Abstract
Purpose of Review
Colorectal cancer is the second deadliest cancer in the world, and its prevalence has been increasing alarmingly in recent years. After researchers discovered the existence of dysbiosis in colorectal cancer, they considered the use of probiotics in the treatment of colorectal cancer. However, for various reasons, including the low safety profile of probiotics in susceptible and immunocompromised patient5s, and the risk of developing antibiotic resistance, researchers have shifted their focus to non-living cells, their components, and metabolites. This review aims to comprehensively evaluate the literature on the effects of diet, microbiota, and postbiotics on colorectal cancer and the future of postbiotics.
Recent Findings
The link between diet, gut microbiota, and colorectal cancer has been established primarily as a relationship rather than a cause-effect relationship. The gut microbiota can convert gastrointestinal tract and dietary factors into either onco-metabolites or tumor suppressor metabolites. There is serious dysbiosis in the microbiota in colorectal cancer. Postbiotics appear to be promising agents in the prevention and treatment of colorectal cancer.
Summary
It has been shown that various postbiotics can selectively induce apoptosis in CRC, inhibit cell proliferation, growth, invasion, and migration, modulate the immune system, suppress carcinogenic signaling pathways, maintain intestinal epithelial integrity, and have a synergistic effect with chemotherapy drugs. However, it is also reported that some postbiotics are ineffective and may be risky in terms of safety profile in some patients. Many issues need to be researched about postbiotics. Large-scale, randomized, double-blind clinical studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC), which includes cancers that occur in the colon and rectum, is the third most common type of cancer in oncologic pathology, following breast and lung cancers. In 2020, nearly 2 million cases were diagnosed. While effective screening techniques exist that could reduce the number of deaths, CRC is still the second most common cause of cancer-related death. The International Agency for Research on Cancer estimates that the global burden of CRC will increase by 56% between 2020 and 2040, with more than 3 million new cases expected annually [https://publications.iarc.fr/, Date of Access: 07.12.2023].
Most CRCs occur sporadically due to genetic mutations and epigenetic modifications in the human genome. These modifications alter the signaling pathways that regulate cell behavior, enabling the progression from normal mucosa to carcinoma [1]. About two-thirds of all CRCs are sporadic, while the remaining third are familial or inherited [2]. The most common hereditary CRC is Lynch syndrome, accounting for 2–3% of cases, followed by familial adenomatous polyposis responsible for 0.5–1% of cases, and other hereditary variants such as MUTYH-associated polyposis, Peutz-Jeghers syndrome, and others, represent less than 1% of cases [3,4,5]. In particular, the high turnover rate of intestinal epithelium makes this tissue a focal point for malignant transformations. However, in the first stage, CRC usually starts as a neoplastic or non-neoplastic polyp in the inner wall of the colon or rectum. The larger the polyps, the greater their malignant potential. The most common type is the adenomatous polyp, which occurs in the glandular cells that produce mucus from these polyps. Adenomas formed in the inner wall of the colorectum are histologically neoplastic. If they become malignant, they are referred to as adenocarcinomas, which make up 96% of all CRCs [6].
It is thought that the development of CRC can result from one or a combination of three different mutational pathways: chromosomal instability (CIN) characterized by an increase in chromosomal aneuploidies; epigenetic changes that cause silencing of tumor suppressor genes, caused by hypermethylation of CpG regions known as the CpG island methylator phenotype (CIMP); and microsatellite instability (MSI) caused by impairment of DNA mismatch repair. Approximately 15% of all CRC cases exhibit MSI, while CIMP is found in 10–40%, and the remaining 85% are associated with CIN. CIN is linked to the mutation of tumor suppressor genes, including adenomatous polyposis coli (APC), P53, Mothers Against Decapentaplegic homolog 2 (SMAD2), SMAD4, deleted in colorectal cancer (DCC), and oncogenes Kirsten rat sarcoma virus (K-ras) and β-catenin [1, 7, 8]
Elective surgery, radiotherapy, and chemotherapy methods are used to treat CRC [9]. Management of CRC is difficult due to late diagnosis, high recurrence rates, and multidrug resistance. Due to the problems caused by the side effects of these treatments and their negative effects on the healing process, different treatment methods are continually being developed. Despite the widespread use of chemotherapy agents in CRC, their limited effectiveness, selectivity, low bioavailability, poor solubility, and non-specific biodistribution between cancer and normal cells/tissues are significant challenges. Increasing cancer deaths and the high cost of treatment increase the interest in new therapeutic approaches and the discovery of chemo-preventive agents from natural sources [10].
Environmental factors and lifestyle, including diet, are crucial in the development of CRC [11]. The interaction between microbiota and CRC has been the focus of research lately [12]. The microbiota refers to the community of microorganisms, including bacteria, fungi, viruses, and yeasts, present in a defined environment and residing on or within human tissues such as the skin, lung, oral mucosa, and the urogenital and gastrointestinal tracts. It has been estimated that close to 95% of these microbes are in our gut, particularly the large intestine [https://internationalprobiotics.org/home/, Date of Access: 07.11.2023]. Only 1.9% of the gut microbiome is estimated to be heritable, and more than 20% of the variance in microbiome β-diversity can be inferred from environmental factors related to diet and lifestyle [12, 13]. Diet can reshape the community structure of the gut microbiota and affect its function by modulating the production of metabolites [12]. CRC is associated with a process known as dysbiosis, which includes the depletion and/or enrichment of certain microbiota members and their metabolic functions [14]. With a better recognition of the role of the microbiota in cancer pathogenesis, the potential of microbiota-based therapeutics has become an increasingly researched topic in cancer therapy. The connection between gut microbiota, dysbiosis, and CRC has led researchers to explore the effects of probiotics in CRC prevention and treatment [15]. The oral use of probiotics is widespread today; however, the safety profile of viable probiotics remains a topic of debate. Therefore, research in this direction seems to be shifting toward postbiotics [16]. Postbiotics are defined as “a preparation of inanimate microorganisms and/or their components that confer a health benefit on the host” [17••]. While postbiotics do not contain live microorganisms, they are thought to exert a beneficial health effect through similar mechanisms characteristic of probiotics while minimizing the risks associated with their intake [18]. Considering all this information, our review focuses on the effects of diet, microbiota, and the increasingly popular postbiotics in CRC.
Diet and CRC
Comprehensive research suggests that diet may play both a causal and protective role in the development of colon cancer [19]. In 2018, the World Cancer Research Fund published a report analyzing 99 studies involving over 29 million adults and more than 247,000 cases of CRC worldwide. This report has identified the following as solid evidence of relationships between diet, nutrition, physical activity, and risk of CRC. Body fatness; consuming processed meat, red meat, and alcoholic beverages; and being tall can increase the risk of CRC. In addition, it has been stated that smoking and inflammatory bowel diseases (such as Chron’s disease and ulcerative colitis) increase the risk of CRC (dietandcancerreport.org, Date of Access: 10.12.2023). In a study involving 2502 CRC cases and 2538 controls, a positive correlation was found between the dietary inflammatory index and CRC [20]. Another study with 400 CRC patients and 400 healthy individuals identified several risk factors for CRC, including obesity, active and passive smoking, low physical activity, and high consumption of red meat and salt [6].
Chronic consumption of the Western diet has been shown to exacerbate colitis and promote colorectal tumor development in mice by activating proinflammatory and abnormal immune response pathways in the colon [21]. A prospective cohort meta-analysis, involving 80,110 cases, determined that CRC had the highest number of diet-related cases. Low consumption of dairy products and whole grains, along with high consumption of processed meat, were contributing factors [22]. In another prospective study, it has been shown that participants who consumed an average of 76 g of processed and red meat per day had a higher risk of CRC compared to those consuming 21 g per day [23].
It is known that cooking meat at high temperatures causes the formation of heterocyclic amines and polycyclic aromatic hydrocarbons, which experimental studies have linked to CRC. Additionally, the high level of heme iron in red meat has been shown to promote CRC by stimulating the endogenous formation of carcinogenic N-nitroso compounds. Processed meat also contains exogenous N-nitroso compounds, which could potentially be carcinogenic [24]. The pre-diagnosis processed meat diet model is associated with higher tumor recurrence, metastasis, and mortality in CRC patients [25]. High consumption of red meat has been shown to increase the levels of oncogenic mature miRNAs, including miR17-92 cluster miRNAs and miR21, in the human rectal mucosa, followed by the addition of butyrylated-resistant starch to the diet, with miR17-92 level returning to baseline and fecal butyrate level increasing [26].
Considering data from three large prospective cohorts, it has been stated that high consumption of ultra-processed foods is associated with the risk of CRC. Among subgroups of ultra-processed foods, higher consumption of meat/poultry/seafood-based ready-to-eat products and sugar-sweetened beverages in men, as well as ready-to-eat/heat-mixed dishes in women, is associated with an increased risk of CRC [27]. Consumption of a plant-based diet high in refined grains and sugar was associated with a high incidence of CRC [28].
According to the report of the World Cancer Research Fund, engaging in physical activity, consuming whole grains, incorporating foods with dietary fiber, consuming dairy products, and taking calcium supplements can reduce the risk of CRC at the level of solid evidence. Furthermore, using anti-inflammatory drugs for 5 years or more and hormone therapy in postmenopausal women have been found to reduce the risk of CRC (dietandcancerreport.org, Date of Access: 10.12.2023).
Increased fiber consumption can potentially reduce the risk of CRC through two non-mutually exclusive mechanisms. First, insoluble fiber accelerates colonic transit and may reduce exposure of colonic epithelial cells to ingested carcinogens such as heterocyclic amines from charred meat. Second, soluble fiber is fermented into butyrate and other potentially beneficial metabolites by certain classes of bacteria [29]. In a prospective study of stages I to III CRC patients, high grain-derived fiber intake was associated with lower CRC-specific mortality and overall mortality [30]. Consumption of a plant-based diet with a predominance of whole grains, fruits, and vegetables was associated with a lower incidence of CRC [28]. Fiber from bread and breakfast cereals was associated with a reduced risk of CRC [23].
It has been found that an increase in fish consumption of 20 g per day was associated with a 2% reduction in gastrointestinal cancer risk in a meta-analysis of 42 cohort studies, including 2,325,040 participants and 24,115 gastrointestinal cancer cases [31]. Yogurt and dairy-based desserts in women are negatively associated with the risk of CRC [27]. In a population of 923 CRC patients and 1846 healthy people, high coffee consumption (3 cups/day) was associated with a lower probability of developing CRC [32]. The relationship between diet, microbiota, and CRC is discussed later in the article.
Microbiota and Its Functions
The microbiota plays an increasingly crucial role in the human digestive system, metabolism, immunity, and various other processes in the body, often referred to as another “organ” of the human body [33]. It has been estimated that close to 95% of these microbes are in the gut, especially the large intestine [https://internationalprobiotics.org/, Date of Access: 31.07.2023]. Considering the high bile concentrations and short transit time, the small intestine provides a more challenging environment for microbial colonization. In contrast, the colon, characterized by a neutral to slightly acidic pH and slow flow rate, hosts the largest microbial community [34].
The International Probiotic Association defines microbiota as follows: “The microbiota refers to the community of microorganisms, including bacteria, fungi, viruses, and yeasts, present in a defined environment and residing on or within human tissues such as the skin, lung, oral mucosa, and the urogenital and gastrointestinal tracts.” [https://internationalprobiotics.org/, Date of Access: 31.07.2023]. The human microbiota consists of bacteria, archaeabiota, virobiota, and micobiota, which form a highly complex network of interactions between each other and the host [35]. The human gastrointestinal system contains more than 100 trillion microorganisms. The density of bacterial cells is estimated to be 1011 to 1012 per milliliter in the colon. In Eubiosis, the predominant gut microbial phyla are Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia. Firmicutes and Bacteroidetes phyla represent 90% of the gut microbiota. The Firmicutes phylum consists of more than 200 species, such as Lactobacillus, Bacillus, Clostridium, Enterococcus, and Ruminicoccus. The genus Clostridium represents 95% of the Firmicutes phylum. It consists of dominant genera such as Bacteroidetes, Bacteroides, and Prevotella. The phylum Actinobacteria is proportionally less abundant and is mainly represented by the genus Bifidobacterium [33]. Most current studies have focused on gut bacteria and neglected the mycobiota, archaeobiota, and virobiota. Chibani et al. [36•] determined 1167 archaeal genomes in human gastrointestinal tract samples. It has been determined NJ-bd that these genomes cover a wide taxonomic diversity and include members of the Methanobacteriales (87.15%), Methanomassiliicoccales (12.43%), Methanomicrobiales (0.26%), and Halobacteriales (0.17%). The predominant Archaea in the human gut are methanogens that anaerobically reduce carbon dioxide to methane gas. The most common methanogen in the gut microbiota is Methanobrevibacter smithii, with a prevalence of about 95.7% [37]. The human virobiota consists primarily of bacteriophages, animal cell viruses, endogenous retroviruses, and viruses that cause persistent and latent infections. Collectively, they contain a more diverse genetic entity than gut bacteria. A healthy human gut virome is predominated by temperate dsDNA Caudovirales and ssDNA Microviridae [38]. In a study examining fecal samples from 147 healthy volunteers, 701 fungal operational taxonomic units (OTUs) were identified, covering 247 genera. Specifically, fungal communities were characterized by a high prevalence of Saccharomyces, Malassezia, and Candida, with S. cerevisiae, M. restricta, and C. albicans OTUs [39].
Microbiota in early life, it has been shown to be affected by type of delivery [40], time of birth [41], breastfeeding, formula feeding [42], and pet ownership [43]. In the later stages of life, it has been determined that the microbiota is affected by diet, using drugs such as antibiotics and proton pump inhibitors [44, 45], stress [46], smoking, alcohol consumption [47], living in rural or urban areas [48], and physical activity [49].
The microbiota synthesizes numerous enzymes for digesting nutrients that escape the human digestive system. The gut microbiome is highly enriched for genes related to carbohydrate metabolism, including ≥ 115 glycoside hydrolase families and ≥ 21 polysaccharide lyase families [50]. Intestinal microbiota can also produce aspartic-, cysteine-, serine-, and metallo-proteases [51]. The microbiota biochemically modifies the substances taken up by the host, both exogenously and endogenously. Furthermore, they generate de novo metabolites [52]. Short-chain fatty acids (SCFAs) are mainly produced by saccharolytic fermentation of carbohydrates that escape digestion and absorption in the small intestine. SCFAs represent the primary carbon source from the diet to the host through the microbiome. The major SCFAs include formate, acetate, propionate, and butyrate. Additionally, amino acid fermentation contributes to SCFA production, mainly yielding acetate and propionate [53]. Butyrate constitutes the critical energy source for human colonocytes, which induces the proliferation of healthy colon cells while inducing apoptosis of colon cancer cells. Propionate directly supports energy homeostasis by reducing hepatic glucose production and adiposity. Acetate is an auxiliary metabolite necessary for the growth of other bacteria. Butyrate, propionate, and acetate show anti-inflammatory effects by regulating T-cell differentiation [54]. The microbiota plays a critical role in the maturation and regulation of the host’s immune functions. It prevents the proliferation of pathobionts and regulates colon cell proliferation and vascularization. It can synthesize vitamins and neurotransmitters, metabolize bile salts and xenobiotics, regulate mucus production, improve intestinal barrier integrity, and provide biotransformation of polyphenols [54,55,56,57]. Considering all these functions, it is not surprising that the microbiota is linked to metabolic, autoimmune, neurological, psychiatric, inflammatory, and cardiovascular diseases and cancer.
Microbiota, Dysbiosis, Diet, and Their Relationship with CRC
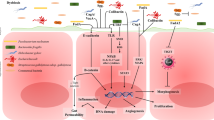
Dysbiosis is generally defined as a cause or consequence of a change and disorder in the gut microbiota composition. Dysbiosis is categorized into three types: (i) the loss of beneficial microbes, (ii) the proliferation of pathogenic microbes, and (iii) reduced microbial diversity [58]. It is often difficult to determine whether the change is beneficial or harmful [34]. CRC is linked to dysbiosis, which includes depletion and/or enrichment of certain intestinal bacterial species and their metabolic functions [14]. We summarized the microbiota identified to be enriched in colorectal cancer [59,60,61,62,63,64,65,66] in Fig. 1.
It is thought that holistic dysbiosis, rather than a specific pathogen in the microbiota, plays a role in the etiology of CRC [67]. The underlying mechanisms during the interaction between microbial dysbiosis and colorectal carcinogenesis include the promotion of inflammation, pathological bacterial adhesion, and induction of tumorigenesis [12]. It has been suggested that the microbiota differs between the early and late stages of CRC. Some pathogenic bacteria rapidly colonize the intestinal epithelium as drivers, while opportunistic microorganisms, namely travelers, further contribute to cancer progression [68]. It is stated that the changed microbiota in CRC can be used in diagnosis in the future and will contribute to the prevention/treatment of CRC [69]. Below, we summarize studies demonstrating changes in microorganisms and their metabolites in the analysis of rectal biopsy and fecal samples from CRC patients and healthy people.
In a study, biopsies were collected from normal rectal mucosa areas ∼10–12 cm from the anal verge in 33 adenoma subjects and 38 non-adenoma subjects, and bacterial genomic DNA was extracted from these biopsies. Biopsy samples from adenoma subjects showed higher relative abundance levels of 30 genera, including Acidovorax, Aquabacterium, Cloacibacterium, Helicobacter, Lactococcus, Lactobacillus, and Pseudomonas, compared to the controls. It has been determined that the bacteria, which were relatively high in the cases, were mostly members of the phylum Firmicutes, Bacteroidetes, and Proteobacteria. The authors suggest it can be used in future microbiota array analysis to identify patients at risk of developing adenomas [70]. In a study that included 203 CRC and 236 healthy controls, totaling 439 participants, higher relative abundances of Fusobacterium nucleatum, Clostridium hathewayi, Bacteroides clarus, and Roseburia intestinalis were observed in the fecal samples of CRC patients compared to the controls [59]. In a meta-analysis of 271 controls and 255 CRC cases, higher levels of Bacteroides fragilis, Fusobacterium nucleatum, Porphyromonas asaccharolytica, Parvimonas micra, Prevotella intermedia, Alistipes finegoldii, and Thermanaeravibrio acidaminovorans were detected in fecal samples from CRC cases compared to control [60].
In a study, microbiota and metabolomic profiling have been performed in fecal samples from 42 CRC cases and 89 controls. The results revealed strong microbe-metabolite correlations in CRC cases, primarily involving Enterobacteriaceae and Actinobacteria. CRC has been independently associated with lower levels of Clostridia, Lachnospiraceae, p-aminobenzoate, and conjugated linoleate and higher levels of Fusobacterium, Porphyromonas, p-hydroxy-benzaldehyde, and palmitoyl-sphingomyelin [71].
According to the results of a systematic analysis examining the relationship between gut microbiota and prognosis after CRC surgery, Fusobacterium nucleatum and Bacteroides fragilis have been associated with a worse prognosis [72].
In a systematic review including nine studies, organisms associated with CRC were identified as Fusobacterium, Enterotoxigenic Bacteroides fragilis, Clostridium, Salmonella, and Peptostreptococcus [73]. A meta-analysis of 57 studies demonstrated a higher presence of Fusobacterium nucleatum in tissue samples with CRC tumors [74].
As a result of the microbiome and metabolome analysis in fecal samples from 50 CRC patients and 50 healthy individuals, it has been determined that fecal samples of patients with CRC showed a unique and distinct metabolic signature. In CRC, there is a high presence of Proteobacteria, Fusobacteria, and lower levels of Firmicutes. In contrast, the control group exhibited higher levels of SCFAs and hydroxycinnamic acid. Additionally, CRC samples showed reduced microbial diversity. Fecal polyamines (cadaverine, putrescine) have been suggested as potential biomarkers for CRC due to their increased levels [67]. Additionally, CRC tissues have been found to be enriched with biofilms of Bacteroides fragilis, Fusobacterium nucleatum, Parvimonas micra, and Peptostreptococcus stomatis [75].
Fungal dysbiosis has been observed in patients with CRC and polyps. This dysbiosis is characterized by decreased fungal diversity, an increased Ascomycota/Basidiomycota ratio, and an elevated proportion of opportunistic fungi such as Trichosporon and Malassezia. These findings may contribute to the progression of CRC [61]. Biopsy was obtained from the adenoma and adjacent tissues of 27 subjects with colorectal adenomas. The analysis revealed a higher prevalence of the Glomeramycota phylum in adenomas [62]. As a result of the analysis of fecal shotgun metagenomic sequences of 184 patients with CRC, 197 patients with adenoma, and 204 control subjects, it has been determined that Basidiomycota-to-Ascomycota ratio was high, Malasseziomycetes increased, and Saccharomycetes and Pneumocystidomycetes decreased in CRC [63]. Archaeal dysbiosis has also been shown to occur in patients with CRC. It has been shown that methanogenic archaea are depleted, and halophilic archaea are enriched in patients with CRC [64]. A study examining fecal samples from three different cohorts showed that bacterial diversity in CRC patients decreased, the diversity of bacteriophage virome increased, and 22 viral taxa separated healthy controls from patients with CRC [65].
Genotoxic substances such as colibactin secreted by Escherichia coli, Bacteroides fragilis toxin produced by Bacteroides fragilis, and typhoid toxin from Salmonella can cause host DNA damage. These bacteria can also indirectly promote CRC by affecting host signaling pathways such as E-cadherin/β-catenin, Toll-like receptor-4 (TLR-4)/myeloid differentiation primary response protein 88 (MyD88)/nuclear factor kappa B (NF-κB), and smoothened (SMO)/rat sarcoma virus (RAS)/p38 mitogen-activated protein kinase (MAPK) pathway [76]. In dysbiosis, there is a decrease in the number of butyrate-producing bacteria such as Bifidobacterium, Lactobacillus, and Bacteroides. Simultaneously, there is an increase in the number of pathogenic bacteria such as Clostridium difficile, Enterobacter, Fusobacterium nucleatum, Escherichia coli, and Klebsiella pneumonia. These changes contribute to the deterioration of cell DNA structure, activation of carcinogenic signaling pathways, and induction of CRC [56]. Intestinal dysbacteriosis stimulates macrophage activation, which promotes the production and secretion of the inflammatory cytokines interleukin-6 (IL-6) and TNF-α (tumor necrosis factor-alpha), and elevated peripheral IL-6 and TNF-α subsequently support the epithelial-mesenchymal transition process of CRC that contributes to tumor progression and metastasis [77].
Microbiota has a bidirectional effect on CRC. The gut microbiota may support or protect against CRC through various mechanisms. The cancer-promoting microbiota adheres directly to epithelial cells via microorganism-associated molecular models and adhesins, such as Wingless-related integration site (Wnt)-β-catenin or oncogenic signal activation. It also produces toxins and harmful metabolites (such as secondary bile acids, polyamines, and hydrogen sulfide) and induces tumor-associated immune cell populations (such as tumor-associated macrophages, tumor-associated neutrophils, Myeloid-derived suppressor cells, and regulatory T cells) to regulate inflammation, tissue damage, cell proliferation and survival, immune evasion, and drug resistance. It can interact with the tumor microenvironment, affecting tumor growth and progression. In contrast, the gut microbiota may play a role in detoxifying dietary components and reducing chronic inflammation. Microbiota that inhibits CRC can directly trigger anti-tumor signal activation in epithelial cells, produce beneficial metabolites (such as SCFAs), and stimulate tumor-preventing immune cells (such as the cluster of differentiation-8 cells, T helper-1, T helper-17, and innate lymphoid cell-3) [69, 78].
Long-term dietary patterns exert a significant influence on our gut microbiome. However, short-term dietary interventions can also impact the gut microbiome [79]. The link between diet, gut microbiota, and CRC has primarily been established as an association rather than a cause-effect relationship. Microbiota can metabolize the gastrointestinal tract and dietary factors to oncometabolites and tumor suppressor metabolites [80]. Soluble fiber increases SCFA synthesis, exhibits prebiotic, anti-inflammatory, and antitumoral effects on microbiota, prevents dysbiosis, and enhances mucus secretion. On the other hand, insoluble fiber increases the rate of colonic transit and reduces the absorption of carcinogenic components. Thus, fiber strengthens the gut microbiota and potentially reduces the risk of developing colon cancer. Butyrate, one of the SCFAs, provides the necessary energy for the proliferation of colonic epithelial cells with rapid turnover. Colorectal tumor cells are exposed to the Warburg effect and switch to glucose utilization and aerobic glycolysis. Butyrate is not metabolized to the same extent and accumulates in the nucleus, which functions as a histone deacetylase (HDAC) inhibitor [81]. A meta-analysis of 17 case-controlled and six cross-sectional studies found that lower fecal SCFA concentrations were associated with a higher risk of CRC and incidence of CRC [82]. Normally, the amounts of proteolytic fermentation in the colon are smaller than those of saccharolytic fermentation. A high-fat, high-meat, low-fiber diet, pro-neoplastic, and pro-inflammatory properties of protein fermentation and bile acid deconjugated residues predominate, increasing CRC risk [83]. These diets produce pro-inflammatory and oncogenic components such as polyamines, ammonia, branched-chain SCFAs, nitrogen-rich metabolites, hydrogen sulfide (especially F. nucleatum and Desulfovibrio vulgaris), and secondary bile acids [84]. High-fat diets increase deoxycholic acid levels, which in turn resist apoptosis, trigger reactive oxygen species (ROS) generation and DNA damage, and activate NF-κB, ultimately increasing the risk of CRC [58]. In mice, dietary deoxycholic acid induced colonic tumors [85]. Trimethylamine-N-oxide (TMAO) is a microbiota-dependent metabolite from protein, particularly red meat, and a high TMAO level is associated with a higher risk of CRC. Firmicutes species contribute to TMAO production, while Eubacterium limosum has the potential to metabolize TMA precursors and reduce TMAO levels in the gut [78].
It is known that a diet rich in fruits, vegetables, and grains suppresses CRC, and not only high fiber content but also polyphenol content is effective in this regard [83]. Phytochemicals such as epigallocatechin-3-gallate, quercetin, ellagic acid, elicithins, proanthocyanidins, resveratrol, and curcumin can directly or indirectly affect the composition/metabolism of the gut microbiota and regulate gene expression epigenetically [86]. For example, the gut microbiota converts ellagitannins in foods such as pomegranates, strawberries, and nuts to a secondary polyphenol metabolite called urolithins. This urolithin has been shown to have anti-carcinogenic effects in CRC [87]. Thioglucosidases derived from the microbiota can convert glucosinolates found in vegetables such as broccoli and cabbage to isothiocyanates, which have anti-carcinogenic properties and act as HDAC inhibitors. The gut microbiota can conjugate linoleic acid [86]. Conjugated linoleic acid is reported to have anti-carcinogenic properties [88].
Current Definitions and Information of Biotics (Probiotic, Prebiotic, and Postbiotic)
The critical relationship between gut microbiota, dysbiosis, and CRC, which we mentioned above, has pushed researchers toward investigating the effects of probiotics, prebiotics, and postbiotics in the prevention and treatment of CRC [15, 89]. The most current and widely accepted definition of probiotics, as articulated by Hill et al. [90], states that probiotics are live microorganisms that, when administered in adequate amounts, confer a health benefit on the host. The current scientific definition of the prebiotic is “A substrate that is selectively utilized by host microorganisms conferring a health benefit” [91].
The safety profile of probiotics in vulnerable and immunocompromised patients, the risk of developing antibiotic resistance and virulence gene transfer, the existence of problems with maintaining viability and stability during production and storage, and the demonstration that health benefits are not necessarily directly related to viable cells have led researchers to study of non-viable cells, especially heat-killed microorganisms, cell extracts, components, and metabolites [16, 92,93,94]. Before 2021, The International Scientific Association for Probiotics and Prebiotics (ISAPP) definition of postbiotics, researchers named them “cell-free supernatant,” “metabiotic,” “paraprobiotic,” “biogenic,” “ghost probiotics,” “abiotic,” “pseudoprobiotic,” and “postbiotic.” In 2019, ISAPP convened a panel of experts specializing in nutrition, microbial physiology, gastroenterology, pediatrics, food science, and microbiology to review and clarify the definition and scope of postbiotics. As a result of this panel, “the ISAPP consensus statement on the definition and scope of postbiotics” was published in 2021 [17••]. The committee defined a postbiotic as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host.” Postbiotics may contain inanimated microbial cells, and/or cell components, with or without metabolites, that contribute to observed health benefits. Considering the data published by ISAPP, we have summarized the current definitions and important notes regarding probiotics, prebiotics, and postbiotics in Table 1.
ISAPP has determined the following issues regarding postbiotics. “Postbiotics do not need to be derived from a probiotic. The domain of action is not just the gut. Purified microbial metabolites, vaccines, and injections are beyond the scope of postbiotics. The host can include humans, companion animals, livestock and other targets. A postbiotic must be safe for the intended use in the target host and this safety must be evaluated. The procedure for inactivating the microorganism needs to be explained in detail and verified that inactivation of the microorganism has occurred. There must be evidence of health benefits in the host from a controlled, high-quality trial. The composition of the postbiotic preparation should be described in detail. The molecular characterization of progenitor microorganisms must be determined to ensure accurate identification and screening of potential genes of safety concern.” [https://isappscience.org/, Date of Access: 07.10.2023]. It is suggested that postbiotics are more advantageous than probiotics due to their stability in industrial processes and storage, long shelf life, not requiring a cold chain, more reliable profile in terms of health, and not having the risk of developing antibiotic resistance [14, 16, 69, 92,93,94,95,96,97,98].
Postbiotics and CRC
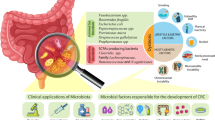
We have collected the types of postbiotics in Fig. 2. The effects of postbiotics in CRC are discussed in detail below.
Techniques such as heat treatment, enzymatic processes, chemical treatments, sonication, hyperbaric conditions, and solvent extraction—either individually or in combination—are applied to inactivate microorganisms to produce postbiotics. Separating the desired postbiotics from the resulting solutions is subjected to steps such as centrifugation, dialysis, lyophilization, and column purification [92•].
Heat-Killed Microorganisms and CRC
Heat-killed microorganisms were accepted as postbiotics by ISAPP in 2021 [17••]. Table 2 provides information about studies examining the relationship between heat-killed bacteria and CRC. In one study, some heat-killed Bifidobacterium and Lactobacillus strains have been shown to induce apoptosis in RKO cells, and administration of L. casei MG4584 and L. reuteri MG5346 (orally) to BALB/c nude mice xenografted with RKO cells significantly has been delaying tumor growth and inducing apoptosis in tumor tissues. Furthermore, when a mixture of L. reuteri MG5346 and L. casei MG4584 (Mix2) and a mixture of B. bifidum MG731, L. reuteri MG5346, and L. casei MG4584 (Mix3) were administered to mice, the tumor inhibitory effect is enhanced, and tumor volume further is decreased [99].
Heat-killed Lactobacillus brevis and Lactobacillus paracasei isolated from the traditional Iranian food “Terxine” have been shown to induce apoptosis in the HT-29 cell line. It has been shown that L. brevis has a greater ability to inhibit the growth of HT-29 cells and induce apoptosis, compared with L. paracasei [100].
Both viable and heat-killed Lactobacillus paracasei MPC2.1 and Lactobacillus GG have been shown to inhibit the growth and proliferation of the human gastric carcinoma HGC-27 and colorectal adenocarcinoma DLD-1 cells. Based on these results, the authors suggested that dead probiotics may also be an effective dietary supplement [101].
Heat-killed cells of Enterococcus faecalis have been shown to effectively reduce the inflammatory activation of NLRP3 (nucleotide-binding-domain- and leucine-rich repeat-containing family, pyrin-domain-containing 3) in macrophages, ameliorating the severity of DSS (dextran sodium sulfate)-induced experimental colitis and the development of colitis-associated CRC [102].
In a xenograft mouse model in which MC38 cells derived from murine colon adenocarcinoma have been inoculated subcutaneously, administration of heat-killed Mycobacterium paragordonae by subcutaneous injection has reduced tumor incidence, tumor progression, and tumor-related mortality. It has induced apoptosis in tumor tissues and the cytotoxic ability of natural killer cells and inhibited tumor progression in a natural killer cell–dependent manner. Moreover, it has been shown that heat-killed M. paragordonae has a synergistic effect with the cancer chemotherapy drug cisplatin. Based on all the above results, the authors have considered that the potential use of heat-killed M. paragordonae as adjunctive immunotherapy can enhance the effect of chemotherapy [103]. In one study, it has been shown that heat-killed Lactobacillus rhamnosus GG remained structurally intact with elongation. Both live and heat-killed L. rhamnosus GG have been shown to induce expression of TNF-α, monocyte chemoattractant protein-1, and IL-1 genes on 5-fluorouracil (5-FU)-induced in vitro mucositis of Caco-2 cells. The authors have proposed that intestinal epithelium may be vulnerable to the post-chemotherapeutic use of live or dead L. rhamnosus GG in 5-FU-induced mucositis [104]. Although it is thought that the use of heat-killed microorganisms instead of probiotics is safer, this seems to be contradictory. More research is needed to elucidate this situation.
The strain, dose, viable and heat-killed state, exopolysaccharide (EPS), and other postbiotics of microorganisms can have different effects on CRC. For this reason, it is necessary to carry out more research on this subject and to provide an adequate database. Viable, heat-killed, and cell-free supernatant (CFS) of Lactobacillus casei 1296-1, 1296-2, and 1296-3 strains showed cytotoxic effects on human colorectal adenocarcinoma cancer Caco2 cells. Moreover, the cytotoxicity effects of live cells on Caco2 cells were found to be significantly higher than on heat-killed cells. The authors suggested that live and CFS of L. casei 1296-2 may be promising candidates for the CRC [105••]. Heat-killed cells and CFS of Lactobacillus plantarum A7 and L. rhamnosus GG strain exhibited anti-proliferative activity on human colon cancer cell lines (Caco-2 and HT-29). In addition, CFS has been shown to further inhibit the growth of cancer cell lines [106]. The authors stated that this higher inhibitory effect of CFS could be attributed to the higher organic acid concentration in the supernatant, and neutralization may also need to be tried in the studies.
Cell Components
Cell components are considered another important type of postbiotic. These may include plasma membrane, cell wall and its components (peptidoglycans), teichoic acid and lipoteichoic acid, surface layers (S-layers) proteins, organelles, flagella, pili, and capsules. Apart from mycoplasmas, both Gram-positive and Gram-negative bacteria have cell wall peptidoglycans that give the characteristic cell shape and provide mechanical protection to the cell. Peptidoglycan gets its name from its two main components: glycan strands made up of repeating disaccharide units and short peptide chains made up of two to five amino acid residues. The negatively charged teichoic acids found in Gram-positive bacteria encompass a diverse family of cell surface glycopolymers containing phosphodiester-linked polyol repeat units. Teichoic acids include both lipoteichoic acids that bind to the bacterial membrane via a glycolipid and wall teichoic acids that covalently bind to peptidoglycan [107, 108]. Table 3 provides information about studies examining the relationship between cell components and CRC.
Monomolecular arrays of protein or glycoprotein subunits that make up the S-layers are one of the most frequently observed prokaryotic cell envelope charges [109]. S-layer protein obtained from Lactobacillus acidophilus CICC 6074 has been shown to induce apoptosis on HT-29 cells and inhibit the phosphatidylinositol 3-kinase (PI3K)/a serine/threonine protein kinase (AKT) pathway and Fas-Ligand synthesis [110].
It has been shown that mucin-binding protein obtained from Lactobacillus casei can inhibit proliferation in HT-29 cells [111]. It has been determined that cytoplasmic extract and cell wall of Lactococcus lactis, and nisin showed antiproliferative effects by reducing cyclin D1 expression in SW480 cells. Nisin has shown the highest antiproliferative effect, followed by cytoplasmic extract and cell wall, respectively [112].
A cell wall extract from Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 has not affected the growth of HT-29 colon cancer cells. However, when combined with cranberry fractions, a significant increase in inhibition rate has been observed. In this study, the authors stated that probiotic components and phenolic compounds in foods may act synergistically in colon cancer [113].
Gavage administration of insoluble glucan extract obtained from Saccharomyces boulardii cell wall in rats on a high-fat, low-fiber diet and treated with 1,2-dimethylhydrazine has been shown to reduce the number of colon abnormal crypt foci, decrease b-glucuronidase activity, and increase nicotinamide adenine dinucleotide phosphate (NADPH):quinone reductase activity in cecum [114].
It has been shown that the Lactobacillus paracasei subsp. paracasei M5 strain obtained from Chinese traditional koumiss has a dose-dependent cytotoxic effect on HT-29 cells of the cell wall, causes G2 phase arrest, alters the mitochondrial membrane potential, activates caspase 3, increases Cyto-C gene expression in the cytosol, increases the expression of BAX- and BCL2-associated agonist of cell death (BAD) genes, and decreases the expression of B-cell lymphoma-extra-large (Bcl-xl) gene [115]. Proteins (12 and 15 kDa) from heat-killed Lactobacillus plantarum L67 have been shown to induce apoptosis in HT-29 cells [116].
Wang et al. [117] obtained a total of 138 Lactobacillus strains from conventional fermented foods and infant feces. They showed that 10 strains had higher anti-proliferative activity and higher adhesion ability on HT-29 cells. They then screened these strains for resistance to acid and bile salts and selected the four most promising strains. The cell walls and cytoplasm extracts of these 4 strains have been shown to cause antiproliferative activity and DNA strand breakage on HT-29 cells. Cell walls extracted from strains X12, M5, and K14 and cytoplasm from strain M5 have been shown to disrupt mitochondrial membrane potential and induce apoptosis in HT-29 cells. However, it was determined to be less harmful to non-cancerous Vero cells than to human colon cancer HT-29 cells.
EPSs and CRC
EPSs are extracellular high-molecular-weight biopolymers composed of sugar residues secreted by a microorganism into the surrounding environment during their growth [119]. EPSs are homopolysaccharides or heteropolysaccharides with different properties in terms of their composition, structural conformation, molecular weight, and functional groups. Microbial EPSs are more cost-effective than polysaccharides of plant and animal origin, and large amounts of EPS can be produced in a short time using low-cost substrates such as microorganism wastes [120].
Besides protecting probiotic organisms against harsh environmental conditions, they also take part in cell recognition and biofilm formation. They play the most prominent role against desiccation, phagocytosis, cell recognition, phage attack, antibiotics or toxic compounds, and osmotic stress. EPS can form a loosely attached layer or be secreted into the extracellular area. In the last few decades, natural polymers have gained much attention among scientific communities owing to their therapeutic potential [121, 122]. EPS is not catabolized by the human digestive system and enters the cecum and colon, where the microbiota ferments EPS to produce beneficial substances, especially SCFA, also lowers pH, inhibits the growth of pathogens, increases the abundance of beneficial bacteria, provides energy for colonic epithelial cells, and increases intestinal barrier function [123]. Studies examining the relationship of EPS with CRC are increasing day by day. Various studies have examined the effect of microbial EPS on CRC (Table 4).
In another study, LP-EPS produced by Lactiplantibacillus plantarum-12 was administered orally daily for 85 days to C57BL/6 mice treated with azoxymethane (AOM)/DSS salt. LP-EPS supplementation increased colon tight junction protein (Claudin-1) expressions, increased goblet cell number, restored crypt structure, significantly decreased serum proinflammatory cytokines TNF-α, IL-8, and IL-1β levels, and increased anti-inflammatory factor IL-10. The authors have suggested that this LP-EPS could be used as a potential active ingredient to alleviate inflammation and colon cancer burden in colon cancer patients [123].
Both viable cells and EPSs of Levilactobacillus brevis LB63, Lactiplantibacillus plantarum GD2, and Lacticaseibacillus rhamnosus E9 bacteria have shown anti-genotoxic, immunomodulatory, and anti-proliferative effects on HT-29. Especially viable LB63 strain and EPS of E9 have been found to have anti-genotoxic activity. It has been found that the effects of EPSs were like viable cells. Based on these results, the authors suggested that live probiotics and EPSs could be natural anti-cancer agents for CRC [124].
Oral administration of EPS obtained from Lactobacillus acidophilus on rats that developed 1,2-dimethyl hydrazine–induced colon cancer decreased the number of polyps, restored the levels of antioxidative enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and vitamin C to near normal levels, which decreased in the colon during carcinogenesis [125].
It has been shown that EPS1-1 isolated from Rhizopus nigricans induced apoptosis in murine colon cancer CT26 cells and tumor tissues of CRC mice treated with AOM/DSS. Based on these findings, the researchers have suggested that EPS1-1 may be a potential anti-CRC drug [126]. In the study of Saadat et al. [128], EPSs from Kluyveromyces marxianus and Pichia kudriavzevii were applied to colon cancer cell lines (SW-480, HT-29, HCT-116) and normal cell line (KDR/293). EPS application has reduced cell viability in colon cancer cell lines, induced apoptosis, inhibited the proliferation of cancer cells like 5-FU, and increased the expression of proapoptotic genes (BAX, caspase-3–8) and anti-apoptotic gene Bcl-2 and the expression of AKT-1, Janus kinase 1, and mTOR genes. A lower cytotoxic effect has been seen in KDR/293 cells compared to 5-FU.
In one study, EPSs have been obtained from 4 strains isolated from healthy infant feces (Lactobacillus plantarum GD2, L. rhamnosus E9, L. brevis LB63) and yogurt (L. delbrueckii ssp. bulgaricus B3). These EPSs have been shown to inhibit proliferation and induce apoptosis in HT-29 cells. EPS produced from L. delbrueckii ssp. bulgaricus B3 has had the highest mannose and lowest glucose and this EPS showed the highest induction of apoptosis. Researchers have demonstrated a relationship between the ability of EPSs to induce apoptosis and the composition of mannose and glucose [129].
MSR101, EPS produced by Lactobacillus kefiri isolated from Chinese kefir grains, has been shown to have an anticancer effect on HT-29 cells and up-regulate the expression of BAX, BAD, and caspase3-8-9 and down-regulate BCl-2 [130].
EPS obtained from Lactobacillus casei M5, L. casei SB27, L. casei × 12, and L. casei K11, especially acidic EPS produced by L. casei SB27, induced apoptosis via caspase-3 activation, and they have been not toxic effects in Vero cells [131]. LA-EPS-20079 produced by L. acidophilus 20,079 has been shown to inhibit cell viability, induce apoptosis (via sub G0/G1 cell cycle arrest in the cell cycle), and up-regulate the expression of IkappaB kinase alpha, P53, and transforming growth factor in Caco-2 cells [132].
It has been shown that cell-bound EPS produced by Lactobacillus plantarum 70810 significantly inhibits proliferation in HepG-2, BGC-823, and especially HT-29 tumor cells. In addition, the authors showed that EPS production method differences may affect EPS yield [133]. The effects of L. casei 01 on HT-29 and intestine-407 cell proliferation, including its heat-inactivated form, cell wall, intracellular extracts, and EPSs, have been investigated. The highest antiproliferation activity was demonstrated by EPSs. It has also been shown that these EPSs reduce the cytotoxic effect of 4-nitroquinoline-1-oxide on intestinal-407 cells [134].
Other Substances Produced by Microorganisms, Not Included in the Definition of Postbiotic, but May Be Present in Postbiotics, and Their Relationships with CRC
Postbiotics must include inanimate microorganisms and/or cell components. These substances may or may not contain metabolites. However, it is essential to note that purified metabolites are not considered postbiotic [17••]. In this part of our review, we focus on CFS and metabolites (enzymes, bacteriocins, SCFA, and others) produced by microorganisms that can be found in postbiotics, although they are no longer considered postbiotics and their relationship with CRC.
CFS and CRC
CFS is obtained from bacterial cultures after the incubation step, by centrifuging the culture medium, removing the pellet, and filtering the supernatant. CFS contains metabolites from microbial growth and residual nutrients of the medium used. It is a complex of enzymes, proteins, SCFA, vitamins, surfactants, amino acids, peptides, organic acids, and metabolic products secreted into CFS [18, 135]. A summary of studies showing the effects of CFSs on CRC is given in Table 5.
Salemi et al. [136] showed that the CFS of Lactobacillus rhamnosus GG reduced the viability of HCT-116, Caco-2, HT-29 (human colon cancer cell lines), and A375 (malignant melanoma cell line). CFS has been shown a positive synergistic effect by sensitizing cancer cells to both 5-FU and irinotecan. The authors have indicated that they propose CFS of L. rhamnosus GG as an ideal candidate for improving therapeutic response in cancer patients.
CFSs and cell walls of Lacticaseibacillus paracasei SD1, L. rhamnosus SD4, SD11, and GG inhibited the release of IL-1β, IL-6, IL-8, and TNFα; stimulated the expression of human β-defensin -2, -4, and IL-10; and inhibited growth in Caco-2 cells [137].
In a study, CFSs have been prepared from 100 gut microorganisms isolated from human feces, and 21 of them have been shown to have antiproliferative effects in HCT-116 cells. Among these CFSs, the CFS of Odoribacter splanchnicus has been identified as exhibiting the highest anti-cancer activity in both HCT-116 and CT 26 mice colon cancer cells. Active molecule believed to be present in this CFS has been suggested to be malic acid. Additionally, in the CRC mice allograft model, peri-tumoral injection of this CFS has demonstrated a reduction in tumor growth, volume, and weight [138].
It has been shown that the heat-killed (100 °C for 2 h) sonicated fraction and CFS of Lactobacillus reuteri reduced the invasion and induced apoptosis in HT29-ShE cells. It has been suggested that these effects arise from secretory macromolecules such as polysaccharides, nucleic acids, or proteins [139].
CFS of Lactobacillus fermentum induces apoptosis in three-dimensional (3D) spheroids of HT-29, DLD1, and WiDr; increases BAX, BAK (Bcl-2 homologous antagonist/killer), and BH3-containing mitochondrial protein; decreases PARP (poly ADP ribose polymerase)-1 and BCL-XL expressions; and inhibits NFκB [140].
Among the CFSs of 60 Lactobacillus strains isolated from different sources, it has been determined that the CFS with the highest antigenotoxicity and cytotoxicity on Caco-2 and HT-29 cells belonged to L. rhamnosus MD14. As a result of the physicochemical characterization of CFS, the presence of heat-sensitive organic acids and proteins has been demonstrated [141]. Yue et al. [142] have found that CFSs of Lactobacillus rhamnosus GG, L. casei M3, and L. plantarum YYC-3 inhibit growth, invasion, migration, and cell metastasis in Caco2 and HT-29 cells. Moreover, they have determined that this inhibitory effect of all CFSs was like that of 5-FU.
It has been shown that oral administration of both the Lactobacillus plantarum YYC-3 strain and its CFS to APCMin/+ mice fed a high-fat diet prevented the formation of colon tumors and mucosal damage, modulated the immune system, reduced the infiltration of inflammatory cells, and improved the gut dysbiosis [143].
It has been shown that CFS of Lactobacillus plantarum inhibits proliferation, Wnt/β-catenin pathway, and colonosphere formation in HT-29 and HCT-116 cells resistant to 5-FU chemotherapy and increases apoptosis and caspase-3 activation when applied together with 5-FU [144].
Nami et al. [145] showed that CFS of Enterococcus lactis IW5 from the human intestine strongly decreased the viability of different cancer cells such as HeLa, MCF-7, AGS, HT-29, and Caco-2 and did not prevent the viability of normal FHs-74 cells.
CFS of L. casei and L. rhamnosus has been determined to reduce the invasion of HCT-116 cells. It has been suggested that the effective compound may be a macromolecule such as a protein, nucleic acid, or polysaccharide [146].
Metabolites
Plantarisin BM-1, a bacteriocin produced by Lactobacillus plantarum, significantly reduces the viability of SW480, Caco-2, and HCT-116 CRC cells, with a more pronounced impact on SW480 [147]. In a study that screened 16,000 bacterial clones from human fecal samples, salivaricin A5 and salivaricin B 243, the bacteriocins of Streptococcus salivarius, were shown to have selective antimicrobial activity against pro-tumorigenic Fusobacterium nucleatum strains and are a biotherapeutic that can target pathogens causing CRC [148]. Reuterin produced by Lactobacillus reuteri has been shown to inhibit colon tumor growth in both in vitro and in vivo settings by modifying the redox balance, inducing protein oxidation, and inhibiting ribosomal biogenesis [149]. Indole-3-lactic acid produced by Lactobacillus gallinarum has significantly reduced tumor number and size in APC Min/+ mice [150].
Mice receiving orally catalase-producing Lactococcus lactis have been shown to have significantly less colonic damage and inflammation and prevent tumorigenesis compared to animals not receiving catalase-producing L. lactis or bacterial supplementation in an experimental DMH-induced colon cancer mouse model [151]. It has been reported that β-galactosidase secreted by Streptococcus thermophilus can prevent colon tumor formation in mice injected with APC min/+ and AOM [152].
It has been shown that SCFA obtained from L. reuteri induces apoptosis in HT-29 cells [153]. Among the SCFAs, butyrate is the most potent in inhibiting HDAC activities both in vitro and in vivo [154]. Butyrate inhibits glucose uptake and membrane abundance of glucose transporter 1 (GLUT-1) in HCT116 and LoVo cells, decreases AKT phosphorylation, and decreases the expressions of ribose-5-phosphate, acetyl-CoA, NADPH, and glucose-6-phosphate dehydrogenase. It has been determined that it inhibits DNA synthesis and has a synergistic effect with 5-FU [155]. Butyrate has been shown to inhibit the invasion of HCT116, HT29, LOVO, and HCT8 CRC cells [156]. In the AOM/DSS-induced CRC mouse model, gavage-ingested butyrate has been reported to modulate the microbiota, attenuate weight loss, disease activity index, and survival and inhibit tumor number and progression [157]. Sodium butyrate has been shown to induce apoptosis in HT29 cells [158]. Butyrate has been shown to increase ROS levels, inhibit growth, decrease cyclin D2 protein levels, and increase P21 and cleaved PARP levels in LT97 and HT29 colon cells [159]. It has been shown that acetate reduces proliferation, decreases glycolysis, and increases oxygen consumption and ROS levels in HT29 and HCT116 colon cancer cells [160].
Future Perspectives
Postbiotics are still in their infancy and more studies need to be done in the future. Some of those: Inactivation steps and techniques of microorganisms should be standardized. The composition of the postbiotic product should be defined. Necessary methods for measuring the amount of postbiotics should be determined and developed. It should not be ignored that lipopolysaccharides present in Gram-negative bacteria can cause sepsis and toxic shock. Attention should be paid to product contamination. The effects of probiotics and postbiotics should be compared. It should be noted that different strains of the same bacteria can produce different postbiotics. A postbiotic can be a complex mixture, but what is responsible for the beneficial effects needs to be determined. The required dose should be determined. Bioavailability and bioaccessibility should be evaluated. How to evaluate the degree of microbial cell disruption after inactivation should be determined. Randomized controlled large-scale clinical studies should be conducted to demonstrate the clinical effects of postbiotics. It should be investigated whether existing postbiotic metabolites may enter the feedback loop and disrupt endogenous production [14, 69, 93,94,95,96,97,98].
Conclusion
The microbiota uses dietary and gastrointestinal system factors in two different ways when dysbiosis is present and absent. Diet alters the microbiota and its metabolites. The link between diet, gut microbiota, and CRC has been established primarily as a relationship rather than a cause-effect relationship. It has been shown that various postbiotics can selectively induce apoptosis in CRC, prevent cell proliferation, growth, invasion, and migration, modulate the immune system, suppress carcinogenic signaling pathways, maintain intestinal epithelial integrity, and have a synergistic effect with chemotherapy drugs. However, it has been reported that some postbiotics are ineffective and may be risky regarding safety profile in some patients. Furthermore, there is insufficient information regarding the necessary dosage and whether it may induce a negative feedback effect in the body, inhibiting endogenous production, bioavailability, and bioaccessibility. Although numerous preclinical studies demonstrate the promising role of postbiotics in the prevention and treatment of CRC, clinical evidence remains scarce and is currently in its early stages. Therefore, there is a necessity for large-scale, randomized, double-blind clinical studies. Considering that not only bacteria but also mycobiota, virobiota, and archaeabiota contribute to both eubiosis and dysbiosis, postbiotic research based on these elements is anticipated to expand in the future.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- 5-FU:
-
5-Fluorouracil
- AKT:
-
A serine/threonine protein kinase
- AOM:
-
Azoxymethane
- APC:
-
Adenomatous polyposis coli
- BAD:
-
BCL2-associated agonist of cell death
- BAK:
-
Bcl-2 homologous antagonist/killer
- BAX:
-
Bcl-2-associated X protein
- Bcl-xl:
-
B-cell lymphoma-extra-large
- Bcl-2:
-
B-cell lymphoma 2
- CFS:
-
Cell-free supernatant
- CIMP:
-
CpG island methylator phenotype
- CIN:
-
Chromosomal instability
- CRC:
-
Colorectal cancer
- DCC:
-
Deleted in colorectal cancer
- DSS:
-
Dextran sodium sulfate
- EPS:
-
Exopolysaccharides
- GLUT-1:
-
Glucose transporter-1
- HDAC:
-
Histone deacetylase
- IL:
-
Interleukin
- MSI:
-
Microsatellite instability
- ISAPP:
-
International Scientific Association for Probiotics and Prebiotics
- SMAD2:
-
Mothers Against Decapentaplegic homolog 2
- K-ras:
-
Kirsten rat sarcoma virus
- MAPK:
-
Mitogen-activated protein kinase
- MyD88:
-
Myeloid differentiation primary response protein 88
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NF-κB:
-
Nuclear factor kappa B
- NLRP3:
-
Nucleotide-binding-domain- and leucine-rich repeat-containing family, pyrin-domain-containing 3
- OTUs:
-
Operational taxonomic units
- PARP:
-
Poly ADP ribose polymerase
- PI3K:
-
Phosphatidylinositol 3-kinase
- RAS:
-
Rat sarcoma virus
- ROS:
-
Reactive oxygen species
- SCFAs:
-
Short-chain fatty acids
- S-layers:
-
Surface layers
- SMO:
-
Smoothened
- TMAO:
-
Trimethylamine-N-oxide
- TLR-4:
-
Toll-like receptor-4
- TNF-α:
-
Tumor necrosis factor alpha
- Wnt:
-
Wingless-related integration site
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hong SN. Genetic and epigenetic alterations of colorectal cancer. Intest Res. 2018. https://doi.org/10.5217/ir.2018.16.3.327.
Marmol I, Sánchez-de-Diego C, Dieste AP, Cerrada E, Yoldi MJR. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017. https://doi.org/10.3390/ijms18010197.
Hanus M, Parada-Venegas D, Landskron G, Wielandt AM, Hurtado C, Alvarez K, Hermoso MA, López-Köstner F, De la Fuente M. Immune system, microbiota, and microbial metabolites: the unresolved triad in colorectal cancer microenvironment. Front Immunol. 2021. https://doi.org/10.3389/fimmu.2021.612826.
Wells K, Wise PE. Hereditary colorectal cancer syndromes. Surg Clin North Am. 2017. https://doi.org/10.1016/j.suc.2017.01.009.
Okugawa Y, Grady WM, Goel A. Epigenetic alterations in colorectal cancer: emerging biomarkers. Gastroenterology. 2015. https://doi.org/10.1053/j.gastro.2015.07.011.
Lewandowska A, Rudzki G, Lewandowski T, Stryjkowska-Góra A, Rudzki S. Risk factors for the diagnosis of colorectal cancer. Cancer Control. 2022. https://doi.org/10.1177/10732748211056692.
Parmar S, Easwaran H. Genetic and epigenetic dependencies in colorectal cancer development. Gastroenterol Rep. 2022. https://doi.org/10.1093/gastro/goac035.
Testa U, Pelosi E, Castelli G. Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel). 2018. https://doi.org/10.3390/medsci6020031.
Granados-Romero JJ, Valderrama-Treviño AI, Contreras-Flores EH, Barrera-Mera B, Enríquez MH, Uriarte-Ruíz K, Ceballos-Villalba JC, Estrada-Mata AG, Alvarado Rodríguez C, Arauz-Peña G. Colorectal cancer: a review. Int J Res Med Sci. 2017. https://doi.org/10.18203/2320-6012.ijrms20174914.
Hossain MS, Kader MA, Goh KW, Islam M, Khan MS, Harun-Ar Rashid M, Ooi J, Melo Coutinho HD, Al-Worafi YM, Moshawih S, Lim YC, Kibria KMK, Ming LC. Herb and spices in colorectal cancer prevention and treatment: a narrative review. Front Pharmacol. 2022. https://doi.org/10.3389/fphar.2022.865801.
Murphy N, Moreno V, Hughes DJ, Vodicka L, Vodicka P, Aglago EK, Gunter MJ, Jenab M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol Aspects Med. 2019. https://doi.org/10.1016/j.mam.2019.06.005.
Yang J, Yu J. The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell. 2018. https://doi.org/10.1007/s13238-018-0543-6.
Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, Shilo S, Lador D, Vila AV, Zmora N, Pevsner-Fischer M, Israeli D, Kosower N, Malka G, Wolf BC, Avnit-Sagi T, Lotan-Pompan M, Weinberger A, Halpern Z, Carmi S, Fu J, Wijmenga C, Zhernakova A, Elinav E, Segal E. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018. https://doi.org/10.1038/nature25973.
Song D, Wang X, Ma Y, Liu NN, Wang H. Beneficial insights into postbiotics against colorectal cancer. Front Nutr. 2023. https://doi.org/10.3389/fnut.2023.1111872.
Dahiya D, Nigam PS. The gut microbiota influenced by the intake of probiotics and functional foods with prebiotics can sustain wellness and alleviate certain ailments like gut-inflammation and colon-cancer. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10030665.
Pique N, Berlanga M, Minana-Galbis D. Health benefits of heat-killed (tyndallized) probiotics: an overview. Int J Mol Sci. 2019. https://doi.org/10.3390/ijms20102534.
•• Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, Sanders ME, Shamir R, Swann JR, Szajevska H, Vinderola G. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. 2021. https://doi.org/10.1038/s41575-021-00440-6. This article establishes consistency, common ground, and clarity for the definition of postbiotics.
Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-a step beyond pre- and probiotics. Nutrients. 2020. https://doi.org/10.3390/nu12082189.
Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019. https://doi.org/10.3390/nu11010164.
Abulimiti A, Zhang X, Shivappa N, Hébert JR, Fang YJ, Huang CY, Feng XL, Chen YM, Zhang CX. The dietary inflammatory index is positively associated with colorectal cancer risk in a Chinese case-control study. Nutrients. 2020. https://doi.org/10.3390/nu12010232.
Benninghoff AD, Hintze KJ, Monsanto SP, Rodriguez DM, Hunter AH, Phatak S, Pestka JJ, Wettere AJV, Ward RE. Consumption of the total Western diet promotes colitis and inflammation-associated colorectal cancer in mice. Nutrients. 2020. https://doi.org/10.3390/nu12020544.
Zhang FF, Cudhea F, Shan Z, Michaud DS, Imamura F, Eom H, Ruan M, Rehm CD, Liu J, Du M, Kim D, Lizewski L, Wilde P, Mozaffarian D. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. 2019. https://doi.org/10.1093/jncics/pkz034.
Bradbury KE, Murphy N, Key TJ. Diet and colorectal cancer in UK Biobank: a prospective study. Int J Epidemiol. 2020. https://doi.org/10.1093/ije/dyz064.
Diakité MT, Diakité B, Koné A, Balam S, Fofana D, Diallo D, Kassogué Y, Traoré CB, Kamaté B, Ba D, Ly M, Ba M, Koné B, Maiga AI, Achenbach C, Holl J, Murphy R, Hou L, Maiga M. Relationships between gut microbiota, red meat consumption and colorectal cancer. J Carcinog Mutagen. 2022;13:1000385.
Zhu Y, Wu H, Wang PP, Savas S, Woodrow J, Wish T, Jin R, Green R, Woods M, Roebothan B, Buehler S, Dicks E, McLaughlin JR, Campbell PT, Parfrey PS. Dietary patterns and colorectal cancer recurrence and survival: a cohort study. BMJ Open. 2013. https://doi.org/10.1136/bmjopen-2012-002270.
Humphreys KJ, Conlon MA, Young GP, Topping DL, Hu Y, Winter JM, Bird AR, Cobiac L, Kennedy NA, Michael MZ, Le Leu RK. Dietary manipulation of oncogenic microRNA expression in human rectal mucosa: a randomized trial. Cancer Prev Res (Phila). 2014. https://doi.org/10.1158/1940-6207.CAPR-14-0053.
Wang L, Du M, Wang K, Khandpur N, Rossato SL, Drouin-Chartier J, Steele EM, Giovannucci E, Song M, Zhang FF. Association of ultra-processed food consumption with colorectal cancer risk among men and women: results from three prospective US cohort studies. BMJ. 2022. https://doi.org/10.1136/bmj-201-068921.
Wang F, Ugai T, Haruki K, Wan Y, Akimoto N, Arima K, Zhong R, Twombly TS, Wu K, Yin K, Chan AT, Giannakis M, Nowak JA, Meyerhardt JA, Liang L, Song M, Smith-Warner SA, Zhang X, Giovannucci EL, Willett WC, Ogino S. Healthy and unhealthy plant-based diets in relation to the incidence of colorectal cancer overall and by molecular subtypes. Clin Transl Med. 2022. https://doi.org/10.1002/ctm2.893.
Bultman SJ. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food Res. 2017. https://doi.org/10.1002/mnfr.201500902.
Song M, Wu K, Meyerhardt JA, Ogino S, Wang M, Fuchs CS, Giovannucci EL, Chan AT. Fiber intake and survival after colorectal cancer diagnosis. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2017.3684.
Yu XF, Zou J, Dong J. Fish consumption and risk of gastrointestinal cancers: a meta-analysis of cohort studies. World J Gastroenterol. 2014. https://doi.org/10.3748/wjg.v20.i41.15398.
Kim Y, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, Kim J. The association between coffee consumption and risk of colorectal cancer in a Korean population. Nutrients. 2021. https://doi.org/10.3390/nu13082753.
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Sig Transduct Target Ther. 2022. https://doi.org/10.1038/s41392-022-00974-4.
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019. https://doi.org/10.3390/microorganisms7010014.
Kapitan M, Niemiec MJ, Steimle A, Frick JS, Jacobsen ID. Fungi as part of the microbiota and interactions with intestinal bacteria. Curr Top Microbiol Immunol. 2019. https://doi.org/10.1007/82_2018_117.
• Chibani CM, Mahnert A, Borrel G, Almeida A, Werner A, Brugère JF, Gribaldo S, Finn RD, Schmitz RA, Moissl-Eichinger C. A catalogue of 1,167 genomes from the human gut archaeome. Nat Microbiol. 2022. https://doi.org/10.1038/s41564-021-01020-9. This article is important in terms of illuminating the archaeal members of the microbiota. Most current studies have focused on gut bacteria and neglected the mycobiota, archaeobiota, and virobiota.
Mafra D, Ribeiro M, Fonseca L, Regis B, Cardozo LFMF. Fragoso Dos Santos H, Emiliano de Jesus H, Schultz J, Shiels PG, Stenvinkel P, Rosado A. Archaea from the gut microbiota of humans: could be linked to chronic diseases? Anaerobe. 2022. https://doi.org/10.1016/j.anaerobe.2022.102629.
Zuo T. Unveiling the gut virome in human health and diseases. Int J Clin Virol. 2018. https://doi.org/10.29328/journal.ijcv.1001002.
Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, Ajami NJ, Petrosino JF. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 2017. https://doi.org/10.1186/s40168-017-0373-4.
Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, Arp K, Watson RL, Sanders EAM, Fuentes S. Debby Bogaert Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-13014-7.
Cuna A, Morowitz MJ, Ahmed I, Umar S, Sampath V. Dynamics of the preterm gut microbiome in health and disease. Am J Physiol Gastrointest Liver Physiol. 2021. https://doi.org/10.1152/ajpgi.00399.2020.
Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, Zhuo N, Wang H, Wang L, Wu D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: a study of 91 term infants. Sci Rep. 2020. https://doi.org/10.1038/s41598-020-72635-x.
Tun HM, Konya T, Takaro TK, Brook JR, Chari R, Field CJ, Guttman DS, Becker AB, Mandhane PJ, Turvey SE, Subbarao P, Sears MR, Scott JA, Kozyrskyj AL. CHILD Study. Investigators Exposure to household furry pets influences the gut microbiota of infant at 3–4 months following various birth scenarios. Microbiome. 2017. https://doi.org/10.1186/s40168-017-0254-x.
Patangia DV, Anthony Ryan C, Dempsey E, Paul Ross R, Stanton C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen. 2022. https://doi.org/10.1002/mbo3.1260.
Bruno G, Zaccari P, Rocco G, Scalese G, Panetta C, Porowska B, Pontone S, Severi C. Proton pump inhibitors and dysbiosis: current knowledge and aspects to be clarified. World J Gastroenterol. 2019. https://doi.org/10.3748/wjg.v25.i22.2706.
Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017. https://doi.org/10.1016/j.ynstr.2017.03.001.
Lin R, Zhang Y, Chen L, Qi Y, He J, Hu M, Zhang Y, Fan L, Yang T, Wang L, Si M, Chen S. The effects of cigarettes and alcohol on intestinal microbiota in healthy men. J Microbiol. 2020. https://doi.org/10.1007/s12275-020-0006-7.
Das B, Ghosh TS, Kedia S, Rampal R, Saxena S, Bag S, Mitra R, Dayal M, Mehta O, Surendranath A, Travis SPL, Tripathi P, Nair GB, Ahuja V. Analysis of the gut microbiome of rural and urban healthy Indians living in sea level and high altitude areas. Sci Rep. 2018. https://doi.org/10.1038/s41598-018-28550-3.
Dziewiecka H, Buttar HS, Kasperska A, Ostapiuk-Karolczuk J, Domagalska M, Cichoń J, Skarpańska-Stejnborn A. Physical activity induced alterations of gut microbiota in humans: a systematic review. BMC Sports Sci Med Rehabil. 2022. https://doi.org/10.1186/s13102-022-00513-2.
Bhatt AP, Redinbo MR, Bultman SJ. The role of the microbiome in cancer development and therapy. CA Cancer J Clin. 2017. https://doi.org/10.3322/caac.21398.
Oliphan K, Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019. https://doi.org/10.1186/s40168-019-0704-8.
Zhang Y, Chen R, Zhang D, Qi S, Liu Y. Metabolite interactions between host and microbiota during health and disease: which feeds the other? Biomed Pharmacother. 2023. https://doi.org/10.1016/j.biopha.2023.114295.
Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016. https://doi.org/10.1080/19490976.2015.1134082.
Rowland I, Gibson G, Heinken A, Scott K, Swann J, Thiele I, Tuohy K. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018. https://doi.org/10.1007/s00394-017-1445-8.
Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023. https://doi.org/10.1016/j.ebiom.2023.104527.
Chen M, Lin W, Li N, Wang Q, Zhu S, Zeng A, Song L. Therapeutic approaches to colorectal cancer via strategies based on modulation of gut microbiota. Front Microbiol. 2022. https://doi.org/10.3389/fmicb.2022.945533.
Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020. https://doi.org/10.1038/s41422-020-0332-7.
Pandey H, Tang DWT, Wong SH, Lal D. Gut microbiota in colorectal cancer: biological role and therapeutic opportunities. Cancers (Basel). 2023. https://doi.org/10.3390/cancers15030866.
Liang Q, Chiu J, Chen Y, Huang Y, Higashimori A, Fang J, Brim H, Ashktorab H, Ng SC, Ng SSM, Zheng S, Chan FKL, Sung JJY, Yu J. Fecal bacteria act as novel biomarkers for noninvasive diagnosis of colorectal cancer. Clin Cancer Res. 2017. https://doi.org/10.1158/1078-0432.CCR-16-1599.
Dai Z, Coker OO, Nakatsu G, Wu WKK, Zhao L, Chen Z, Chan FKL, Kristiansen K, Sung JJY, Wong SH, Yu J. Multi-cohort analysis of colorectal cancer metagenome identified altered bacteria across populations and universal bacterial markers. Microbiome. 2018. https://doi.org/10.1186/s40168-018-0451-2.
Gao R, Kong C, Li H, Huang L, Qu X, Qin N, Qin H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur J Clin Microbiol Infect Dis. 2017. https://doi.org/10.1007/s10096-017-3085-6.
Luan C, Xie L, Yang X, Miao H, Lv N, Zhang R, Xiao X, Hu Y, Liu Y, Wu N, Zhu Y, Zhu B. Dysbiosis of fungal microbiota in the intestinal mucosa of patients with colorectal adenomas. Sci Rep. 2015. https://doi.org/10.1038/srep07980.
Coker OO, Nakatsu G, Dai RZ, Wu WKK, Wong SH, Ng SC, Chan FKL, Sung JJY, Yu J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut. 2019. https://doi.org/10.1136/gutjnl-2018-317178.
Coker OO, Wu WKK, Wong SH, Sung JJY, Yu J. Altered gut archaea composition and interaction with bacteria are associated with colorectal cancer. Gastroenterology. 2020. https://doi.org/10.1053/j.gastro.2020.06.042.
Nakatsu G, Zhou H, Wu WKK, Wong SH, Coker OO, Dai Z, Li X, Szeto CH, Sugimura N, Lam TY, Yu AC, Wang X, Chen Z, Wong MC, Ng SC, Chan MTV, Chan PKS, Chan FKL, Sung JJ, Yu J. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology. 2018. https://doi.org/10.1053/j.gastro.2018.04.018.
Drewes JL, Housseau F, Sears CL. Sporadic colorectal cancer: microbial contributors to disease prevention, development and therapy. Br J Cancer. 2016. https://doi.org/10.1038/bjc.2016.189.
Yang Y, Misra BB, Liang L, Bi D, Weng W, Wu W, Cai S, Qin H, Goel A, Li X, Ma Y. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics. 2019. https://doi.org/10.7150/thno.35186.
Tjalsma H, Boleij A, Marchesi JR, Dutilh BE. A bacterial driver-passenger model for colorectal cancer: beyond the usual suspects. Nat Rev Microbiol. 2012. https://doi.org/10.1038/nrmicro2819.
Kvakova M, Kamlarova A, Stofilova J, Benetinova V, Bertkova I. Probiotics and postbiotics in colorectal cancer: prevention and complementary therapy. World J Gastroenterol. 2022. https://doi.org/10.3748/wjg.v28.i27.3370.
Sanapareddy N, Legge RM, Jovov B, McCoy A, Burcal L, Araujo-Perez F, Randall TA, Galanko J, Benson A, Sandler RS, Rawls JF, Abdo Z, Fodor AA, Keku TO. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012. https://doi.org/10.1038/ismej.2012.43.
Sinha R, Ahn J, Sampson JN, Shi J, Yu G, Xiong X, Hayes RB, Goedert JJ. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLoS ONE. 2016. https://doi.org/10.1371/journal.pone.0152126.
Colov EP, Degett TH, Raskov H, Gögenur I. The impact of the gut microbiota on prognosis after surgery for colorectal cancer-a systematic review and meta-analysis. APMIS. 2020. https://doi.org/10.1111/apm.13032.
Tabowei G, Gaddipati GN, Mukhtar M, Alzubaidee MJ, Dwarampudi RS, Mathew S, Bichenapally S, Khachatryan V, Muazzam A, Hamal C, Velugoti LSDR, Mohammed L. Microbiota dysbiosis a cause of colorectal cancer or not? A systematic review Cureus. 2022. https://doi.org/10.7759/cureus.30893.
Villar-Ortega P, Expósito-Ruiz M, Gutiérrez-Soto M, Ruiz-Cabello JM, Navarro-Marí JM, Gutiérrez-Fernández J. The association between Fusobacterium nucleatum and cancer colorectal: a systematic review and meta-analysis. Enferm Infecc Microbiol Clin (Engl Ed). 2022. https://doi.org/10.1016/j.eimce.2022.02.007.
Drewes JL, White JR, Dejea CM, Fathi P, Iyadorai T, Vadivelu J, Roslani AC, Wick EC, Mongodin EF, Loke MF, Thulasi K, Gan HM, Goh KL, Chong HY, Kumar S, Wanyiri JW, Sears CL. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017. https://doi.org/10.1038/s41522-017-0040-3.
Li S, Liu J, Zheng X, Ren L, Yang Y, Li W, Fu W, Wang J, Du G. Tumorigenic bacteria in colorectal cancer: mechanisms and treatments. Cancer Biol Med. 2021. https://doi.org/10.20892/j.issn.2095-3941.2020.0651.
Wan G, Xie M, Yu H, Chen H. Intestinal dysbacteriosis activates tumor-associated macrophages to promote epithelial-mesenchymal transition of colorectal cancer. Innate Immun. 2018. https://doi.org/10.1177/1753425918801496.
Xing C, Du Y, Duan T, Nim K, Chu J, Wang HY, Wang RF. Interaction between microbiota and immunity and its implication in colorectal cancer. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.963819.
Ecklu-Mensah G, Gilbert J, Devkota S. Dietary selection pressures and their impact on the gut microbiome. Cell Mol Gastroenterol Hepatol. 2022. https://doi.org/10.1016/j.jcmgh.2021.07.009.
Loke YL, Chew MT, Ngeow YF, Lim WWD, Peh SC. Colon carcinogenesis: the interplay between diet and gut microbiota. Front Cell Infect Microbiol. 2020. https://doi.org/10.3389/fcimb.2020.603086.
Bultman SJ. The microbiome and its potential as a cancer preventive intervention. Semin Oncol. 2016. https://doi.org/10.1053/j.seminoncol.2015.09.001.
Alvandi E, Wong WKM, Joglekar MV, Spring KJ, Hardikar AA. Short-chain fatty acid concentrations in the incidence and risk-stratification of colorectal cancer: a systematic review and meta-analysis. BMC Med. 2022. https://doi.org/10.1186/s12916-022-02529-4.
O’Keefe SJD. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016. https://doi.org/10.1038/nrgastro.2016.165.
Sun D, Chen Y, Fang J-Y. Influence of the microbiota on epigenetics in colorectal cancer. Natl Sci Rev. 2019. https://doi.org/10.1093/nsr/nwy160.
Bernstein C, Holubec H, Bhattacharyya AK, Nguyen H, Payne CM, Zaitlin B, Bernstein H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch Toxicol. 2011. https://doi.org/10.1007/s00204-011-0648-7.
Del Cornò M, Donninelli G, Conti L, Gessani S. Linking diet to colorectal cancer: the emerging role of microRNA in the communication between plant and animal kingdoms. Front Microbiol. 2017. https://doi.org/10.3389/fmicb.2017.00597.
Al-Harbi SA, Abdulrahman AO, Zamzami MA, Khan MI. Urolithins: the gut based polyphenol metabolites of ellagitannins in cancer prevention, a review. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.647582.
Dachev M, Bryndová J, Jakubek M, Moučka Z, Urban M. The effects of conjugated linoleic acids on cancer. Processes. 2021. https://doi.org/10.3390/pr9030454.
Wierzbicka A, Mańkowska-Wierzbicka D, Mardas M, Stelmach-Mardas M. Role of probiotics in modulating human gut microbiota populations and activities in patients with colorectal cancer-a systematic review of clinical trials. Nutrients. 2021. https://doi.org/10.3390/nu13041160.
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014. https://doi.org/10.1038/nrgastro.2014.66.
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G. Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017. https://doi.org/10.1038/nrgastro.2017.75.
• Liang B, Xing D. The current and future perspectives of postbiotics. Probiotics Antimicrob Proteins. 2023. https://doi.org/10.1007/s12602-023-10045-x. This article provides detailed information about the current and future market of postbiotics.
Thorakkattu P, Khanashyam AC, Shah K, Babu KS, Mundanat AS, Deliephan A, Deokar GS, Santivarangkna C. Nirmal NP Postbiotics: current trends in food and pharmaceutical industry. Foods. 2022. https://doi.org/10.3390/foods11193094.
Nataraj BH, Ali SA. Behare PV Yadav H Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact. 2020. https://doi.org/10.1186/s12934-020-01426-w.
Vinderola G, Sanders ME, Salminen S. The concept of postbiotics. Foods. 2022. https://doi.org/10.3390/foods11081077.
Scott E, De Paepe K, Van de Wiele T. Postbiotics and their health modulatory biomolecules. Biomolecules. 2022. https://doi.org/10.3390/biom12111640.
Jastrząb R, Graczyk D, Siedlecki P. Molecular and cellular mechanisms influenced by postbiotics. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms222413475.
Doron S, Snydman DR. Risk and safety of probiotics. Clin Infect Dis. 2015. https://doi.org/10.1093/cid/civ085.
Kim SJ, Kang CH, Kim GH, Cho H. Anti-tumor effects of heat-killed L. reuteri MG5346 and L. casei MG4584 against human colorectal carcinoma through caspase-9-dependent apoptosis in xenograft model. Microorganisms. 2022. https://doi.org/10.3390/microorganisms10030533.
Karimi Ardestani S, Tafvizi F, Tajabadi EM. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum Exp Toxicol. 2019. https://doi.org/10.1177/0960327119851255.
Orlando A, Refolo MG, Messa C, Amati L, Lavermicocca P, Guerra V. Russo F Antiproliferative and proapoptotic effects of viable or heat-killed Lactobacillus paracasei IMPC2.1 and Lactobacillus rhamnosus GG in HGC-27 gastric and DLD-1 colon cell lines. Nutr Cancer. 2012. https://doi.org/10.1080/01635581.2012.717676.
Chung IC, OuYang CN, Yuan SN, Lin HC, Huang KY, Wu PS, Liu CY, Tsai KJ, Loi LK, Chen YJ, Chung AK, Ojcius DM, Chang YS, Chen LC. Pretreatment with a heat-killed probiotic modulates the NLRP3 inflammasome and attenuates colitis-associated colorectal cancer in mice. Nutrients. 2019. https://doi.org/10.3390/nu11030516.
Lee SY, Yang SB, Choi YM, Oh SJ, Kim BJ, Kook YH, Kim BJ. Heat-killed Mycobacterium paragordonae therapy exerts an anti-cancer immune response via enhanced immune cell mediated oncolytic activity in xenograft mice model. Cancer Lett. 2020. https://doi.org/10.1016/j.canlet.2019.12.028.
Fang SB, Shih HY, Huang, Li LT, Fang HW. Live and heat-killed Lactobacillus rhamnosus GG upregulate gene expression of pro-inflammatory cytokines in 5-fluorouracil-pretreated Caco-2 cells. Support Care Cancer. 2014. https://doi.org/10.1007/s00520-014-2137-z.
•• Elham N, Naheed M, Elahe M, Hossein MM, Majid T. Selective cytotoxic effect of probiotic, paraprobiotic and postbiotics of L.casei strains against colorectal cancer cells: Invitro studies Braz. J Pharm Sci. 2022. https://doi.org/10.1590/s2175-97902022e19400. This article showed that different strains of bacteria and that viable, heat-killed bacteria and their CFS will have different effects on colorectal cancer. It has shown how important strain is and that probiotics and postbiotics can have different effects.
Sadeghi-Aliabadi H, Mohammadi F, Fazeli H, Mirlohi M. Effects of Lactobacillus plantarum A7 with probiotic potential on colon cancer and normal cells proliferation in comparison with a commercial strain. Iran J Basic Med Sci. 2014;17:815–9.
Garde S, Chodisetti PK, Reddy M. Peptidoglycan: structure, synthesis, and regulation. EcoSal Plus. 2021. https://doi.org/10.1128/ecosalplus.ESP-0010-2020.
Brown S, Santa Maria JP, Wakler S Jr. Wall teichoic acids of gram-positive bacteria. Annu Rev Microbiol. 2013. https://doi.org/10.1146/annurev-micro-092412-155620.
Sleytr UB, Schuster B, Egelseer EM, Pum D. S-layers: principles and applications. FEMS Microbiol Rev. 2014. https://doi.org/10.1111/1574-6976.12063.
Zhang T, Pan D, Yang Y, Jiang X, Zhang J, Zeng X, Wu Z, Sun Y, Guo Y. Effect of Lactobacillus acidophilus CICC 6074 S-layer protein on colon cancer HT-29 cell proliferation and apoptosis. J Agric Food Chem. 2020. https://doi.org/10.1021/acs.jafc.9b06909.
Ju X, Wu X, Chen Y, Cui S, Cai Z, Zhao L, Hao Y, Zhou F, Chen F, Yu Z, Yang D. Mucin binding protein of Lactobacillus casei inhibits HT-29 colorectal cancer cell proliferation. Nutrients. 2023. https://doi.org/10.3390/nu15102314.
Hosseini SS, Goudarzi H, Ghalavand Z, Hajikhani B, Rafeieiatani Z, Hakemi-Vala M. Anti-proliferative effects of cell wall, cytoplasmic extract of Lactococcus lactis and nisin through down-regulation of cyclin D1 on SW480 colorectal cancer cell line. Iran J Microbiol. 2020. https://doi.org/10.18502/ijm.v12i5.4603.
Desrouillères K, Millette M, Bagheri L, Maherani B, Jamshidian M, Lacroix M. The synergistic effect of cell wall extracted from probiotic biomass containing Lactobacillus acidophilus CL1285, L. casei LBC80R, and L. rhamnosus CLR2 on the anticancer activity of cranberry juice-HPLC fractions. J Food Biochem. 2020. https://doi.org/10.1111/jfbc.13195.
Fortin O, Aguilar-Uscanga BR, Vu KD, Salmieri S, Lacroix M. Effect of Saccharomyces boulardii cell wall extracts on colon cancer prevention in male F344 rats treated with 1,2-dimethylhydrazine. Nutr Cancer. 2018. https://doi.org/10.1080/01635581.2018.1460672.
Wang S, Han X, Zhang L, Zhang Y, Li H, Jiao Y. Whole peptidoglycan extracts from the Lactobacillus paracasei subsp. paracasei M5 strain exert anticancer activity in vitro. Biomed Res Int. 2018. https://doi.org/10.1155/2018/2871710.
Song S, Oh S, Lim KT. The proteins (12 and 15 kDa) isolated from heat-killed Lactobacillus plantarum L67 induces apoptosis in HT-29 cells. Cell Biochem Funct. 2015. https://doi.org/10.1002/cbf.3094.
Wang SM, Zhang LW, Fan RB, Han X, Yi HX, Zhang LL, Xue CH, Li HB, Zhang YH, Shigwedha N. Induction of HT-29 cells apoptosis by Lactobacilli isolated from fermented products. Res Microbiol. 2014. https://doi.org/10.1016/j.resmic.2014.02.004.
Nozari S, Faridvand Y, Etesami A, Ahmad Khan Beiki M, Miresmaeili Mazrakhondi SA, Abdolalizadeh J. Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett Appl Microbiol. 2019. https://doi.org/10.1111/lam.13198.
Kawanabe-Matsuda H, Takeda K, Nakamura M, Makino S, Karasaki T, Kakimi K, Nishimukai M, Ohno T, Omi J, Kano K, Uwamizu A, Yagita H, Boneca IG, Eberl G, Aoki J, Smyth MJ, Okumura K. Dietary Lactobacillus-derived exopolysaccharide enhances immune-checkpoint blockade therapy. Cancer Discov. 2022. https://doi.org/10.1158/2159-8290.CD-21-0929.
Salimi F, Farrokh P. Recent advances in the biological activities of microbial exopolysaccharides. World J Microbiol Biotechnol. 2023. https://doi.org/10.1007/s11274-023-03660-x.
Korcz E, Varga L. Exopolysaccharides from lactic acid bacteria: techno-functional application in the food industry. Trends Food Sci Technol. 2021. https://doi.org/10.1016/j.tifs.2021.02.014.
Angelin J, Kavitha M. Exopolysaccharides from probiotic bacteria and their health potential. Int J Biol Macromol. 2020. https://doi.org/10.1016/j.ijbiomac.2020.06.190.
Ma F, Song Y, Sun M, Wang A, Jiang S, Mu G, Tuo Y. Exopolysaccharide produced by Lactiplantibacillus plantarum-12 alleviates intestinal inflammation and colon cancer symptoms by modulating the gut microbiome and metabolites of C57BL/6 mice treated by azoxymethane/dextran sulfate sodium salt. Foods. 2021. https://doi.org/10.3390/foods.10123060.
Nigdelioglu-Dolanbay S, Aslim B. Comparison of the anti-carcinogenic effects of some probiotic bacteria and their postbiotics on colorectal cancer cells. J Appl Biol Sci. 2022. https://doi.org/10.5281/zenodo.6590524.
Deepak V, Sundar WA, Pandian SRK, Sivasubramaniam SD, Hariharan N, Sundar K. Exopolysaccharides from Lactobacillus acidophilus modulates the antioxidant status of 1,2-dimethyl hydrazine-induced colon cancer rat model. Biotech. 2021. https://doi.org/10.1007/s13205-021-02784-x.
Lu Y, Zhang X, Wang J, Chen K. Exopolysaccharides isolated from Rhizopus nigricans induced colon cancer cell apoptosis in vitro and in vivo via activating the AMPK pathway. Biosci Rep. 2020. https://doi.org/10.1042/BSR20192774.
Yu W, Chen G, Zhang P, Chen K. Purification, partial characterization and antitumor effect of an exopolysaccharide from Rhizopus nigricans. Int J Biol Macromol. 2016. https://doi.org/10.1016/j.ijbiomac.2015.10.005.
Saadat YR, Khosroushahi AY, Movassaghpour AA, Talebi M, Gargari BP. Modulatory role of exopolysaccharides of Kluyveromyces marxianus and Pichia kudriavzevii as probiotic yeasts from dairy products in human colon cancer cells. J Funct Foods. 2020. https://doi.org/10.1016/j.jff.2019.103675.
Tukenmez U, Aktas B, Aslim B, Yavuz S. The relationship between the structural characteristics of Lactobacilli-EPS and its ability to induce apoptosis in colon cancer cells in vitro. Sci Rep. 2019. https://doi.org/10.1038/s41598-019-44753-8.
Rajoka MSR, Mehwish HM, Fang H, Padhiar AA, Zeng X, Khurshid M, He Z, Zhao L. Characterization and anti-tumor activity of exopolysaccharide produced by Lactobacillus kefiri isolated from Chinese kefir grains. J Funct Foods. 2019. https://doi.org/10.1016/j.jff.2019.103588.
Di W, Zhang L, Yi H, Han X, Zhang Y, Xin L. Exopolysaccharides produced by Lactobacillus strains suppress HT-29 cell growth via induction of G0/G1 cell cycle arrest and apoptosis. Oncol Lett. 2018. https://doi.org/10.3892/ol.2018.912.
El-Deeb NM, Yassin AM, Al-Madboly LA, El-Hawiet A. A novel purified Lactobacillus acidophilus 20079 exopolysaccharide, LA-EPS-20079, molecularly regulates both apoptotic and NF-κB inflammatory pathways in human colon cancer. Microb Cell Fact. 2018. https://doi.org/10.1186/s12934-018-0877-z.
Wang K, Li W, Rui X, Chen X, Jiang M, Dong M. Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol. 2014. https://doi.org/10.1016/j.ijbiomac.2013.10.036.
Liu CT, Chu FJ, Chou CC, Yu RC. Antiproliferative and anticytotoxic effects of cell fractions and exopolysaccharides from Lactobacillus casei 01. Mutat Res. 2011. https://doi.org/10.1016/j.mrgentox.2011.01.005.
Mani-López E, Arrioja-Bretón D, López-Malo A. The impacts of antimicrobial and antifungal activity of cell-free supernatants from lactic acid bacteria in vitro and foods. Compr Rev Food Sci Food Saf. 2022. https://doi.org/10.1111/1541-4337.12872.
Salemi R, Vivarelli S, Ricci D, Scillato M, Santagati M, Gattuso G, Falzone L, Libra M. Lactobacillus rhamnosus GG cell-free supernatant as a novel anti-cancer adjuvant. J Transl Med. 2023. https://doi.org/10.1186/s12967-023-04036-3.
Pahumunto N, Teanpaisan R. Anti-cancer properties of potential probiotics and their cell-free supernatants for the prevention of colorectal cancer: an in vitro study. Probiotics Antimicrob Proteins. 2022. https://doi.org/10.1007/s12602-022-09972-y.
Oh BS, Choi WJ, Kim JS, Ryu SW, Yu SY, Lee JS, Park SH, Kang SW, Lee J, Jung WY, Kim YM, Jeong JH, Lee JH. Cell-free supernatant of Odoribacter splanchnicus isolated from human feces exhibits anti-colorectal cancer activity. Front Microbiol. 2021. https://doi.org/10.3389/fmicb.2021.736343.
Maghsood F, Johari B, Rohani M, Madanchi H, Saltanatpour Z, Kadivar M. Anti-proliferative and anti-metastatic potential of high molecular weight secretory molecules from probiotic Lactobacillus reuteri cell-free supernatant against human colon cancer stem-like cells (HT29-ShE). Int J Pept Res. 2020. https://doi.org/10.1007/s10989-020-10049-z.
Lee J, Lee JE, Kim S, Kang D, Yoo HM. Evaluating cell death using cell-free supernatant of probiotics in three-dimensional spheroid cultures of colorectal cancer cells. J Vis Exp. 2020. https://doi.org/10.3791/61285.
Sharma M, Chandel D, Shukla G. Antigenotoxicity and cytotoxic potentials of metabiotics extracted from isolated probiotic, Lactobacillus rhamnosus MD 14 on Caco-2 and HT-29 human colon cancer cells. Nutr Cancer. 2020. https://doi.org/10.1080/01635581.2019.1615514.
Yue YC, Yang BY, Lu J, Zhang SW, Liu L, Nassar K, Xu XX, Pang XY, Lv JP. Metabolite secretions of Lactobacillus plantarum YYC-3 may inhibit colon cancer cell metastasis by suppressing the VEGF-MMP2/9 signaling pathway. Microb Cell Fact. 2020. https://doi.org/10.1186/s12934-020-01466-2.
Yue Y, Ye K, Lu J, Wang X, Zhang S, Liu L, Yang B, Nassar K, Xu X, Pang X, Lv J. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. Biomed Pharmacother. 2020. https://doi.org/10.1016/j.biopha.2020.110159.
An J, Ha EM. Combination therapy of Lactobacillus plantarum supernatant and 5-fluouracil increases chemosensitivity in colorectal cancer cells. J Microbiol Biotechnol. 2016. https://doi.org/10.4014/jmb.1605.05024.
Nami Y, Haghshenas B, Haghshenas M, Abdullah N, Yari KA. The prophylactic effect of probiotic Enterococcus lactis IW5 against different human cancer cells. Front Microbiol. 2015. https://doi.org/10.3389/fmicb.2015.01317.
Escamilla J, Lane MA, Maitin V. Cell-free supernatants from probiotic Lactobacillus casei and Lactobacillus rhamnosus GG decrease colon cancer cell invasion in vitro. Nutr Cancer. 2012. https://doi.org/10.1080/01635581.2012.700758.
Wang H, Jin J, Pang X, Bian Z, Zhu J, Hao Y, Zhang H, Xie Y. Plantaricin BM-1 decreases viability of SW480 human colorectal cancer cells by inducing caspase-dependent apoptosis. Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2022.1103600.
Lawrence GW, McCarthy N, Walsh CJ, Kunyoshi TM, Lawton EM, O’Connor PM, Begley M, Cotter PD, Guinane CM. Effect of a bacteriocin-producing Streptococcus salivarius on the pathogen Fusobacterium nucleatum in a model of the human distal colon. Gut Microbes. 2022. https://doi.org/10.1080/19490976.2022.2100203.
Bell HN, Rebernick RJ, Goyert J, Singhal R, Kuljanin M, Kerk SA, Huang W, Das NK, Andren A, Solanki S, Miller SL, Todd PK, Fearon ER, Lyssiotis CA, Gygi SP, Mancias JD, Shah YM. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell. 2022. https://doi.org/10.1016/j.ccell.2021.12.001.
Sugimura N, Li Q, Chu ESH, Lau HCH, Fong W, Liu W, Liang C, Nakatsu G, Su ACY, Coker OO, Wu WKK, Chan FKL, Yu J. Lactobacillus gallinarum modulates the gut microbiota and produces anti-cancer metabolites to protect against colorectal tumourigenesis. Gut. 2021. https://doi.org/10.1136/gutjnl-2020-323951.
de Moreno de LeBlanc A, LeBlanc JG, Perdigón G, Miyoshi A, Langella P, Azevedo V, Sesma F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J Med Microbiol. 2008. https://doi.org/10.1099/jmm.0.47403-0.
Li Q, Hu W, Liu WX, Zhao LY, Huang D, Liu XD, Chan H, Zhang Y, Zeng JD, Coker OO, Kang W, Ng SSM, Zhang L, Wong SH, Gin T, Chan MTV, Wu JL, Yu J, Wu WKK. Streptococcus thermophilus inhibits colorectal tumorigenesis through secreting β-galactosidase. Gastroenterology. 2021. https://doi.org/10.1053/j.gastro.2020.09.003.
Darendelioğlu E, Çiftçi M, Baydaş G. The apoptotic effects of SCFAs from Lactobacillus reuteri on (HT29) human colon cancer cells. Tr J Nat Bilim. 2017;6:11–9.
Liu H, Wang J, He T, Becker S, Zhang G, Li D, Ma X. Butyrate: a double-edged sword for health? Adv Nutr. 2018. https://doi.org/10.1093/advances/nmx009.
Geng HW, Yin FY, Zhang ZF, Gong X, Yang Y. Butyrate suppresses glucose metabolism of colorectal cancer cells via GPR109a-AKT signaling pathway and enhances chemotherapy. Front Mol Biosci. 2021. https://doi.org/10.3389/fmolb.2021.634874.
Li Q, Ding C, Meng T, Lu W, Liu W, Hao H, Cao L. Butyrate suppresses motility of colorectal cancer cells via deactivating Akt/ERK signaling in histone deacetylase dependent manner. J Pharmacol Sci. 2017. https://doi.org/10.1016/j.jphs.2017.11.004.
Kang J, Sun M, Chang Y, Chen H, Zhang J, Liang X, Xiao T. Butyrate ameliorates colorectal cancer through regulating intestinal microecological disorders. Anticancer Drugs. 2023. https://doi.org/10.1097/CAD.0000000000001413.
Wang L, Luo HS, Xia H. Sodium butyrate induces human colon carcinoma HT-29 cell apoptosis through a mitochondrial pathway. J Int Med Res. 2009. https://doi.org/10.1177/147323000903700323.
Schlörmann W, Horlebein C, Hübner SM, Wittwer E, Glei M. Potential role of ROS in butyrate- and dietary fiber-mediated growth inhibition and modulation of cell cycle-, apoptosis- and antioxidant-relevant proteins in LT97 colon adenoma and HT29 colon carcinoma cells. Cancers. 2023. https://doi.org/10.3390/cancers15020440.
Sahuri-Arisoylu M, Mould RR, Shinjyo N, Bligh SWA, Nunn AVW, Guy GW, Thomas EL, Bell JD. Acetate induces growth arrest in colon cancer cells through modulation of mitochondrial function. Front Nutr. 2021. https://doi.org/10.3389/fnut.2021.588466.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. The idea of the article was put forward by R.K.Y. and Ö.Ü.A. Literature search, data collection and analysis, and critical revisions were performed by R.K.Y. and Ö.Ü.A. The first draft of the manuscript was written by R.K.Y. and Ö.Ü.A. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuru-Yaşar, R., Üstün-Aytekin, Ö. The Crucial Roles of Diet, Microbiota, and Postbiotics in Colorectal Cancer. Curr Nutr Rep 13, 126–151 (2024). https://doi.org/10.1007/s13668-024-00525-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-024-00525-z