and US are performed 3 months after varicocele repair. In other centres sperm analysis and US are performed 3 months and 1 year after varicocele repair, with the rationale that recurrent varicocele may only appear one year or later after the procedure [39, 134, 138].
If varicocele repair is performed for treatment of subfertility, then sperm analysis must form the basis of follow-up. If semen analysis improves, scanning is usually unhelpful for the patient, while if semen analysis remains unsatisfactory, US can be considered to look for persistent reflux or recurrent disease.
Systematic review to address question 14
It is commonly thought that US should be extended to the abdomen in newly discovered varicoceles to identify tumours. This practice, however, is not fully supported by the available literature. The classic triad of symptoms of renal cell carcinoma (RCC) is haematuria, flank pain, and a palpable abdominal mass. Other paraneoplastic signs such as fever or general malaise may also be present with retroperitoneal tumours. A number of case reports and small case series suggest that varicoceles are a possible mode of presentation of retroperitoneal tumours [148–152]. This led to the dogma that an extended abdominal ultrasound examination should be performed in all patients with a newly diagnosed varicocele. Varicocele is, however, very common, whereas retroperitoneal tumours are rare. The incidence of renal tumours is 13.4 per 100,000 per year, and that of retroperitoneal tumours is 0.3 per 100,000 per year in the male population [148]. There is no data to suggest that finding a retroperitoneal or renal tumour as a cause for a varicocele is any more likely than discovering an incidental tumour in a male patient without a varicocele, or in any other patient group. In a much-quoted series detailing the clinical manifestations of renal cell carcinoma with emphasis on the presence of varicoceles [149, 151, 153], the prevalence of co-existing retroperitoneal tumours and a varicocele is low (incidence of 2.8–3.3%) [149, 151]. Moreover, varicocele, whether acute, symptomatic or an incidental finding, is almost never a sole feature of a renal or retroperitoneal tumour and some other features of the tumour are usually evident from the history or examination. Ding et al. reported a retrospective analysis which included 1028 patients with pathologically confirmed unilateral RCC [154]. In 333 patients, paraneoplastic signs were present as the initial symptoms comprising pyrexia in 175 cases (52.6%), anaemia in 146 cases (43.8%), hypertension in 101 cases (30.3%), and varicocele in 12 cases (3.6%). In this study, varicocele was found to be related significantly to advanced cancer stage and therefore other clinical signs (abdominal mass, flank pain) and paraneoplastic signs more likely co-existed at presentation. Conversely, Pauruso et al. found no abdominal tumours at US in all their patients examined for varicoceles [49].
The mechanism for the development of a symptomatic varicocele is not always extension of tumour/thrombus along the renal vein. Pathological extrinsic compression of the venous drainage of the pampiniform plexus by the primary tumour or enlarged lymph nodes, or an increased blood supply to the tumour may also lead to a local increase in venous pressure, or an increase of reflux of blood into collaterals [155].
Besides advanced retroperitoneal tumours, a variety of non-tumour lesions have been associated with secondary varicoceles including non-neoplastic enlarged lymph nodes, non-neoplastic renal vein thrombosis, large aneurysmal dilatations, pancreatic pseudocysts, renal arteriovenous malformations, and the nutcracker (left renal vein entrapment) syndrome [156–158].
Anecdotally, a secondary varicocele is often considered when the disease is found solely on the right side. Isolated right-side varicocele occurs in a minority of patients with clinical varicoceles [110, 115]. Tumour thrombus in the right renal vein seldom results in the development of a right-sided varicocele, because the right spermatic vein commonly drains into the inferior vena cava, with the incidence of the right spermatic vein draining into the right renal vein reported in less than 5% of individuals [159–161]. Moreover, it has been shown that a secondary varicocele is as likely on the left side as the right, therefore, considering a secondary varicocele only in the rare situation in which it develops on the right is misleading [162].
There are no data that currently support extended US examination to the abdomen in all patients with a newly diagnosed varicocele. An exception to this is in children less than 9 years of age presenting with a varicocele. Epidemiological studies have demonstrated that varicoceles develop at puberty. Oster et al. observed that no varicoceles were detected in 188 boys 6–9 years of age [163]. Akbay et al. evaluated the prevalence of varicoceles in 4052 boys aged 2–19 [164]. They reported that the prevalence of varicoceles was < 1% in boys aged 2–10. In the child less than 9 years of age, where acute varicoceles are normally not seen, a renal or retroperitoneal tumour is a possibility and should be excluded [165].
In conclusion, suspicion of a secondary varicocele can arise when a clinically evident varicocele presents acutely, and remains tense in a supine position. Secondary subclinical varicocele has not been reported. The ESUR-SPIWG believes that US examination should always be extended to the abdomen in children less than 9 years presenting with an acute varicocele, to rule-out a renal or retroperitoneal tumour. In adults, the alleged association between varicoceles and retroperitoneal masses is based on a limited number of series and case reports in which the varicocele was a late sign of a symptomatic, palpable lesion [152, 166–169].
Although the possibility of missing an undetected retroperitoneal lesion during a Doppler investigation for varicocele when the abdomen is not evaluated in an adult is very unlikely, it cannot be completely excluded (Fig. 7). Patients often undergo US examinations without a full clinical evaluation, and even obvious abdominal findings can be missed. As a consequence, the ESUR-SPIWG believes that the sonologist is fully justified in extending the US examination to the abdomen when the varicocele is of acute onset, whatever the side (left or right), and particularly when it remains unchanged in the supine position. Otherwise, the US practitioner should refer the patient to an internal medicine specialist for a complete diagnostic evaluation including an abdominal examination (Table 2, recommendation 21–22).
Secondary varicocele in an 85-year-old patient presenting with a right scrotal lump. a Colour Doppler ultrasound of the right spermatic cord shows markedly dilated pampiniform plexus with basal reflux. b Spectral Doppler interrogation obtained in an upright position shows no changes during Valsalva’s maneuver (arrowhead). c Ultrasound interrogation of the right kidney shows a large renal tumour. d Contrast-CT scanning showing a large right renal tumour growing into the right renal vein (curved arrow) and inferior vena cava (asterisks)
Systematic review to address question 15
The ESUR-SPIWG recommend considering other pathologies when performing US in patients with clinical diagnosis of varicoceles (Table 2, recommendation 23). Not all tubular extratesticular structures, either palpated or identified at US, are varicoceles. Colour Doppler interrogation is important to differentiate between varicocele and other tubular structures on grey-scale US, such as spermatoceles and cyst clusters, tubular ectasia and postvasectomy changes.
Patients presenting with uncommon conditions [170, 171], as well as patients with extratesticular cavernous haemangiomas, lymphangiomas, and arteriovenous malformations can present with a non-specific diagnosis, or with suspicion of a varicocele based on their clinical presentation [172–174]. Although MRI is the modality of choice for delineating the extent of these abnormalities, in clinical practice, the diagnosis is usually obtained with US. On US, cavernous haemangiomas present with a heterogeneous echo texture and increased through transmission, showing septa and enlarged vascular spaces. Phleboliths may be seen as foci with distal acoustic shadowing. The tortuous vessels inside the lesion may mimic a varicocele on grey-scale US. At Doppler interrogation, flow velocity is often too slow to be detected, even with the patient standing and during the Valsalva manoeuvre. MRI shows intermediate-to-low signal intensity on T1-weighted images, and very high signal intensity on T2-weighted images. The lesion and serpiginous vessels show enhancement after contrast medium administration. Focal signal voids may be seen, consistent with thrombi [172, 173]. Lymphangiomas are composed of dilated lymphatic channels,they may present as lobulated cystic masses or with findings similar to those of haemangiomas. The dilated lymphatic channels of lymphangiomas do not enhance after contrast medium injection. Lymphangiomas present on MRI as lobulated scrotal masses with intermediate-to-low signal intensity on T1-weighted images and high signal intensity on T2-weighted images [172]. Arteriovenous malformations are characterized by abnormal connections between arteries and veins. At colour Doppler and duplex Doppler imaging, they show prominent vessels with low-resistance arterial flow signal and high-velocity venous components. These features allow differentiation from varicoceles, in which only venous flow are recorded [174]. Arteriovenous malformations appear at MRI as a tangle of abnormal vessels, frequently with internal flow voids on both T1- and T2-weighted images produced by high-velocity flow [175].
Zinner syndrome has also been described as a mimic for varicocele. It is a rare developmental anomaly of the mesonephric duct consisting of unilateral renal agenesis, ipsilateral seminal vesicle cyst, and ipsilateral ejaculatory duct obstruction resulting in dilatation of the epididymis and vas deferens. In Zinner syndrome, the dilated vas deferens and epididymis can simulate venous dilatation. Reflux can be artefactually recorded at colour Doppler interrogation, due to sperm movement during the Valsalva manoeuvre [176].
A few uncommon pathological conditions can mimic intratesticular varicocele [177]. The Valsalva manoeuvre can be very important to differentiate between intratesticular varicocele adjacent to the mediastinum testis and tubular ectasia of the rete testis. Intratesticular arteriovenous malformations and intratesticular haemangiomas can be similar morphologically to intratesticular varicocele, but present with arterial flows and an arterialized-venous spectral waveform.
Conclusion
Guidelines have increasingly become a familiar part of clinical practice. They can improve the quality and consistency of care, promoting use of procedures of proved benefit and discouraging use of ineffective ones [178]. Imaging guidelines, in particular, have been developed to address specific and controversial problems on how to perform imaging procedures [179–182], and to offer explicit recommendations for operators who are uncertain about how to proceed [183–189]. They fix technical standards, and provide authoritative recommendations that reassure practitioners about the appropriateness of their work [190–192].
This systematic literature review of US imaging for varicocele shows significant variability. There is, however, good evidence to support the contention that an accurate imaging diagnosis of patients with varicoceles is important to guide clinicians in making effective treatment decisions. The recommendations and guidelines released by the ESUR-SPIWG are aimed to produce a framework that allows standardisation across future studies with the intention of clarifying the role of US in varicocele assessment.
References
Clavijo RI, Carrasquillo R, Ramasamy R (2017) Varicoceles: prevalence and pathogenesis in adult men. Fertil Steril 108:364–369
Lotti F, Maggi M (2015) Ultrasound of the male genital tract in relation to male reproductive health. Hum Reprod Update 21:56–83
Paick S, Choi WS (2019) Varicocele and testicular pain: a review. World J Mens Health 37:4–11
Dubin L, Amelar RD (1970) Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril 21:606–609
Salonia A, Bettochi C, Carvalho J, Corona G, Jones TH, Kadioğlu A, Martinez-Salamanca JI, Minhas S, Serefoğlu EC, Verze P (2020) EAU guidelines on sexual and reproductive health 2020. In: Presented at the EAU annual congress Amsterdam 2020. European Association of Urology Guidelines Office, Arnhem, The Netherlands
Freeman S, Bertolotto M, Richenberg J, Belfield J, Dogra V, Huang DY, Lotti F, Markiet K, Nikolic O, Ramanathan S, Ramchandani P, Rocher L, Secil M, Sidhu PS, Skrobisz K, Studniarek M, Tsili A, Tuncay Turgut A, Pavlica P, Derchi LE, members of the ESUR-SPIWG WG (2020) Ultrasound evaluation of varicoceles: guidelines and recommendations of the European Society of Urogenital Radiology Scrotal and Penile Imaging Working Group (ESUR-SPIWG) for detection, classification, and grading. Eur Radiol 30:11–25
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 6:e1000100
Dalkey N, Helmer O (1963) An experimental application of the delphi method to the use of experts. Manage Sci 9:458–467
Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Vanderpoel S, International Committee for Monitoring Assisted Reproductive T, World Health O (2009) International committee for monitoring assisted reproductive technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril 92:1520–1524
Lotti F, Maggi M (2018) Sexual dysfunction and male infertility. Nat Rev Urol 15:287–307
Alsaikhan B, Alrabeeah K, Delouya G, Zini A (2016) Epidemiology of varicocele. Asian J Androl 18:179–181
Dubin L, Amelar RD (1971) Etiologic factors in 1294 consecutive cases of male infertility. Fertil Steril 22:469–474
Kibar Y, Seckin B, Erduran D (2002) The effects of subinguinal varicocelectomy on Kruger morphology and semen parameters. J Urol 168:1071–1074
Mihmanli I, Kurugoglu S, Cantasdemir M, Zulfikar Z, Halit Yilmaz M, Numan F (2000) Color Doppler ultrasound in subclinical varicocele: an attempt to determine new criteria. Eur J Ultrasound 12:43–48
Jarow JP, Sharlip ID, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, Schlegel PN, Howards SS, Nehra A, Damewood MD, Overstreet JW, Sadovsky R (2002) Male infertility best practice policy committee of the American Urological Association I best practice policies for male infertility. J Urol 167:2138–2144
Weidner W, Colpi GM, Hargreave TB, Papp GK, Pomerol JM, Ghosh C, Infertility EAUWGOM (2002) EAU guidelines on male infertility. Eur Urol 42:313–322
Agarwal A, Sharma R, Harlev A, Esteves SC (2016) Effect of varicocele on semen characteristics according to the new 2010 World Health Organization criteria: a systematic review and meta-analysis. Asian J Androl 18:163–170
Lipshultz LI, Corriere JN Jr (1977) Progressive testicular atrophy in the varicocele patient. J Urol 117:175–176
Zini A, Buckspan M, Berardinucci D, Jarvi K (1997) The influence of clinical and subclinical varicocele on testicular volume. Fertil Steril 68:671–674
Kolon TF (2015) Evaluation and management of the adolescent varicocele. J Urol 194:1194–1201
Marmar JL (2001) The pathophysiology of varicoceles in the light of current molecular and genetic information. Hum Reprod Update 7:461–472
Will MA, Swain J, Fode M, Sonksen J, Christman GM, Ohl D (2011) The great debate: varicocele treatment and impact on fertility. Fertil Steril 95:841–852
Naughton CK, Nangia AK, Agarwal A (2001) Pathophysiology of varicoceles in male infertility. Hum Reprod Update 7:473–481
Papanikolaou F, Chow V, Jarvi K, Fong B, Ho M, Zini A (2000) Effect of adult microsurgical varicocelectomy on testicular volume. Urology 56:136–139
Jung A, Schuppe HC (2007) Influence of genital heat stress on semen quality in humans. Andrologia 39:203–215
Mallidis C, Czerwiec A, Filippi S, O’Neill J, Maggi M, McClure N (2011) Spermatogenic and sperm quality differences in an experimental model of metabolic syndrome and hypogonadal hypogonadism. Reproduction 142:63–71
Saleh RA, Agarwal A, Sharma RK, Said TM, Sikka SC, Thomas AJ Jr (2003) Evaluation of nuclear DNA damage in spermatozoa from infertile men with varicocele. Fertil Steril 80:1431–1436
Cervellione RM, Cervato G, Zampieri N, Corroppolo M, Camoglio F, Cestaro B, Ottolenghi A (2006) Effect of varicocelectomy on the plasma oxidative stress parameters. J Pediatr Surg 41:403–406
Shiraishi K, Naito K (2006) Generation of 4-hydroxy-2-nonenal modified proteins in testes predicts improvement in spermatogenesis after varicocelectomy. Fertil Steril 86:233–235
Zorgniotti AW, Macleod J (1973) Studies in temperature, human semen quality, and varicocele. Fertil Steril 24:854–863
Camoglio FS, Zampieri N, Corroppolo M, Chironi C, Dipaola G, Giacomello L, Ottolenghi A (2004) Varicocele and retrograde adrenal metabolites flow. An experimental study on rats. Urol Int 73:337–342
Agarwal A, Deepinder F, Cocuzza M, Agarwal R, Short RA, Sabanegh E, Marmar JL (2007) Efficacy of varicocelectomy in improving semen parameters: new meta-analytical approach. Urology 70:532–538
Smit M, Romijn JC, Wildhagen MF, Veldhoven JL, Weber RF, Dohle GR (2010) Decreased sperm DNA fragmentation after surgical varicocelectomy is associated with increased pregnancy rate. J Urol 183:270–274
Baazeem A, Belzile E, Ciampi A, Dohle G, Jarvi K, Salonia A, Weidner W, Zini A (2011) Varicocele and male factor infertility treatment: a new meta-analysis and review of the role of varicocele repair. Eur Urol 60:796–808
Macleod R, Biyani CS, Cartledge J, Eardley I (2015) Varicocele. BMJ Clin Evid 2015
Kroese ACJ, de Lange NM, Collins J, Evers JL (2012) Surgery or embolization for varicoceles in subfertile men. Cochrane Database Syst Rev (10):CD000479. https://doi.org/10.1002/14651858.CD000479.pub5
Kim KH, Lee JY, Kang DH, Lee H, Seo JT, Cho KS (2013) Impact of surgical varicocele repair on pregnancy rate in subfertile men with clinical varicocele and impaired semen quality: a meta-analysis of randomized clinical trials. Korean J Urol 54:703–709
Kirby EW, Wiener LE, Rajanahally S, Crowell K, Coward RM (2016) Undergoing varicocele repair before assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligospermic men with a varicocele: a systematic review and meta-analysis. Fertil Steril 106:1338–1343
Practice Committee of the American Society for Reproductive M, Society for Male R, Urology (2014) Report on varicocele and infertility: a committee opinion. Fertil Steril 102:1556–1560
Mahdavi A, Heidari R, Khezri M, Shiravi A, Pirjani R, Saheb Kashaf R (2016) Can ultrasound findings be a good predictor of sperm parameters in patients with varicocele? a cross-sectional study. Nephrourol Mon 8:e37103
Liguori G, Ollandini G, Pomara G, Amodeo A, Bertolotto M, Mazzon G, de Concilio B, Bucci S, Gattuccio I, Turchi P, Belgrano E, Trombetta C (2010) Role of renospermatic basal reflow and age on semen quality improvement after sclerotization of varicocele. Urology 75:1074–1078
Hirsh AV, Cameron KM, Tyler JP, Simpson J, Pryor JP (1980) The Doppler assessment of varicoceles and internal spermatic vein reflux in infertile men. Br J Urol 52:50–56
Dhabuwala CB, Kumar AB, Kerkar PD, Bhutawala A, Pierce J (1989) Patterns of Doppler recordings and its relationship to varicocele in infertile men. Int J Androl 12:430–438
Sarteschi LM, Paoli R, Bianchini M, Menchini Fabris GF (1993) Lo studio del varicocele con eco-color-Doppler. G Ital Ultrasonologia 4:43–49
Hoekstra T, Witt MA (1995) The correlation of internal spermatic vein palpability with ultrasonographic diameter and reversal of venous flow. J Urol 153:82–84
Chiou RK, Anderson JC, Wobig RK, Rosinsky DE, Matamoros A Jr, Chen WS, Taylor RJ (1997) Color Doppler ultrasound criteria to diagnose varicoceles: correlation of a new scoring system with physical examination. Urology 50:953–956
Cornud F, Belin X, Amar E, Delafontaine D, Helenon O, Moreau JF (1999) Varicocele: strategies in diagnosis and treatment. Eur Radiol 9:536–545
Oyen RH (2002) Scrotal ultrasound. Eur Radiol 12:19–34
Pauroso S, Di Leo N, Fulle I, Di Segni M, Alessi S, Maggini E (2011) Varicocele: Ultrasonographic assessment in daily clinical practice. J Ultrasound 14:199–204
Iosa G, Lazzarini D (2013) Hemodynamic classification of varicoceles in men: our experience. J Ultrasound 16:57–63
Patil V, Shetty SM, Das SK (2016) Redefining the criteria for grading varicoceles based on reflux times: a clinicoradiological correlation. Ultrasound Q 32:82–85
Liguori G, Trombetta C, Garaffa G, Bucci S, Gattuccio I, Salame L, Belgrano E (2004) Color Doppler ultrasound investigation of varicocele. World J Urol 22:378–381
Wolverson MK, Houttuin E, Heiberg E, Sundaram M, Gregory J (1983) High-resolution real-time sonography of scrotal varicocele. AJR Am J Roentgenol 141:775–779
Gonda RL Jr, Karo JJ, Forte RA, O’Donnell KT (1987) Diagnosis of subclinical varicocele in infertility. AJR Am J Roentgenol 148:71–75
Orda R, Sayfan J, Manor H, Witz E, Sofer Y (1987) Diagnosis of varicocele and postoperative evaluation using inguinal ultrasonography. Ann Surg 206:99–101
McClure RD, Khoo D, Jarvi K, Hricak H (1991) Subclinical varicocele: the effectiveness of varicocelectomy. J Urol 145:789–791
Eskew LA, Watson NE, Wolfman N, Bechtold R, Scharling E, Jarow JP (1993) Ultrasonographic diagnosis of varicoceles. Fertil Steril 60:693–697
Pilatz A, Altinkilic B, Kohler E, Marconi M, Weidner W (2011) Color Doppler ultrasound imaging in varicoceles: is the venous diameter sufficient for predicting clinical and subclinical varicocele. World J Urol 29:645–650
Jarow JP, Ogle SR, Eskew LA (1996) Seminal improvement following repair of ultrasound detected subclinical varicoceles. J Urol 155:1287–1290
Schiff JD, Li PS, Goldstein M (2006) Correlation of ultrasound-measured venous size and reversal of flow with Valsalva with improvement in semen-analysis parameters after varicocelectomy. Fertil Steril 86:250–252
Karami M, Mazdak H, Khanbabapour S, Adibi A, Nasr N (2014) Determination of the best position and site for color Doppler ultrasonographic evaluation of the testicular vein to define the clinical grades of varicocele ultrasonographically. Adv Biomed Res 3:17
Kim YS, Kim SK, Cho IC, Min SK (2015) Efficacy of scrotal Doppler ultrasonography with the Valsalva maneuver, standing position, and resting-Valsalva ratio for varicocele diagnosis. Korean J Urol 56:144–149
Cina A, Minnetti M, Pirronti T, Vittoria Spampinato M, Canade A, Oliva G, Ribatti D, Bonomo L (2006) Sonographic quantitative evaluation of scrotal veins in healthy subjects: normative values and implications for the diagnosis of varicocele. Eur Urol 50:345–350
Metin A, Bulut O, Temizkan M (1991) Relationship between the left spermatic vein diameter measured by ultrasound and palpated varicocele and Doppler ultrasound findings. Int Urol Nephrol 23:65–68
Cornud F, Amar E, Hamida K, Thiounn N, Helenon O, Moreau JF (2000) Imaging in male hypofertility and impotence. BJU Int 86(Suppl 1):153–163
Goede J, Hack WW, Sijstermans K, van der Voort-Doedens LM, Van der Ploeg T, Meij-de Vries A, Delemarre-van de Waal HA (2011) Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm Res Paediatr 76:56–64
Atkinson GO, Patrick LE, Ball TI, Stephenson CA, Broecker BH, Woodard JR (1992) The normal and abnormal scrotum in children: evaluation with color Doppler sonography. Am J Roentgenol 158:613–617
Lambert B (1951) The frequency of mumps and of mumps orchitis and the consequences for sexuality and fertility. Acta Genet Stat Med 2:1–166
Mbaeri TU, Orakwe JC, Nwofor AME, Oranusi CK, Mbonu OO (2013) Ultrasound measurements of testicular volume: comparing the three common formulas with the true testicular volume determined by water displacement. Afri J Urol 19:69–73
Sakamoto H, Saito K, Oohta M, Inoue K, Ogawa Y, Yoshida H (2007) Testicular volume measurement: comparison of ultrasonography, orchidometry, and water displacement. Urology 69:152–157
Diamond JM (1986) Ethnic differences: variation in human testis Size. Nature 320:488
Takihara H, Cosentino MJ, Sakatoku J, Cockett ATK (1987) Significance of testicular size measurement in andrology: II. Correlation of testicular size with testicular function. J Urol 137:416–419
Bahk JY, Jung JH, Jin LM, Min SK (2010) Cut-off value of testes volume in young adults and correlation among testes volume, body mass index, hormonal level, and seminal profiles. Urology 75:1318–1323
Tijani KH, Oyende BO, Awosanya GO, Ojewola RW, Yusuf AO (2014) Assessment of testicular volume: a comparison of fertile and sub-fertile West African men. Afr J Urol 20:136–140
Sakamoto H, Yajima T, Nagata M, Okumura T, Suzuki K, Ogawa Y (2008) Relationship between testicular size by ultrasonography and testicular function: measurement of testicular length, width, and depth in patients with infertility. Int J Urol 15:529–533
Condorelli R, Calogero AE, La Vignera S (2013) Relationship between testicular volume and conventional or nonconventional sperm parameters. Int J Endocrinol 2013:6
Kim ED, Lipshultz LI (1996) Role of ultrasound in the assessment of male infertility. J Clin Ultrasound 24:437–453
Lenz S, Thomsen JK, Giwercman A, Hertel NT, Hertz J, Skakkebaek NE (1994) Ultrasonic texture and volume of testicles in infertile men. Hum Reprod 9:878–881
Kim S-O, Ryu KH, Hwang IS, Jung SI, Oh KJ, Park K (2011) Penile growth in response to human chorionic gonadotropin (hCG) treatment in patients with idiopathic hypogonadotrophic hypogonadism. Chonnam Med J 47:39–42
Nerli RB, Guntaka AK, Patne PB, Hiremath MB (2013) Penile growth in response to hormone treatment in children with micropenis. Indian J Urol 29:288–291
Zampieri N, Cervellione RM (2008) Varicocele in adolescents: a 6-year longitudinal and followup observational study. J Urol 180:1653–6 discussion 1656
Zhou T, Zhang W, Chen Q, Li L, Cao H, Xu CL, Chen GH, Sun YH (2015) Effect of varicocelectomy on testis volume and semen parameters in adolescents: a meta-analysis. Asian J Androl 17:1012–1016
Sakamoto H, Saito K, Ogawa Y, Yoshida H (2008) Effects of varicocele repair in adults on ultrasonographically determined testicular volume and on semen profile. Urology 71:485–489
Liguori G, Trombetta C, Ollandini G, Pomara G, Gattuccio I, Turchi P, Bertolotto M, Bucci S, Belgrano E (2009) Predictive factors of better improvement in semen quality after sclerotization of varicocele: preliminary report. J Androl Sci 16:47–53
Abdel-Meguid TA, Al-Sayyad A, Tayib A, Farsi HM (2011) Does varicocele repair improve male infertility? An evidence-based perspective from a randomized, controlled trial. Eur Urol 59:455–461
Lomboy JR, Coward RM (2016) The varicocele: clinical presentation, evaluation, and surgical management. Semin Intervent Radiol 33:163–169
Pathak P, Chandrashekar A, Hakky TS, Pastuszak AW (2016) Varicocele management in the era of in vitro fertilization/intracytoplasmic sperm injection. Asian J Androl 18:343–348
Kohn TP, Kohn JR, Pastuszak AW (2017) Varicocelectomy before assisted reproductive technology: are outcomes improved. Fertil Steril 108:385–391
Cornud F, Amar E, Hamida K, Helenon O, Moreau JF (2001) Ultrasound findings in male hypofertility and impotence. Eur Radiol 11:2126–2136
Kocakoc E, Serhatlioglu S, Kiris A, Bozgeyik Z, Ozdemir H, Bodakci MN (2003) Color Doppler sonographic evaluation of inter-relations between diameter, reflux and flow volume of testicular veins in varicocele. Eur J Radiol 47:251–256
Kozakowski KA, Gjertson CK, Decastro GJ, Poon S, Gasalberti A, Glassberg KI (2009) Peak retrograde flow: a novel predictor of persistent, progressive and new onset asymmetry in adolescent varicocele. J Urol 181:2717–22 discussion 2723
Tasci AI, Resim S, Caskurlu T, Dincel C, Bayraktar Z, Gurbuz G (2001) Color doppler ultrasonography and spectral analysis of venous flow in diagnosis of varicocele. Eur Urol 39:316–321
Mohseni MJ, Nazari H, Amini E, Javan-Farazmand N, Baghayee A, Farzi H, Kajbafzadeh AM (2011) Shunt-type and stop-type varicocele in adolescents: prognostic value of these two different hemodynamic patterns. Fertil Steril 96:1091–1096
Glassberg KI (2014) My indications for treatment of the adolescent varicocele (and why?). Transl Androl Urol 3:402–412
Paduch DA, Niedzielski J (1997) Repair versus observation in adolescent varicocele: a prospective study. J Urol 158:1128–1132
Poon SA, Gjertson CK, Mercado MA, Raimondi PM, Kozakowski KA, Glassberg KI (2010) Testicular asymmetry and adolescent varicoceles managed expectantly. J Urol 183:731–734
Tahmasbi M, Assare E (2011) Quantitative Doppler assessment of flow characteristics in pampiniform venous plexus, in patients with varicocele: is there any relationship with semen analysis. PJR Pak J Radiol 20:121–124
Van Batavia JP, Badalato G, Fast A, Glassberg KI (2013) Adolescent varicocele-is the 20/38 harbinger a durable predictor of testicular asymmetry. J Urol 189:1897–1901
Kocakoc E, Kiris A, Orhan I, Bozgeyik Z, Kanbay M, Ogur E (2002) Incidence and importance of reflux in testicular veins of healthy men evaluated with color duplex sonography. J Clin Ultrasound 30:282–287
Zhang M, Du L, Liu Z, Qi H, Chu Q (2014) The effects of varicocelectomy on testicular arterial blood flow: laparoscopic surgery versus microsurgery. Urol J 11:1900–1906
Grasso Leanza F, Pepe P, Panella P, Pepe F (1997) Volocimetric evaluation of spermatic vessels with echo color doppler in patients with idiopathic varicocele. Minerva Urol Nefrol 49:179–182
Ross JA, Watson NE Jr, Jarow JP (1994) The effect of varicoceles on testicular blood flow in man. Urology 44:535–539
Tarhan S, Gumus B, Gunduz I, Ayyildiz V, Goktan C (2003) Effect of varicocele on testicular artery blood flow in men–color Doppler investigation. Scand J Urol Nephrol 37:38–42
Biagiotti G, Cavallini G, Modenini F, Vitali G, Gianaroli L (2002) Spermatogenesis and spectral echo-colour Doppler traces from the main testicular artery. BJU Int 90:903–908
Unsal A, Turgut AT, Taskin F, Kosar U, Karaman CZ (2007) Resistance and pulsatility index increase in capsular branches of testicular artery: indicator of impaired testicular microcirculation in varicocele. J Clin Ultrasound 35:191–195
Hsu HS, Chang LS, Chen MT, Wei YH (1994) Decreased blood flow and defective energy metabolism in the varicocele-bearing testicles of rats. Eur Urol 25:71–75
Akcar N, Turgut M, Adapinar B, Ozkan IR (2004) Intratesticular arterial resistance and testicular volume in infertile men with subclinical varicocele. J Clin Ultrasound 32:389–393
Baigorri BF, Dixon RG (2016) Varicocele: a review. Semin Intervent Radiol 33:170–176
Organization WH (1985) Comparison among different methods for the diagnosis of varicocele. Fertil Steril 43:575–582
Cariati M, Pieri S, Agresti P, Cariati M, Candito DF, Damiani G, Marzano D (2012) Diagnosis of right-sided varicocele: a retrospective comparative study between clinical examination, Doppler findings, US imaging and vascular anatomy at phlebography. Eur J Radiol 81:1998–2006
Decastro GJ, Shabsigh A, Poon SA, Laor L, Glassberg KI (2009) Adolescent varicocelectomy—is the potential for catch-up growth related to age and/or Tanner stage. J Urol 181:322–7 discussion 327
Gat Y, Bachar GN, Zukerman Z, Belenky A, Gorenish M (2004) Physical examination may miss the diagnosis of bilateral varicocele: a comparative study of 4 diagnostic modalities. J Urol 172:1414–1417
Pfeiffer D, Berger J, Schoop C, Tauber R (2006) A Doppler-based study on the prevalence of varicocele in German children and adolescents. Andrologia 38:13–19
Trussell JC, Haas GP, Wojtowycz A, Landas S, Blank W (2003) High prevalence of bilateral varicoceles confirmed with ultrasonography. Int Urol Nephrol 35:115–118
Gat Y, Bachar GN, Zukerman Z, Belenky A, Gornish M (2004) Varicocele: a bilateral disease. Fertil Steril 81:424–429
Gat Y, Zukerman ZVI, Bachar GN, Feldberg DOV, Gornish M (2003) Adolescent varicocele: is it a unilateral disease. Urology 62:742–746
Sun XL, Wang JL, Peng YP, Gao QQ, Song T, Yu W, Xu ZP, Chen Y, Dai YT (2018) Bilateral is superior to unilateral varicocelectomy in infertile males with left clinical and right subclinical varicocele: a prospective randomized controlled study. Int Urol Nephrol 50:205–210
Elbendary MA, Elbadry AM (2009) Right subclinical varicocele: how to manage in infertile patients with clinical left varicocele. Fertil Steril 92:2050–2053
Fujisawa M, Ishikawa T, Takenaka A (2003) The efficacy of bilateral varicocelectomy in patients with palpable bilateral varicoceles: comparative study with unilateral varicocele. Urol Res 31:407–409
Pasqualotto FF, Lucon AM, de Goes PM, Sobreiro BP, Hallak J, Pasqualotto EB, Arap S (2005) Is it worthwhile to operate on subclinical right varicocele in patients with grade II–III varicocele in the left testicle. J Assist Reprod Genet 22:227–231
Zheng YQ, Gao X, Li ZJ, Yu YL, Zhang ZG, Li W (2009) Efficacy of bilateral and left varicocelectomy in infertile men with left clinical and right subclinical varicoceles: a comparative study. Urology 73:1236–1240
Damsgaard J, Joensen UN, Carlsen E, Erenpreiss J, Blomberg Jensen M, Matulevicius V, Zilaitiene B, Olesen IA, Perheentupa A, Punab M, Salzbrunn A, Toppari J, Virtanen HE, Juul A, Skakkebaek NE, Jorgensen N (2016) Varicocele is associated with impaired semen quality and reproductive hormone levels: a study of 7035 healthy young men from six European countries. Eur Urol 70:1019–1029
Preziosi P, Miano R, Bitelli M, Ciolfi MG, Micali S, Micali F (2001) Right varicocele associated with inferior vena cava malformation in situs inversus: percutaneous treatment with retrograde sclerotherapy. J Endourol 15:1001–1003
Yamamoto M, Hibi H, Hirata Y, Miyake K, Ishigaki T (1996) Effect of varicocelectomy on sperm parameters and pregnancy rate in patients with subclinical varicocele: a randomized prospective controlled study. J Urol 155:1636–1638
Breznik R, Vlaisavljevic V, Borko E (1993) Treatment of varicocele and male fertility. Arch Androl 30:157–160
Ding H, Tian J, Du W, Zhang L, Wang H, Wang Z (2012) Open non-microsurgical, laparoscopic or open microsurgical varicocelectomy for male infertility: a meta-analysis of randomized controlled trials. BJU Int 110:1536–1542
Cervellione RM, Corroppolo M, Bianchi A (2008) Subclinical varicocele in the pediatric age group. J Urol 179:717–9 discussion 719
Zampieri N, Dall’Agnola A (2011) Subclinical varicocele and sports: a longitudinal study. Urology 77:1199–1202
Sakamoto H, Ogawa Y, Yoshida H (2008) Relationship between testicular volume and varicocele in patients with infertility. Urology 71:104–109
Diamond DA (2007) Adolescent varicocele. Curr Opin Urol 17:263–267
Practice Committee of American Society for Reproductive M (2008) Report on varicocele and infertility. Fertil Steril 90:S247–S249
Malekzadeh S, Fraga-Silva RA, Morere PH, Sorega A, Produit S, Stergiopulos N, Constantin C (2016) Varicocele percutaneous embolization outcomes in a pediatric group: 7-year retrospective study. Int Urol Nephrol 48:1395–1399
Cvitanic OA, Cronan JJ, Sigman M, Landau ST (1993) Varicoceles: postoperative prevalence—a prospective study with color Doppler US. Radiology 187:711–714
Rotker K, Sigman M (2016) Recurrent varicocele. Asian J Androl 18:229–233
Glassberg KI, Badalato GM, Poon SA, Mercado MA, Raimondi PM, Gasalberti A (2011) Evaluation and management of the persistent/recurrent varicocele. Urology 77:1194–1198
Kwak N, Siegel D (2014) Imaging and interventional therapy for varicoceles. Curr Urol Rep 15:399
El-Haggar S, Nassef S, Gadalla A, Latif A, Mostafa T (2012) Ultrasonographic parameters of the spermatic veins at the inguinal and scrotal levels in varicocele diagnosis and post-operative repair. Andrologia 44:210–213
Winkelbauer FW, Ammann ME, Karnel F, Lammer J (1994) Doppler sonography of varicocele: long-term follow-up after venography and transcatheter sclerotherapy. J Ultrasound Med 13:953–958
Winkelbauer F, Karnel F, Ammann ME, Hofbauer J (1994) Ultrasound diagnosis of persistent varicocele after sclerotherapy. Ultraschall Med 15:29–32
Palazzo S, Boezio F, Mancini V, Martino P, Battaglia M, Garofalo L, Selvaggi FP (2002) Significance of scrotal Doppler color ultrasonography in the postoperative control of patients treated with varicocelectomy. Arch Ital Urol Androl 74:247–249
Hauser R, Yogev L, Greif M, Hirshenbein A, Botchan A, Gamzu R, Paz G, Yavetz H (1997) Sperm binding and ultrasound changes after operative repair of varicocele: correlation with fecundity. Andrologia 29:145–147
Zucchi A, Mearini L, Mearini E, Fioretti F, Bini V, Porena M (2006) Varicocele and fertility: relationship between testicular volume and seminal parameters before and after treatment. J Androl 27:548–551
Trombetta C, Salisci E, Deriu M, Paoni A, Sanna M, Ganau A, Belgrano E (1993) Echo-flowmetric control 6 years after percutaneous treatment of varicocele. Arch Ital Urol Androl 65:363–367
Haller J, Gritzmann N, Czembirek H, Kumpan W, Schramek P, Leitner H, Karnel F (1986) Pulsed duplex sonographic follow-up of varicoceles following sclerotherapy. Rofo 145:547–550
Hamm B, Sorensen R, Berger T (1986) Non-invasive imaging procedures in the diagnosis and control of therapy of varicoceles. 2. Importance of thermography and sonography for therapy control following sclerotherapy of varicoceles. Rofo 144:665–669
Mastrojeni C, Pagano G, Scalisi M, Leonello G, Manfré A, Versaci A (2001) Idiopathic varicocele. Diagnosis and follow-up with CW-Doppler and echo-color-doppler: our experience. G Chir Giornale di chirurgia 22:41–44
Cil AS, Bozkurt M, Kara Bozkurt D, Gok M (2015) Investigating the relationship between persistent reflux flow on the first postoperative day and recurrent varicocele in varicocelectomy patients. J Clin Med Res 7:29–32
El-Saeity NS, Sidhu PS (2006) Scrotal varicocele, exclude a renal tumour. Is this evidence based. Clin Radiol 61:593–599
Spittel JA Jr, Deweerd JH, Shick RM (1959) Acute varicocele: a vascular clue to renal tumor. Proc Staff Meet Mayo Clin 34:134–137
Gibbons RP, Monte JE, Correa RJ Jr, Mason JT (1976) Manifestations of renal cell carcinoma. Urology 8:201–206
Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF (1971) Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer 28:1165–1177
El Abiad Y, Qarro A (2016) Images in clinical medicine. Acute varicocele revealing renal cancer. N Engl J Med 374:2075
Bate J (1927) Symptomatic varicocele. J Urol 18:649–662
Ding GX, Song NH, Feng CC, Xia GW, Jiang HW, Hua LX, Ding Q (2012) Is there an association between advanced stage of renal cell carcinoma and paraneoplastic syndrome. Med Princ Pract 21:370–374
Gieseler B (1984) Pathogenesis of symptomatic varicocele in kidney tumor. Z Urol Nephrol 77:585–587
Li PC, Zhang JY, Xiu YY, Liu S, Xia JG, Shi HB, Song NH (2018) Varicocele due to renal arteriovenous malformation mimicking a renal tumor: a case report. J Med Case Rep 12:2
Chi AC, Hairston JC (2015) Acute right varicocele: a clue to congenital vascular anomaly. Urology 85:e39–40
Lewis DS, Grimm LJ, Kim CY (2015) Left renal vein compression as cause for varicocele: prevalence and associated findings on contrast-enhanced CT. Abdom Imaging 40:3147–3151
Asala S, Chaudhary SC, Masumbuko-Kahamba N, Bidmos M (2001) Anatomical variations in the human testicular blood vessels. Ann Anat 183:545–549
Bigot JM, Utzmann O (1983) Right varicocele: contribution of spermatic phlebography. Results on 250 cases. J Urol (Paris) 89:121–131
Piffer CR, Piffer MI (1988) Anatomical observations of the testicular veins. Anat Anz 166:249–255
Robson J, Wolstenhulme S, Knapp P (2012) Is there a co-association between renal or retroperitoneal tumours and scrotal varicoceles? A systematic review. Ultrasound 20:182–191
Oster J (1971) Varicocele in children and adolescents. An investigation of the incidence among Danish school children. Scand J Urol Nephrol 5:27–32
Akbay E, Cayan S, Doruk E, Duce MN, Bozlu M (2000) The prevalence of varicocele and varicocele-related testicular atrophy in Turkish children and adolescents. BJU Int 86:490–493
Erginel B, Vural S, Akin M, Karadag CA, Sever N, Yildiz A, Tanik C, Demir AA, Yanar O, Dokucu AI (2014) Wilms’ tumor: a 24-year retrospective study from a single center. Pediatr Hematol Oncol 31:409–414
Ates N, Yuksel M, Ylmaz S, Habibi M, Ipekci T (2016) Retroperitoneal paraganglioma presenting as right-sided varicocele: case report. Ann Saudi Med 36:148–151
Arrabal-Polo MA, Merino-Salas S (2013) Acute left varicocele in an adult. CMAJ 185:323
Chandramohan S, Chakravertry S (2007) Re: “scrotal varicocoele, exclude a renal tumour”. Is this evidence based. Clin Radiol 62:192–193
Espinosa Bravo R, Lemourt Oliva M, Pèrez Monzûn AF, Puente Guillèn M, Navarro Cutiòo M, Sandoval Lûpez O (2003) de la C Fuentes Milera A Carcinoma de cèlulas renales y varicocele izquierdo de forma simultánea. Archivos Españoles de Urologìa 56:533–535
Tufano A, Minelli R, Rossi E, Brillantino C, Di Serafino M, Zeccolini M, Cantisani V, Vallone G (2020) Inferior epigastric artery pseudoaneurysm secondary to port placement during a robot-assisted laparoscopic radical cystectomy. J Ultrasound. https://doi.org/10.1007/s40477-020-00442-1
Zawaideh JP, Bertolotto M, Giannoni M, Piaggio G, Durand F, Derchi LE (2020) Tension hydrocele as an additional cause of acute scrotum: case series and literature review. Abdom Radiol (NY) 45:2082–2086
Conzi R, Damasio MB, Bertolotto M, Secil M, Ramanathan S, Rocher L, Cuthbert F, Richenberg J, Derchi LE, Scrotal Imaging Group of the European Society of Urogenital R (2017) Sonography of scrotal wall lesions and correlation with other modalities. J Ultrasound Med 36:2149–2163
Secil M, Bertolotto M, Rocher L, Pekindil G, Stocca T, Richenberg J, Ramchandani P, Derchi LE, European Society of Urogenital Radiology Scrotal Imaging S (2017) Imaging features of paratesticular masses. J Ultrasound Med 36:1487–1509
Yilmaz C, Arslan M, Arslan M (2009) Intrascrotal arteriovenous malformation simulating varicocele. AJR Am J Roentgenol 192:W351
Zachariah JR, Gupta AK, Lamba S (2012) Arteriovenous malformation of the scrotum: is preoperative angioembolization a necessity. Indian J Urol IJU J Urol Soc India 28:329–334
Pavan N, Bucci S, Mazzon G, Bertolotto M, Trombetta C, Liguori G (2015) It’s not always varicocele: a strange case of zinner syndrome. Can Urol Assoc J 9:E535–E538
Bhatt S, Jafri SZ, Wasserman N, Dogra VS (2011) Imaging of non-neoplastic intratesticular masses. Diagn Interv Radiol 17:52–63
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J (1999) Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ 318:527–530
Thomsen HS, Morcos SK, Almén T, Bellin MF, Bertolotto M, Bongartz G, Clement O, Leander P, Heinz-Peer G, Reimer P, Stacul F, van der Molen A, Webb JA, ESUR CMSC (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 23:307–318
Stacul F, Bertolotto M, Thomsen HS, Pozzato G, Ugolini D, Bellin MF, Bongartz G, Clement O, Heinz-Peer G, van der Molen A, Reimer P, Webb JAW, ESUR CMSC (2018) Iodine-based contrast media, multiple myeloma and monoclonal gammopathies: literature review and ESUR Contrast Media Safety Committee guidelines. Eur Radiol 28:683–691
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS (2018) Post-contrast acute kidney injury—part 1: definition, clinical features, incidence, role of contrast medium and risk factors: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28:2845–2855
van der Molen AJ, Reimer P, Dekkers IA, Bongartz G, Bellin MF, Bertolotto M, Clement O, Heinz-Peer G, Stacul F, Webb JAW, Thomsen HS (2018) Post-contrast acute kidney injury. Part 2: risk stratification, role of hydration and other prophylactic measures, patients taking metformin and chronic dialysis patients: recommendations for updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 28:2856–2869
Piscaglia F, Nolsøe C, Dietrich CF, Cosgrove DO, Gilja OH, Bachmann Nielsen M, Albrecht T, Barozzi L, Bertolotto M, Catalano O, Claudon M, Clevert DA, Correas JM, D’Onofrio M, Drudi FM, Eyding J, Giovannini M, Hocke M, Ignee A, Jung EM, Klauser AS, Lassau N, Leen E, Mathis G, Saftoiu A, seidel G, sidhu ps, ter haar g, timmerman d, weskott hp (2012) the efsumb guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 33:33–59
Richenberg J, Belfield J, Ramchandani P, Rocher L, Freeman S, Tsili AC, Cuthbert F, Studniarek M, Bertolotto M, Turgut AT, Dogra V, Derchi LE (2015) Testicular microlithiasis imaging and follow-up: guidelines of the ESUR scrotal imaging subcommittee. Eur Radiol 25:323–330
Vidili G, De Sio I, D’Onofrio M, Mirk P, Bertolotto M, Schiavone C, SIUMB EC, (2019) SIUMB guidelines and recommendations for the correct use of ultrasound in the management of patients with focal liver disease. J Ultrasound 22:41–51
Sidhu PS, Brabrand K, Cantisani V, Correas JM, Cui XW, D’Onofrio M, Essig M, Freeman S, Gilja OH, Gritzmann N, Havre RF, Ignee A, Jenssen C, Kabaalioğlu A, Lorentzen T, Mohaupt M, Nicolau C, Nolsøe CP, Nürnberg D, Radzina M, Saftoiu A, Serra C, Spârchez Z, Sporea I, Dietrich CF (2015) EFSUMB guidelines on interventional ultrasound (INVUS), part II. Diagnostic ultrasound-guided interventional procedures (Long Version). Ultraschall Med 36:E15–35
Sidhu PS, Brabrand K, Cantisani V, Correas JM, Cui XW, D’Onofrio M, Essig M, Freeman S, Gilja OH, Gritzmann N, Havre RF, Ignee A, Jenssen C, Kabaalioğlu A, Lorentzen T, Mohaupt M, Nicolau C, Nolsøe CP, Nürnberg D, Radzina M, Saftoiu A, Serra C, Spârchez Z, Sporea I, Dietrich CF (2015) EFSUMB guidelines on interventional ultrasound (INVUS), part II. Diagnostic ultrasound-guided interventional procedures (Short Version). Ultraschall Med 36:566–580
Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D’Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H (2018) The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (long version). Ultraschall Med 39:e2–e44
Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, Bertolotto M, Calliada F, Clevert DA, Cosgrove D, Deganello A, D’Onofrio M, Drudi FM, Freeman S, Harvey C, Jenssen C, Jung EM, Klauser AS, Lassau N, Meloni MF, Leen E, Nicolau C, Nolsoe C, Piscaglia F, Prada F, Prosch H, Radzina M, Savelli L, Weskott HP, Wijkstra H (2018) The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (short version). Ultraschall Med 39:154–180
Rocher L, Ramchandani P, Belfield J, Bertolotto M, Derchi LE, Correas JM, Oyen R, Tsili AC, Turgut AT, Dogra V, Fizazi K, Freeman S, Richenberg J (2016) Incidentally detected non-palpable testicular tumours in adults at scrotal ultrasound: impact of radiological findings on management Radiologic review and recommendations of the ESUR scrotal imaging subcommittee. Eur Radiol 26:2268–2278
Tsili AC, Bertolotto M, Turgut AT, Dogra V, Freeman S, Rocher L, Belfield J, Studniarek M, Ntorkou A, Derchi LE, Oyen R, Ramchandani P, Secil M, Richenberg J (2018) MRI of the scrotum: Recommendations of the ESUR Scrotal and Penile Imaging Working Group. Eur Radiol 28:31–43
Ramanathan S, Bertolotto M, Shamsodini A, Heidous MA, Dogra V, Ramchandani P (2018) Introduction to imaging of penile prostheses: a primer for the radiologist. AJR Am J Roentgenol 210:1192–1199
Acknowledgements
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement.
The ESUR-SPIWG WG: Jane Belfield, Mustafa Secil, Michele Bertolotto, Agnieszka Bianek-Bodzan, Irene Campo, Beniamino Corcioni, Lorenzo Derchi, Pieter De Visschere, Vikram Dogra, Simon Freeman, Caterina Gaudiano, Dean Huang, Oliwia Kozak, Francesco Lotti, Karolina Markiet, Olivera Nikolic, Alexandra Ntorkou, Raymond Oyen, Nicola Pavan, Pietro Pavlica, Thierry Puttemans, Subramaniyan Ramanathan, Parvati Ramchandani, Jonathan Richenberg, Laurence Rocher, Camilla Sachs, Paul Sidhu, Katarzyna Skrobisz, Michal Studniarek, Athina Tsili, Ahmet Turgut, Massimo Valentino.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The members of “the ESUR-SPIWG WG" are listed in Acknowledgement section.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bertolotto, M., Freeman, S., Richenberg, J. et al. Ultrasound evaluation of varicoceles: systematic literature review and rationale of the ESUR-SPIWG Guidelines and Recommendations. J Ultrasound 23, 487–507 (2020). https://doi.org/10.1007/s40477-020-00509-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40477-020-00509-z