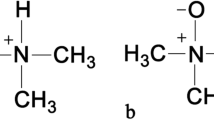Abstract
Purpose of Review
Choline is an essential nutrient for human health and cellular homeostasis as it is necessary for the synthesis of lipid cell membranes, lipoproteins, and the synthesis of the neurotransmitter acetylcholine. The aim of this review is to analyze the beneficial effects of choline and its significance in cellular metabolism and various inflammatory pathways, such as the inflammasome. We will discuss the significance of dietary choline in cardiometabolic disorders, such as non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and chronic kidney disease (CKD) as well as in cognitive function and associated neuropsychiatric disorders.
Recent Findings
Choline deficiency has been related to the development of NAFLD and cognitive disability in the offspring as well as in adulthood. In sharp contrast, excess dietary intake of choline mediated via the increased production of trimethylamine by the gut microbiota and increased trimethylamine-N-oxide (TMAO) levels has been related to atherosclerosis in most studies. In this context, CVD and CKD through the accumulation of TMAO, p-Cresyl-sulfate (pCS), and indoxyl-sulfate (IS) in serum may be the result of the interplay between excess dietary choline, the increased production of TMAO by the gut microbiota, and the resulting activation of inflammatory responses and fibrosis.
Summary
A balanced diet, with no excess nor any deficiency in dietary choline, is of outmost importance regarding the prevention of cardiometabolic disorders as well as cognitive function. Large-scale studies with the use of next-generation probiotics, especially Akkermansia muciniphila and Faecalibacterium prausnitzii, should further examine their therapeutic potential in this context.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Choline or 2-hydroxyethyl-trimethyl-ammonium salt is considered an essential nutrient, as only a small proportion can be synthetized by endogenous processes in the human body [1]. Due to the small amounts of de novo biosynthesis of choline, humans should receive choline from their diet. Eggs, red meat, milk, and cheese are the richest in choline dietary products. Choline is necessary for the function of various molecular pathways. In particular, it is prerequisite for the synthesis of the cells’ lipid membrane in the form of phosphatidylcholine and sphingomyelin. In addition, it is the precursor of the neurotransmitter acetylcholine, thus, being implicated in central nervous system (CNS) development and evolution throughout lifetime [1, 2]. Furthermore, choline is oxidized to betaine, which, in turn, contributes to the synthesis of S-adenosyl-methionine (SAM). SAM is the major methyl group donor in the human body. In this context, SAM plays a pivotal role in epigenetic DNA methylation and modulation [3, 4].
The Institute of Medicine suggested that choline is an essential nutrient in 1998, while in 2016, the European Food Safety Authority (EFSA) set dietary reference values for choline [5, 6]. According to the Institute of Medicine, daily adequate intakes (AIs) for adults are 550 mg for males and 425 mg for females, respectively [7]. Nevertheless, the EFSA in 2016 set the daily AIs to 400 mg for adults, 480 mg for pregnant women, and 520 mg for lactating women [6].
Notably, although there is no doubt that choline is an essential nutrient, there are many parameters, which induce modulations regarding its metabolism in the human body. The type and amount of dietary choline intake and genetic factors as well as the gut microbiota all constitute distinct features that interact with each other to further influence serum choline’s levels. In this review, we will focus on the aforementioned factors and we will discuss the significance of dietary choline in cardiometabolic disorders, such as non-alcoholic fatty liver disease (NAFLD), cardiovascular disease (CVD), and chronic kidney disease (CKD) as well as in cognitive function and associated disorders. In addition, we will elaborate upon the preventive and therapeutic potential of dietary changes and probiotics administration in terms of beneficial outcomes regarding human health.
Choline: The Interplay Between Host Factors and Gut Microbiota
Dietary Choline and Host Factors Affecting Its Metabolism
Dietary choline is classified in either water-soluble, such as free-choline, phosphocholine, and glycerophosphocholine, and lipid-soluble, such as phosphatidylcholine and sphingomyelin [1, 8]. The water-soluble form via portal circulation reaches the liver, whereas the lipid-soluble form by means of the lymphatic system participates into chylomicron formation [9]. Differences in dietary sources of choline play a crucial role in terms of water-soluble and lipid-soluble choline. A simple paradigm is the content of water-soluble and lipid-soluble choline in milk. Maternal milk has a different impact on choline absorption than the frequently consumed pasteurized milk [1, 9]. It is noteworthy that consumption of eggs, which are a well-known source of choline, may result in increases in serum cholesterol among individuals who have polymorphisms in the ATP-binding cassette (ABC) transporters (ABCG) 5 and ABCG8 genes. The above-mentioned polymorphisms account for the increased levels of cholesterol due to enhanced cholesterol absorption in the intestine [10•, 11]. Furthermore, it has been suggested that differences in age, sex, and sex hormones, such as estrogens, may influence the individuals’ needs for dietary choline. In particular, postmenopausal women need larger amounts of dietary choline in order to avoid organ dysfunction attributable to lower dietary choline intake. Postmenopausal women have lower serum estrogen levels, which, in turn, may affect the expression of phosphatidylethanolamine-N-methyltransferase (PEMT) gene [12, 13]. PEMT is the gene responsible for the breakdown of phosphatidylcholine into choline, leading to de novo choline production in humans [12, 13]. Fischer et al. have demonstrated that polymorphisms in the PEMT gene (rs12325817) are also implicated in the vulnerability of women regarding organ dysfunction due to lower serum choline levels [12].
Overall, apart from the water-soluble and lipid-soluble amount in dietary choline, other host factors, such as age, sex hormones, or gene polymorphisms, are involved in the metabolism of choline.
Choline and the Gut Microbiota
The microbiota refers to the sum of bacteria, archaea, viruses, and fungi inhabiting the human body. It has been estimated that there are trillions of microorganisms, approximately 1013 to 1014 in the human body. As the vast majority of microbiota in humans resides in the gut, we often use the term “gut microbiota” instead [14, 15]. Under normal circumstances, there is homeostasis between the human gut microbiota and its host. However, under the influence of various factors, such as senescence, drugs, especially antibiotics, Western diet, or sedentary lifestyle, the phenomenon of “dysbiosis” occurs. Dysbiosis characterizes the imbalance between the gut microbiota and its host and has been suggested to be implicated in several diseases [14,15,16,17,18]. Metabolic disorders, such as obesity, hypertension, diabetes mellitus type 2, and NAFLD, together with other diseases, such as cancer, have been suggested to be also mediated by dysbiosis [14,15,16,17,18].
Regarding choline, it is widely known that it is converted to trimethylamine (TMA) by the gut microbiota. In turn, TMA is oxidized to trimethylamine-N-oxide (TMAO) via mono-oxygenases in the liver, which is then released into the systemic circulation [19,20,21]. Increased serum TMAO levels have been related to the development of atherosclerosis. In particular, elevated TMAO may promote the formation of foam cells from macrophages. Recently, it has been documented that increased TMAO is associated with the polarization of macrophages from the M2 phenotype, which exerts anti-inflammatory effects to the M1 phenotype, which exhibits pro-inflammatory properties. This phenotypic switch from M2 to M1 may be a plausible explanation for the atherogenic features of TMAO [22•, 23,24,25, 26•, 27]. Increased serum levels of TMAO may be attributed to an excess in dietary choline intake and/or abundant production of TMAO by the gut microbiota. Indeed, alterations in the composition and function of gut microbiota seem to play a pivotal role on the amount of gut-produced TMAO [28,29,30].
The Role of Dietary Choline in Cardiometabolic Disorders
Choline and NAFLD
NAFLD poses a global public health issue, as its prevalence has recently been estimated approximately 32% worldwide [31]. Among patients with NAFLD, non-alcoholic steatohepatitis (NASH) will develop in up to 25%. NASH has been suggested as the most common rising cause of hepatocellular carcinoma [32, 33]. NAFLD results from an imbalance between the de novo lipogenesis in the liver and the lipid hepatic excretion, leading to fat accumulation. It is associated with various degrees of liver fat deposition. It is a metabolic disorder that ranges from simple hepatic steatosis to the addition of inflammation (NASH), the development of cirrhosis, and even hepatocellular carcinoma [32, 33]. For the development of NAFLD, the most dominant hypothesis is the “multi-hit hypothesis.” This consists of the involvement of various genetic and epigenetic factors, apart from nutritional aspects, hormonal components, the implication of adipose tissue, and the modulation of the gut microbiota [21, 32, 33].
Choline-deficient diet and choline-methionine-deficient diet have long been used in animal models to provoke NAFLD [34]. Choline is a widely known methyl group donor in humans. As such, choline is necessary for the biosynthesis of phosphatidylcholine in the liver. In turn, phosphatidylcholine is a prerequisite for the synthesis of very low-density lipoproteins (VLDL). A choline-deficient diet leads to the decreased synthesis of phosphatidylcholine in the liver and, thus, to the aggregation of triglycerides in liver cells. Therefore, lipid accumulation in the liver occurs resulting gradually in the development of NAFLD [35, 36].
In addition, choline-deficient diet has been related to mitochondrial dysfunction due to endoplasmic reticulum (ER) stress [37, 38]. ER, among other functions, is responsible for protein maturation. Excess lipid deposition in the liver leading to hepatic steatosis is characterized by a subsequent enhancement in the needs for protein maturation by the ER. This increased burden cannot be satisfied, thus, accounting for unmet protein folding. The whole process results in ER stress, i.e., an abundance of unfolded proteins in the ER. Then, a cascade, which is widely known as unfolded protein response (UPR), is activated to maintain homeostasis. However, if the ER stress becomes chronic, this UPR cascade may lead to significant inflammation, increased oxidative stress, and even cell death [39, 40]. Therefore, while the aim of the UPR is to achieve cell homeostasis, chronic ER stress, such as in the case of NAFLD, may result in the activation of inflammatory responses [39, 40]. Inflammation is further augmented by the binding of reactive oxygen species (ROS) to the inflammasome (nucleotide-binding domain-like receptor protein 3) NLRP3. The inflammasome is a protein complex, which plays a crucial role in the pathogenesis of inflammation. More specifically, this binding of ROS to NLRP3 leads to the activation of the NLRP3. Activation of NLRP3 is achieved by a transformational change in the structure of NLRP3, which is attributed to the activation of caspase-1 from procaspase. In turn, the activated NLRP3 results in the release of various pro-inflammatory cytokines, especially interleukin (IL) 1β and IL-18 [41, 42]. This vicious cycle accounts for the development of inflammation seen in NASH, whereas the release of tumor growth factor β (TGF-β) is responsible for collagen deposition and the progression of NAFLD to cirrhosis [43, 44]. Figure 1 depicts the whole process, from chronic ER stress to the UPR, inflammation, and fibrosis in the context of NAFLD.
Dietary choline deficiency as a part of the “multi-hit hypothesis” in the development of NAFLD. Abbreviations: NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; NLRP3, nucleotide-binding domain-like receptor protein 3; ROS, reactive oxygen species; TGF-β, transforming growth factor β. All free elements in the figure are originated from the free medical site http://smart.servier.com/ (accessed on January 1, 2024) by Servier licensed under a Creative Commons Attribution 3.0 Unported License
Apart from chronic ER stress and the UPR cascade, the activation of NLRP3, and the subsequent inflammatory responses, the gut microbiota is also deeply involved in the pathogenesis of NAFLD [45•]. In particular, using 16S rRNA sequencing techniques, it has been documented that there is an increase in Bacteroidetes and modulations in the abundance of Firmicutes among patients with NAFLD/NASH. In most of the studies regarding NAFLD/NASH, a decrease in the ratio of Firmicutes/Bacteroidetes (F/B) has been demonstrated [21]. Nevertheless, among patients with different stages of NAFLD/NASH, differences in the composition of the gut microbiota have been observed and the different stages of NAFLD may account for these reported discrepancies [21].
Overall, dietary choline deficiency by means of chronic ER stress, activation of the NLRP3, and modulations in the gut microbiota has been suggested to be part of the “multi-hit hypothesis” in the development of NAFLD. Even though NAFLD is a multifactorial process, it seems likely that a balanced diet, by avoiding deficiency or excess of choline intake, plays a key role in the prevention of NAFLD. Regarding therapeutic agents, it has been advocated that polyene phosphatidylcholine supplementation may offer a beneficial effect on NAFLD progression [46]. Notably, Zhang et al. have very recently reported significant improvement in the lipidomics and metabolomics in mice fed a choline-deficient diet, when treated with polyene phosphatidylcholine [47]. In particular, they documented changes in 14 lipids and 19 metabolites, in total [47]. Therefore, a balanced diet together with polyene phosphatidylcholine supplementation may represent novel and advantageous ways for the prevention and treatment of NAFLD.
Choline and CVD
CVD, including heart disease, stroke, and peripheral artery disease, is the leading cause of death worldwide [48]. According to the CDC, 1 out of 5 deaths in the USA is attributed to CVD [49]. As already mentioned above, TMAO, a metabolite of choline derived in the gut, has been related to atherosclerosis and CVD risk [50,51,52,53,54,55,56,57,58,59,60]. TMA is produced directly from the gut microbiota and is further oxidized in the liver to TMAO. This oxidation is catalyzed by flavin mono-oxygenases, especially flavin mono-oxygenase 3 (FMO3).
Animal and Experimental Studies
In rodents, TMAO has been associated with an increased CVD risk. This association has been confirmed by fecal microbiota transplantation (FMT) from mice with CVD to previously health mice [61, 62]. The molecular mechanisms underlying the association between elevated serum TMAO levels and CVD remain largely unknown, so far. However, in 2021, Díez-Ricote et al. demonstrated a relationship between TMAO and specific microRNAs. miRNAs are small, non-coding RNA molecules, which are implicated in the regulation and expression of genes at the post-transcriptional level [63]. Astudillo and Mayrovitz have documented that TMAO levels increased the expression of miR-21-5p and miR-30c-5p. Through this molecular mechanism, TMAO is correlated to lipid metabolism, inflammation and, thus, to atherosclerosis [63]. They also identified that the aforementioned microRNAs modulated the expression of Period Circadian Regulator 2 (PER2), their target gene [63]. Besides, more recently, in 2022, Díez-Ricote et al. have demonstrated another molecular pathway linking increased TMAO levels to atherosclerosis [64]. More specifically, they reported that TMAO may upregulate members of the miR-17/92 cluster, and this upregulation is associated with an enhancement in IL-12A. The release of IL-12A is strongly related to inflammation, while TMAO is also associated with increased levels of plasminogen activator inhibitor 1 (PAI-1) or SERPINE1, which in turn possess pro-thrombotic properties. Therefore, by documenting a relationship between TMAO, IL-12A, and PAI-1, Díez-Ricote et al. have managed to demonstrate another pathway of miR-17/92 cluster with inflammation and fibrin deposition in the atherogenic plaque [64].
Human Studies
Apart from animal models, there are many studies in humans as well. Díez-Ricote et al. have conducted a study among 20 male patients with metabolic syndrome who received lean donor FMT and found alterations in the composition of the gut microbiota, but not significant changes in TMAO levels [65]. On the contrary, Kanitsoraphan et al. have demonstrated an association of TMAO levels with CVD risk together with modifications in the gut microbiota [60]. In their meta-analysis, Smits et al. concluded that they did not find any significant relationship between dietary choline or betaine and incident CVD. However, they noted that further research should be performed regarding choline intake and CVD mortality [66]. In particular, in their meta-analysis, they found only two studies that met their inclusion criteria. One study by Meyer and Shea reported a positive association between dietary phosphatidylcholine intake and CVD mortality [67]. In sharp contrast, the second study by Zheng et al. did not confirm any association between dietary phosphatidylcholine intake and CVD mortality in a Japanese population [68]. However, Meyer and Shea concluded that the fact that only two studies were included in their meta-analysis and these studies had conflicting results is a severe limitation of their work. They pointed out the need for further large-scale studies in order to shed light upon the possible association between dietary choline intake and incident CVD or CVD mortality [69].
Although the results are inconclusive, most studies support the notion that TMAO levels are related to atherosclerosis and CVD. More specifically, TMAO levels may be associated with CVD regardless of other well-known CVD risk factors, such as serum low-density lipoprotein (LDL) cholesterol concentrations and chronic low-grade inflammation [70]. It is noteworthy that elevated serum TMAO levels may be predictive of increased CVD risk, even though other well-established CVD risk factors, such as lipid parameters, are within the normal range [71]. Moreover, TMAO levels are suggested to be related to the formation and progression of the atherosclerotic plaque [22•, 23,24,25, 26•, 27, 69, 70].
Nevertheless, very recently, Zhou et al. have enrolled 14,323 adults in the National Health and Nutrition Examination Survey (NHANES) between 2011 and 2016 and have found that a higher choline intake was related to a lower risk of CVD, especially stroke [71]. Although this is a rather unexpected finding, the authors conclude that further studies are needed to confirm or refute their findings [71].
Regarding nutritional aspects, a Western diet is rich in eggs and red meat, which are well-known sources of dietary choline. Thus, a Western diet is typically associated with increased TMAO production, whereas a healthier diet, such as the Mediterranean diet, has been inversely related to serum and urinary levels of TMAO [72]. Apart from the Mediterranean diet, probiotics, prebiotics, and their combination synbiotics might play a unique role in this context. The administration of probiotics, especially next-generation probiotics (NGP), such as Akkermansia muciniphila, has already been suggested to offer beneficial effects [73,74,75]. In particular, Akkermansia muciniphila has been documented to decrease the formation of abdominal aortic aneurysm, when administered in a mouse model. Furthermore, supplementation of rodents with Akkermansia muciniphila has resulted in an amelioration of the increased TMAO levels and blood pressure as well. It is noteworthy that Depommier et al. in their landmark study among humans have demonstrated that supplementation with this NGP has been a safe and well-tolerated method to improve parameters of the metabolic syndrome among overweight/obese patients [76].
Overall, although the debate regarding TMAO as a biomarker of incident CVD or CVD mortality is still on, there is no doubt that TMAO, as a product of dietary choline, will be a subject of ongoing research. Excess dietary choline intake, which may lead to increased serum TMAO levels through metabolism by the gut microbiota, should generally be avoided.
Choline and CKD
CKD poses a serious public health problem affecting approximately 15% of the population in the USA, as has recently been estimated [77]. It is noteworthy that CVD accounts for the majority of mortality among patients with CKD [78]. TMAO is mainly excreted by the kidneys; therefore, in cases of CKD, serum TMAO levels are elevated. As already mentioned, increased TMAO levels have been associated with CVD. In particular, Go et al. have only recently documented a relationship between increased dietary choline intake and CKD-associated CVD [79]. Notably, they have demonstrated that excess choline intake by means of increased TMAO production results in decreased angiogenesis in the heart vessels. According to Xie et al., this reduction in the formation of new heart vessels is mediated by hypoxia-induced factor 1α (HIF-1α). More specifically, they documented that increased choline intake via a decrease in the release of HIF-1α may lead to ischemic cardiac disease in a mouse model of CKD-induced CVD [79]. In ischemic heart disease, angiogenesis plays a crucial role [80, 81]. The generation of new vessels in the heart through the release of compounds, such as HIF-1α, HIF-2α, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and angiopoieitin-2, is cardioprotective [82]. Therefore, this molecular mechanism of decreased production of HIF-1α as a result of excess choline intake may provide a plausible explanation for the CKD-induced CVD [79,80,81,82]. Very recently, Li et al. in their meta-analysis have documented a relationship between serum TMAO levels and CVD as well as all-cause mortality among patients with CKD [83].
Apart from CKD-induced CVD, choline has been suggested to be related to CKD per se. Serum TMAO levels are elevated among CKD patients due to the decreased renal clearance. In addition, gut microbiota alterations have been documented to occur in patients with CKD [18, 84]. The brain-gut-kidney axis supports the notion that changes in the composition of the gut microbiota are involved in the increased production and decreased renal elimination of uremic toxic metabolites, such as TMAO, p-Cresyl-sulfate (p-CS), and indoxyl-sulfate (IS) [18, 84]. Figure 2 describes the main mechanisms through which CVD and CKD are suggested to be associated with choline intake.
Main mechanisms explaining the association of excess choline intake with CVD and CKD. Abbreviations: HIF, hypoxia-induced factor; IL, interleukin; miRNAs, microRNAs; PAI-1, plasminogen activator inhibitor 1; TGF-β, transforming growth factor β; TMA, trimethylamine; TMAO, trimethylamine-N-oxide. All free elements in the figure are originated from the free medical site http://smart.servier.com/ (accessed on January 1, 2024) by Servier licensed under a Creative Commons Attribution 3.0 Unported License
In animal models, rodents that had been fed with choline or TMAO were documented to develop renal fibrosis and renal impairment [85]. Besides, in another study, Qu et al. have fed mice with choline or TMAO and have reported an upregulation of the transforming growth factor β (TGF-β), which is a widely known factor contributing to fibrosis [86]. This upregulation of TGF-β in the kidneys has been attributed to an increase in the phosphorylation of SMAD3, which is involved in the SMAD3/TGF-β molecular pathway [86]. SMAD3 is a protein transducer of the activation of TGF-β, resulting in increases in the production of TGF-β and subsequently in renal fibrosis [86]. Xie et al. have also demonstrated that mice fed with choline displayed increased renal fibrosis, which was reduced with the use of antibiotics. Antibiotic usage resulted in alterations in the composition of the gut microbiota and, thus, in the reduction of the microbiota-derived TMAO levels [87]. Notably, Zhang et al. reported an improvement in renal fibrosis in mice with CKD, when administered an inhibitor of TMAO, iodomethylcholine [23].
Despite the fact that most studies on TMAO and CKD studies have been performed in animal models, there are a few related human studies as well. Tang et al. followed 521 patients with CKD for 5 years and found that increased TMAO levels were associated with a 2.8 times increased mortality rate [85]. Albeit the rarity of human studies, elevated serum TMAO levels have been associated with CKD [83, 88,89,90,91,92]. Interestingly, Mediterranean diet has not been documented to improve significantly CKD in one study. The authors concluded that the use of probiotics could be more beneficial in this context [93]. The role of NGPs, such as Faecalibacterium prausnitzii, is currently being further investigated [93]. Nevertheless, excess dietary choline in CKD patients should be avoided, as there seems to be a causative role of increased dietary choline intake and progression to CKD. Figure 2 shows the association between dietary choline intake and CKD and CVD.
Dietary Choline, Cognitive Function, and Associated Disorders
Choline is a major element of the membrane component phosphatidylcholine and of the neurotransmitter acetylcholine [94]. Lately, there is a growing body of evidence advocating the maternal choline supplementation in order to improve cognitive ability in their offspring [95,96,97,98]. This advantageous supplementation has been suggested to act via an enhancement in the function of the hippocampus. Moreover, it has been documented to last throughout lifetime [95,96,97,98]. It is noteworthy that maternal choline supplementation is likely to protect from Alzheimer’s disease (AD) later in life as well [95,96,97,98]. It has been demonstrated that prenatal choline supplementation has been associated with increased levels of nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), VEGF, and insulin-like growth factor 2 (IGF2) in the hippocampus. All these factors seem to be implicated in the increased function of hippocampus and the amelioration in cognitive ability [96,97,98,99]. In addition, it has been shown that supplementation with choline during adulthood has been related to improved cognitive ability. Besides, choline supplementation has been associated with less white matter hyperintensity in magnetic resonance imaging, the latter being an imaging feature suggestive of AD [96]. Apart from the amelioration in cognitive ability, maternal choline intake has been associated with less neural tube defects, a better outcome in patients with Down and Rett syndromes, which are characterized by cognitive impairment and improved effects regarding autism and schizophrenia disorders [96, 97, 100]. Table 1 shows main studies in animal and humans that have been performed between 2013 and 2023 regarding dietary choline supplementation and neuropsychiatric disorders.
Perspectives and Challenges
Choline metabolism in humans is a complex process. Apart from the amount of dietary choline intake, other parameters, such as its bioavailability, the gut microbiota composition, and genetic host factors, are involved. The gut microbiota depending on their microbial TMA lyases produce different amounts of TMA. TMA lyases are encoded by the CutC/D genes (chronic utilization cluster genes C and D), which are mainly responsible for TMA production [113]. Moreover, hormonal factors as well as host gene polymorphisms, such as rs12325817 gene, are also implicated [12]. Therefore, a more personalized dietary approach depending on the individuals’ hormonal and health status, the knowledge of the gut microbiota composition and the genes implicated in choline metabolism, is necessary. To this end, multi-omics analysis may help identify important players and interactions. In particular, omics technologies comprise genomics (polymorphisms and other structural genetic variants), epigenomics (DNA methylation, histone modifications, microRNAs), metagenomics (gut microbiota composition), transcriptomics (RNA expression patterns), proteomics (protein profiles), and metabolomics (metabolite profiles), as well as the study of interactions with dietary factors. The field of microbiome research was revolutionized by using modern molecular methods and bioinformatics. The same holds true for lipidomics, i.e., the methodology of analyzing lipids, such as phosphatidylcholine and lysophosphatidylcholine. The combination of multiple omics represents an innovative holistic approach to provide a more integrated view of the molecular and physiological events underlying human disorders (including obesity, metabolic syndrome, NAFLD, CVD, and CKD) as well as for deciphering unique and specific metabolic phenotypes. Although more data are still necessary, it is expected that the incorporation of integrative omics may be useful not only for early diagnosis and risk prognosis but also for guiding tailored dietary treatments and prediction schemes [114,115,116,117,118,119,120,121,122]. As there are significant inter-individual variations, all the above-mentioned parameters should be considered to make the right decisions in an updated and personalized manner. However, there are many challenges that include the lack of robust and reproducible results due to methodological parameters, the elevated cost of omics methodologies, and the presence of high-dimensional data analyses and interpretation as well as ethical and regulatory issues.
Conclusion
Choline is an essential nutrient for human health. Choline deficiency has been related to the development of NAFLD and cognitive disability in the offspring as well as in adulthood. In sharp contrast, excess dietary intake of choline mediated via the increased production of TMA by the gut microbiota and the subsequent increased levels of TMAO has been associated with atherosclerosis in most studies. In this context, CVD and CKD through the accumulation of TMAO, p-CS, and IS in serum may be the result of the interplay between excess dietary choline, the increased production of TMAO by the gut microbiota, and the resulting activation of inflammatory responses and fibrosis. Therefore, a balanced diet, with no excess nor any deficiency in dietary choline, is of outmost importance. Apart from a balanced diet, the potential benefit of NGPs, such as Akkermansia muciniphila and Faecalibacterium prausnitzii, should be further examined in large-scale randomized controlled studies.
Data Availability
Not applicable.
Abbreviations
- ABC:
-
ATP-binding cassette
- AD:
-
Alzheimer disease
- BDNF:
-
Brain-derived neurotrophic factor
- CDC:
-
Centers for Disease Control
- CKD:
-
Chronic kidney disease
- CNS:
-
Central nervous system
- CVD:
-
Cardiovascular disease
- EFSA:
-
European Food Safety Authority
- ER:
-
Endoplasmic stress
- FMO3:
-
Flavin mono-oxygenase 3
- FMT:
-
Fecal microbiota transplantation
- HIF-1a:
-
Hypoxia-induced factor 1a
- IGF-2:
-
Insulin-like growth factor 2
- IL:
-
Interleukin
- IS:
-
Indoxyl-sulfate
- miRNAs:
-
MicroRNAs
- NAFLD:
-
Non-alcoholic fatty liver disease
- NASH:
-
Non-alcoholic steatohepatitis
- NGF:
-
Nerve growth factor
- NGP:
-
Next-generation probiotic
- NHANES:
-
National Health And Nutrition Examination Survey
- NLRP3:
-
Nucleotide-binding domain-like receptor protein 3 inflammasome
- PAI-1:
-
Plasminogen activator inhibitor-1
- pCS:
-
P-Cresyl-sulfate
- PDGF:
-
Platelet-derived growth factor
- PEMT:
-
Phosphatidylethanolamine-N-methyltransferase
- PER-2:
-
Period Circadian Regulator 2
- ROS:
-
Reactive oxygen species
- SAM:
-
S-Adenosyl-methionine
- TGF-β:
-
Tumor growth factor β
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine-N-oxide
- VEGF:
-
Vascular endothelial growth factor
- VLDL:
-
Very low-density lipoproteins
- UPR:
-
Unfolded protein responses
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wiedeman AM, Barr SI, Green TJ, Xu Z, Innis SM, Kitts DD. Dietary choline intake: current state of knowledge across life cycle. Nutrients. 2018;10(10):1513. https://doi.org/10.3390/nu10101513.
Leermakers ET, Moreira EM, Kiefte-de JC, Darweesh SK, Visser T, Voortman T, Bautista PK, Chowdhury R, Gorman D, Bramer WM, Felix JF, Frasco HO. Effects of choline on health across the life course. A systematic review Nutr Rev. 2015;73:500–22. https://doi.org/10.1093/nutrit/nyv010.
Arumugam MK, Paal MC, Donohue TM Jr, Ganesan M, Osna NA, Kharbanda KK. Beneficial effects of betaine: a comprehensive review. Biology (Basel). 2021;10(6):456. https://doi.org/10.3390/biology10060456.
Bekdash RA. Early life nutrition and mental health: the role of DNA methylation. Nutrients. 2021;13(9):3111. https://doi.org/10.3390/nu13093111.
Institute of Medicine. Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline; The National Academy Press: Washington. USA: DC; 1998.
European Food Safety Authority. Dietary reference values for choline. EFSA J. 2016;14:e04484.
NIH. Choline Factsheet. Assessed 25th October 2023.
Patterson YK, Bhagwat AS, Williams RJ, Howe CJ, Holden MJ. USD database for the choline content of common foods, release 2; Agricultural Research Service: Washington. USA: DC; 2008.
Lewis ED, Field CJ, Jacobs RL. Should the forms of dietary choline also be considered when estimating dietary intake and the implications for health? Lipid Technol. 2015;27:227–30.
• Kang JW, Zivkovic AM. Are eggs good again? A precision nutrition perspective on the effects of eggs on cardiovascular risk, taking into account plasma lipid profiles and TMAO. J Nutr Biochem. 2022;100:108906. https://doi.org/10.1016/j.jnutbio.2021.108906. The authors have elaborated upon the complex interplay between dietary choline, the gut microbiome, and TMAO levels.
Gylling H, Hallikainen M, Pihlajamäki J, Agren J, Laakso M, Rajaratnam RA, Rauramaa R, Miettinen TA. Polymorphisms in the ABCG5 and ABCG8 genes associate with cholesterol absorption and insulin sensitivity. J Lipid Res. 2004;45:1660–5. https://doi.org/10.1194/jlr.M300522-JLR200.
Fischer LM, da Costa KA, Kwock L, Galanko J, Zeisel SH. Dietary choline requirements of women: effects of estrogen and genetic variation. Am J Clin Nutr. 2010;92(5):1113–9. https://doi.org/10.3945/ajcn.2010.30064.
Resseguie M, Song J, Niculescu MD, da Costa KA, Randall TA, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) gene expression is induced by estrogen in human and mouse primary hepatocytes. FASEB J. 2007;21:2622–32. https://doi.org/10.1096/fj.07-8227com.
Vallianou N, Stratigou T, Christodoulatos GS, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in obesity and obesity related metabolic disorders. Current concepts and future perspectives. Curr Obes Rep. 2019;8(3):317–32. https://doi.org/10.1007/s13679-019-00352-2.
Vallianou N, Liu J, Dalamaga M. What are the key points in the association between the gut microbiome and non-alcoholic fatty liver disaese? Metabol Open. 2019;1:9–10. https://doi.org/10.1016/j.metop.2019.02.003.
Vallianou N, Stratigou T, Tsagarakis S. Microbiome and diabetes: where are we now? Diabetes Res Clin Pract. 2018;146:111–8. https://doi.org/10.1016/j.diabres.2018.10.008.
Vallianou NG, Tzortzatou-Stathopoulou F. Microbiota and cancer: an update. J Chemother. 2019;31(2):59–63. https://doi.org/10.1080/1120009X.2018.1541046.
Vallianou NG, Kounatidis D, Panagopoulos F, Evangelopoulos A, Stamatopoulos V, Papagiorgos A, Geladari E, Dalamaga M. Gut microbiota and its role in the brain gut kidney axis in hypertension. Curr Hypertens Rep. 2023;25(11):367–76. https://doi.org/10.1007/s11906-023-01263-3.
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. https://doi.org/10.1038/nature09922.
Hoyles L, Jiménez-Pranteda ML, Chilloux J, Bria F, Myridakis A, Aranias T, Magnan C, Gibson GR, Sanderson JD, Nicholson JK, Gauguier D, McCartney AL, Dumas ME. Metabolic retroconversion of trimethylamine N-oxide and the gut microbiota. Microbiome. 2018;6:73. https://doi.org/10.1186/s40168-018-0461-0.
Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, Dalamaga M. Understanding the role of the gut microbiome and microbial metabolites in non-alcoholc fatty liver disease: current concepts and perspectives. Biomolecules. 2021;12(1):56. https://doi.org/10.3390/biom12010056.
• Barrea L, Annunziata G, Muscogiuri G, Di Somma C, Laudisio D, Maisto M, de Alteriis G, Tenore GC, Colao A, Savastano S. Trimethylamine-N-oxide (TMAO) as novel potential biomarker of early predictors of metabolic syndrome. Nutrients. 2018;10:1971. https://doi.org/10.3390/nu10121971. The authors have performed a cross-sectional observational study regarding TMAO and demonstrated that TMAO could serve as a biomarker of metabolic syndrome.
Xie Y, Hu X, Li S, Qiu Y, Cao R, Xu C, Lu C, Wang Z, Yang J. Pharmacological targeting macrophage phenotype via gut kidney axis ameliorates renal fibrosis in mice. Pharmacol Res. 2022;178: 106161. https://doi.org/10.1016/j.phrs.2022.106161.
Shi W, Huang Y, Yang Z, Zhu L, Yu B. Reduction of TMAO level enhances the stability of carotid atherosclerotic plaque through promoting M2 polarization and efferocytosis. Biosci Rep. 2021;41(6):BSR20204250. https://doi.org/10.1042/BSR20204250.
Agus A, Clément K, Sokol H. Gut microbiota derived metabolites as central regulators in metabolic disorders. Gut. 2021;70(6):1174–82. https://doi.org/10.1136/gutjnl-2020-323071.
• Benson TW, Conrad KA, Li XS, Wang Z, Helsley RN, Schugar RC, Coughlin TM, Wadding-Lee C, Fleifil S, Russell HM, Stone T, Brooks M, Buffa JA, Mani K, Björck M, Wanhainen A, Sangwan N, Biddinger S, Bhandari R, Ademoya A, Pascual C, Tang WHW, Tranter M, Cameron SJ, Brown JM, Hazen SL, Owens AP 3rd. Gut microbiota derived trimethylamine N-oxide contributes to abdominal aortic aneurysm through inflammatory and apoptotic mechanisms. Circulation. 2023;147(14):1079–96. https://doi.org/10.1161/CIRCULATIONAHA.122.060573. The authors have performed an original research study regarding the role of TMAO in abdominal aneurysm in terms of inflammatory and apoptotic mechanisms.
Liu ZY, Tan XY, Li QJ, Liao GC, Fang AP, Zhang DM, Chen PY, Wang XY, Luo Y, Long JA, Zhong RH, Zhu HL. Trimethylamine-N-oxide, a gut microbiota-dependent metabolite of choline, is positively associated with the risk of primary liver cancer: a case-control study. Nutr Metab. 2018;15:81. https://doi.org/10.1186/s12986-018-0319-2.
Li XX, Su CY, Jiang ZB, Yang YX, Zhang Y, Yang MX, Zhang XM, Du Y, Zhang J, Wang L, Jiang J, Hong B. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes. 2021;7(1):36. https://doi.org/10.1038/s41522-021-00205-8.
Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr. 2017;37:157–81. https://doi.org/10.1146/annurev-nutr-071816-064732.
Luo T, Guo Z, Liu D, Guo Z, Wu Q, Li Q, Lin R, Chen P, Ou C, Chen M. Deficiency of PSRC1 accelerates atherosclerosis by increasing TMAO production via manipulating gut microbiota and flavin mono-oxygenase 3. Gut Microbes. 2022;14(1):2077602. https://doi.org/10.1080/19490976.2022.2077602.
Le MH, Yeo YH, Li X, Li J, Zou B, Wu Y, Ye Q, Huang DQ, Zhao C, Zhang J, Liu C, Chang N, Xing F, Yan S, Wan ZH, Tang NSY, Mayumi M, Liu X, Liu C, Rui F, Yang H, Yang Y, Jin R, Le RHX, Xu Y, Le DM, Barnett S, Stave CD, Cheung R, Zhu Q, Nguyen MH. 2019 Global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:2809-2817.e28. https://doi.org/10.1016/j.cgh.2021.12.002.
Teng ML, Ng CH, Huang DQ, Chan KE, Tan DJ, Lim WH, Yang JD, Tan E, Muthiah MD. Global incidence and prevalence of non alcoholic fatty liver disease. Clin Mol Hepatol. 2023;29(Suppl):S32–42. https://doi.org/10.3350/cmh.2022.0365.
Huang DQ, Singal AG, Kono Y, Tan DJ, El-Serag HB, Loomba R. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab. 2022;34:969-977.e2. https://doi.org/10.1016/j.cmet.2022.05.003.
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038–48.
Teodoro JS, Rolo AP, Duarte FV, Simoes AM, Palmeira CM. Differential alterations in mitochondrial function induced by a choline-deficient diet: understanding fatty liver disease progression. Mitochondrion. 2008;8:367–76. https://doi.org/10.1016/j.mito.2008.07.008.
Sherriff JL, O’Sullivan TA, Properzi C, Oddo JL, Adams LA. Choline, its potential role in nonalcoholic fatty liver disease, and the case for human and bacterial genes. Adv Nutr. 2016;7(1):5–13. https://doi.org/10.3945/an.114.007955.
Mokhtari Z, Gibson DL, Hekmatdoost A. Non alcoholic fattyliver disease, the gut microbiome and diet. Adv Nutr. 2017;8(2):240–52. https://doi.org/10.3945/an.116.013151.
Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184:2537–64. https://doi.org/10.1016/j.cell.2021.04.015.
Baiceanu A, Mesdom P, Lagouge M, Foufelle F. Endoplasmic reticulum proteostasis in hepatic steatosis. Nat Rev Endocrinol. 2016;12(12):710–22. https://doi.org/10.1038/nrendo.2016.124.
Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr. 2018;18:5–17. https://doi.org/10.3727/105221617X15093707969658.
Lebeaupin C, Vallee D, Hazari Y, Hetz C, Chevet E, Bailly-Maitre B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J Hepatol. 2018;69(4):927–47. https://doi.org/10.1016/j.jhep.2018.06.008.
Kounatidis D, Vallianou N, Evangelopoulos A, Vlahodimitris I, Grivakou E, Kotsi E, Dimitriou K, Skourtis A, Mourouzis I. SGLT-2 inhibitors and the inflammasome: what’s next in the 21st century? Nutrients. 2023;15:2294. https://doi.org/10.3390/nu15102294.
Rivera-Iñiguez I, Panduro A, Roman S, González-Aldaco K. What do we know about nutrient based strategies targeting molecular mechanisms associated with obesity related fatty liver disease? Ann Hepatol. 2023;28(1):100874. https://doi.org/10.1016/j.aohep.2022.100874.
Hetz C, Zhang K, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol. 2020;21(8):421–38. https://doi.org/10.1038/s41580-020-0250-z.
• Zhou L, Shen H, Li X, Wang H. Endoplasmic reticulum stress in innate immune cells - a significant contribution to non-alcoholic fatty liver disease. Front Immunol. 2022;13 The authors have written an interesting review regarding the role of endoplasmic reticulum stress in innate immune cell response in NAFLD.
Ye J, Li YT, Wu WR, Shi D, Fang DQ, Yang LY, Bian XY, Wu JJ, Wang Q, Jiang XW. Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced non-alcoholic steatohepatitis. World J Gastroenterol. 2018;24:2468–81. https://doi.org/10.3748/wjg.v24.i23.2468.
Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. 2022;126:154925. https://doi.org/10.1016/j.metabol.2021.154925.
Zhang J, Zang X, Lv J, Zhang Y, Lv Z, Yu M. Changes in lipidomics, metabolomics, and the gut microbiota in CDAA-induced NAFLD mice after polyene phosphatidylcholine Treatment. Int J Mol Sci. 2023;24:1502. https://doi.org/10.3390/ijms24021502.
Shanthi M, Pekka P, Norving B. Global atlas on cardiovascular disease prevention and control. WHO in collaboration with World Hear Federation and the World Stroke Association, 2011;3–18.
CDC. Heart disease facts, assessed 26th December 2023.
Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J Clin Invest. 2014;124:4204–11. https://doi.org/10.1172/JCI72331.
Wang Z, Tang WH, Buffa J, Fu X, Britt EB, Koeth RA, Levison BS, Fan Y, Wu Y, Hazen SL. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J. 2014;35:904–10. https://doi.org/10.1093/eurheartj/ehu002.
Yoo W, Zieba JK, Foegeding NJ, Torres TP, Shelton CD, Shealy NG, Byndloss AJ, Cevallos SA, Gertz E, Tiffany CR, Thomas JD, Litvak Y, Nguyen H, Olsan EE, Bennett BJ, Rathmell JC, Major AS, Bäumler AJ, Byndloss MX. High fat diet induced colonocyte dysfunction escalates microbiota derived trimethylamine N- oxide. Science. 2021;373(6556):813–818. https://doi.org/10.1126/science.aba3683.
Canyelles M, Tondo M, Cedó L, Farràs M, Escolà-Gil JC, Blanco-Vaca F. Trimethylamine N-oxide: a link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int J Mol Sci. 2018;19:3228. https://doi.org/10.3390/ijms19103228.
Ziesel S. Choline, other methyl donors. Nutrients. 2017;9(5):445. https://doi.org/10.3390/nu9050445.
Gallo M, Gámiz F. Choline: an essential nutrient for human health. Nutrients. 2023;15(13):2900. https://doi.org/10.3390/nu15132900.
Senthong V, Li XS, Hudec T, Coughlin J, Wu Y, Levison B, Wang Z, Hazen SL, Tang WH. Plasma trimethylamine n-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67:2620–8. https://doi.org/10.1016/j.jacc.2016.03.546.
Senthong V, Wang Z, Li XS, Fan Y, Wu Y, Tang WH, Hazen SL. Intestinal microbiota-generated metabolite trimethylamine-n-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a courage-like patient cohort. J Am Heart Assoc. 2016;5. https://doi.org/10.1161/JAHA.115.002816.
Senthong V, Kiatchoosakun S, Wongvipaporn C, Phetcharaburanin J, Tatsanavivat P, Sritara P, Phrommintikul A. Gut microbiota generated metabolite trimethylamine-N-oxide and subclinical myocardial damage: a multicenter study from Thailand. Sci Rep. 2021;11(1):14963. https://doi.org/10.1038/s41598-021-93803-7.
Kanitsoraphan C, Rattanawong P, Charoensri S, Senthong V. Trimethylamine N-oxide and risk of cardiovascular disease and mortality. Curr Nutr Rep. 2018;7(4):207–13. https://doi.org/10.1007/s13668-018-0252-z.
Fu BC, Hullar MAJ, Randolph TW, Franke AA, Monroe KR, Cheng I, Wilkens LR, Shepherd JA, Madeleine MM, Le Marchand L, Lim U, Lampe JW. Associations of plasma trimethylamine N-oxide, choline, carnitine and betaine with inflammatory and cardiometabolic risk biomarkers and the fecal microbiome in the Multiethnic Cohort Adiposity Phenotype Study. Am J Clin Nutr. 2020;111(6):1226–34. https://doi.org/10.1093/ajcn/nqaa015.
Gregory JC, Buffa JA, Org E, Wang Z, Levison BS, Zhu W, Wagner MA, Bennett BJ, Li L, DiDonato JA, Lusis AJ, Hazen SL. Transmission of atherosclerosis susceptibility with gut microbial transplantation. J Biol Chem. 2015;290:5647–60. https://doi.org/10.1074/jbc.M114.618249.
Astudillo AA, Mayrovitz HN. The gut microbiome and cardiovascular disease. Cureus. 2021;13(4):e14519. https://doi.org/10.7759/cureus.14519.
Díez-Ricote L, Ruiz-Valderrey P, Micó V, Blanco-Rojo R, Tomé-Carneiro J, Dávalos A, Ordovás JM, Daimiel L. TMAO modulates the expression of cardiovascular disease related microRNAs and their targets. Int J Mol Sci. 2021;22(20):11145. https://doi.org/10.3390/ijms222011145.
Díez-Ricote L, Ruiz-Valderrey P, Micó V, Blanco R, Tomé-Carneiro J, Dávalos A, Ordovás JM, Daimiel L. TMAO upregulates members of the miR-17/92 cluster and impacts targets associated with atherosclerosis. Int J Mol Sci. 2022;23(20):12107. https://doi.org/10.3390/ijms232012107.
Smits LP, Kootte RS, Levin E, Prodan A, Fuentes S, Zoetendal EG, Wang Z, Levison BS, Cleophas MCP, Kemper EM, Dallinga-Thie GM, Groen AK, Joosten LAB, Netea MG, Stroes ESG, de Vos WM, Hazen SL, Nieuwdorp M. Effect of vegan fecal microbiota transplantation on carnitine- and choline-derived trimethylamine-N-oxide production and vascular inflammation in patients with metabolic syndrome. J Am Heart Assoc. 2018;7(7):e008342. https://doi.org/10.1161/JAHA.117.008342.
Meyer KA, Shea JW. Dietary choline and betaine and risk of CVD: a systematic review and meta-analysis of prospective studies. Nutrients. 2017;9(7):711. https://doi.org/10.3390/nu9070711.
Zheng Y, Li Y, Rimm EB, Hu FB, Albert CM, Rexrode KM, Manson JE, Qi L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among us women and men. Am J Clin Nutr. 2016;104:173–80.
Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, Tsuji M, Nakamura K. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. 2015;145:1787–92.
Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition. 2015;31:1317–23.
Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–84.
Zhou R, Yang M, Yue C, Shi Y, Tan Y, Zha L, Zhang J, Chen S. Association between dietary choline intake and cardiovascular diseases: National Health and Nutrition Examination Survey 2011–2016. Nutrients. 2023;15(18):4036. https://doi.org/10.3390/nu15184036.
Arias N, Arboleya S, Allison J, Kaliszewska A, Higarza SG, Gueimonde M, Arias JL. The relationship between choline bioavailability from diet, intestinal microbiota composition and its modulation of human diseases. Nutrients. 2020;12(8):2340. https://doi.org/10.3390/nu12082340.
Pellegrino A, Coppola G, Santopaolo F, Gasbarrini A, Ponziani FR. Role of Akkermansia in human disease: from causation to therapeutic properties. Nutrients. 2023;15(8):1815. https://doi.org/10.3390/nu15081815.
Centner AM, Khalili L, Ukhanov V, Kadyan S, Nagpal R, Salazar G. The role of phytochemicals and gut microbiome in atherosclerosis in preclinical mouse models. Nutrients. 2023;15(5):1212. https://doi.org/10.3390/nu15051212.
Khalili L, Centner AM, Salazar G. Effects of berries, phytochemicals and probiotics on atherosclerosis through gut microbiota modification: a meta analysis of animal studies. Int J Mol Sci. 2023;24(4):3084. https://doi.org/10.3390/ijms24043084.
Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–103. https://doi.org/10.1038/s41591-019-0495-2.
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study. Lancet. 2020;2017(395):709–33. https://doi.org/10.1016/S0140-6736(20)30045-3.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. https://doi.org/10.1056/NEJMoa041031.
Xie F, Zhen X, Liu Z, Chen X, Liu Z, Zhou M, Zhou Z, Hu Z, Zhu F, Huang Q, Zhang L, Nie J. Dietary choline, via gut microbe-generated trimethylamine-N-oxide, aggravates chronic kidney disease-induced cardiac dysfunction by inhibiting hypoxia induced factor 1α. Front Physiol. 2022;13:996166. https://doi.org/10.3389/fphys.2022.996166.
Bi X, Yang K, Zhang B, Zhao J. The protective role of klotho in CKD-associated cardiovascular disease. Kidney Dis. 2020;6:395–406. https://doi.org/10.1159/000509369.
Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat Rev Nephrol. 2019;15:159–75. https://doi.org/10.1038/s41581-018-0101.
Chumakova SP, Urazova OI, Shipulin VM, Andreev SL, Denisenko OA, Gladkovskaya MV, Litvinova LS, Bubenchikov MA. Role of angiopoietic coronary endothelial dysfunction in the pathogenesis of ischemic cardiomyopathy. Biomedicines. 2023;11(7):1950. https://doi.org/10.3390/biomedicines11071950.
Li Y, Lu H, Guo J, Zhang M, Zheng H, Liu Y, Liu W. Gut microbiota derived trimethylamine N oxide is associated with the risk of all cause and cardiovascular mortality in patients with chronic kidney disease: a systematic review and meta-analysis. Ann Med. 2023;55(1):2215542. https://doi.org/10.1080/07853890.2023.2215542.
Nakano T, Katsuki S, Chen M, Decano JL, Halu A, Lee LH. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation via the interplay of OATP2B1 and dll4-notch signaling. Circulation. 2019;139:78–96. https://doi.org/10.1161/CIRCULATIONAHA.118.034588.
Tang WH, Wang Z, Kennedy DJ, Wu Y, Buffa JA, Agatisa-Boyle B, Li XS, Levison BS, Hazen SL. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116:448–55. https://doi.org/10.1161/CIRCRESAHA.116.305360.
Qu X, Li X, Zheng Y, Ren Y, Puelles VG, Caruana G, Nikolic-Paterson DJ, Li J. Regulation of renal fibrosis by Smad3 Thr388 phosphorylation. Am J Pathol. 2014;184(4):944–52. https://doi.org/10.1016/j.ajpath.2013.12.003.
Zhang W, Miikeda A, Zuckerman J, Jia X, Charugundla S, Zhou Z, Kaczor-Urbanowicz KE, Magyar C, Guo F, Wang Z, Pellegrini M, Hazen SL, Nicholas SB, Lusis AJ, Shih DM. Inhibition of microbiota dependent TMAO production attenuates chronic kidney disease in mice. Sci Rep. 2021;11(1):518. https://doi.org/10.1038/s41598-020-80063-0.
Zeng Y, Guo M, Fang X, Teng F, Tan X, Li X, Wang M, Long Y, Xu Y. Gut microbiota derived trimethylamine N oxide and kidney function: a systematic review and meta-analysis. Adv Nutr. 2021;12(4):1286–304. https://doi.org/10.1093/advances/nmab010.
Guo F, Dai Q, Zeng X, Liu Y, Tan Z, Zhang H, Ouyang D. Renal function is associated with plasma trimethylamine N oxide, choline, L carnitine and betaine: a pilot study. Int Urol Nephrol. 2021;53(3):539–51. https://doi.org/10.1007/s11255-020-02632-6.
Zhang J, Zhu P, Li S, Gao Y, Xing Y. From heart failure and kidney dysfunction to cardiorenal syndrome. Front Pharmacol. 2023;21(14):1291922. https://doi.org/10.3389/fphar.2023.1291922.
Xu KY, Xia GH, Lu JQ, Chen MX, Zhen X, Wang S, You C, Nie J, Zhou HW, Yin J. Impaired renal function and dysbiosis of gut microbiota contribute to increased trimethylamine Noxide in chronic kidney disease patients. Sci Rep. 2017;7(1):1445. https://doi.org/10.1038/s41598-017-01387-y.
Mafra D, Cardozo L, Ribeiro-Alves M, Bergman P, Shiels PG, Stenvinkel P. Short report: choline plasma levels are related to Nrf2 transcriptional expression in chronic kidney disease? Clin Nutr ESPEN. 2022;50:318–21. https://doi.org/10.1016/j.clnesp.2022.06.008.
Pignanelli M, Bogiatzi C, Gloor G, Allen-Vercoe E, Reid G, Urquhart BL, Ruetz KN, Velenosi TJ, Spence JD. Moderate renal impairment and toxic metabolites produced by the intestinal microbiome: dietary implications. J Ren Nutr. 2018;10(6):779. https://doi.org/10.1053/j.jrn.2018.05.007.
Li HB, Xu ML, Xu XD, Tang YY, Jiang HL, Li L, Xia WJ, Cui N, Bai J, Dai ZM, Han B, Li Y, Peng B, Dong YY, Aryal S, Manandhar I, Eladawi MA, Shukla R, Kang YM, Joe B, Yang T. Faecalibacterium prausnitzii attenuates CKD via butyrate renal GOR43 axis. Circ Res. 2022;131(9):e120–34. https://doi.org/10.1161/CIRCRESAHA.122.320184.
Van Echten-Deckert G, Alam S. Sphingolipid metabolism-an ambiguous regulator of autophagy in the brain. Biol Chem. 2018;399:837–50. https://doi.org/10.1515/hsz-2018-0237.
Blusztajn JK, Slack BE, Mellott TJ. Neuroprotective actions of dietary choline. Nutrients. 2017;9(8):815. https://doi.org/10.3390/nu9080815.
Strupp BJ, Powers BE, Velazquez R, Ash JA, Kelley CM, Alldred MJ, Strawderman M, Caudill MA, Mufson EJ, Ginsberg SD. Maternal choline supplementation: a potential prenatal treatment for Down syndrome and Alzheimer’s disease. Curr Alzheimer Res. 2016;13:97–106. https://doi.org/10.2174/1567205012666150921100311.
Mellott TJ, Huleatt OM, Shade BN, Pender SM, Liu YB, Slack BE, Blusztajn JK. Perinatal choline supplementation reduces amyloidosis and increases choline acetyltransferae expression in the hippocampus of the APPswePS1dE9 Alzheimer’s disease model mice. PLoS ONE. 2017;12:e0170450. https://doi.org/10.1371/journal.pone.0170450.
Halder R, Hennion M, Vidal RO, Shomroni O, Rahman RU, Rajput A, Centeno TP, van Bebber F, Capece V, Garcia Vizcaino JC, Schuetz AL, Burkhardt S, Benito E, Navarro Sala M, Javan SB, Haass C, Schmid B, Fischer A, Bonn S. DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat Neurosci. 2016;19:102–10. https://doi.org/10.1038/nn.4194.
Ricceri L, De Filippis B, Fuso A, Laviola G. Cholinergic hypofunction in MeCP2–308 mice: beneficial neurobehavioural effects of choline supplementation. Behav Brain Res. 2011;221(2):623–629. https://doi.org/10.1016/j.bbr.2011.03.051.
Velazquez R, Ash JA, Powers BE, Kelley CM, Strawderman M, Luscher ZI, Ginsberg SD, Mufson EJ, Strupp BJ. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in Ts65Dn mouse model of Down syndrome. Neurobiol Dis. 2013;58:92–101. https://doi.org/10.1016/j.nbd.2013.04.016.
Kelley CM, Powers BE, Velazquez R, Ash JA, Ginsberg SD, Strupp BJ, Mufson EJ. Maternal choline supplementation differentially alters the basal forebrain cholinergic system of young adult Ts65Dn and disomic mice. J Comp Neurol. 2014;522(6):1390–410. https://doi.org/10.1002/cne.23492.
Ash JA, Velazquez R, Kelley CM, Powers BE, Ginsberg SD, Mufson EJ, Strupp BJ. Maternal choline supplementation improves spatial mapping and increases basal forebrain cholinergic neuron number and size in aged Ts65Dn mice. Neurobiol Dis. 2014;70:32–42. https://doi.org/10.1016/j.nbd.2014.06.001.
Borges AA, El-Batah PN, Yamashita LF, Santana Ados S, Lopes AC, Freymuller-Haapalainen E, Coimbra CG, Sinigaglia-Coimbra R. Neuro[rotective effects of oral choline administration after global brain ischemia in rats. Nutr Neurosci. 2015;18(6):265–74. https://doi.org/10.1179/1476830514Y.0000000125.
Langley E, Krykbaeva M, Blusztajn JK, Mellott TJ. High maternal choline consumption during pregnancy and nursing alleviates deficits in social interaction and improves anxiety like behaviors in the BTBRT+itpr3tf/J mouse model of autism. Behav Brain Res. 2015;278:210–20. https://doi.org/10.1016/j.bbr.2014.09.043.
Powers BE, Kelley CM, Velazquez R, Ash JA, Strawderman MS, Alldred MJ, Ginsberg SD, Mufson EJ, Strupp BJ. Maternal choline supplementation in a mouse model of Down syndrome. Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience. 2017;340:501–514. https://doi.org/10.1016/j.neuroscience.2016.11.001.
Jadavji NM, Emmerson JT, MacFarlane AJ, Willmore G, Smith PD. B vitamin and choline supplementation increases neuroplasticity and recovery after stroke. Neurobiol Dis. 2017;103:89–100. https://doi.org/10.1016/j.nbd.2017.04.001.
Hurley L, Jauhal J, Ille S, Pull K, Malysheva OV, Jadavji NM. Maternal dietary deficiencies in folic acid and choline result in larger volume, reduced neuro-degeneration and inflammation and changes in choline metabolites after ischemic stroke in middle-ages offspring. Nutrients. 2023;15(7):1556. https://doi.org/10.3390/nu15071556.
Clementson M, Hurley L, Coonrod S, Bennett C, Marella P, Pascual AS, Pull K, Wasek B, Bottiglieri T, Malysheva O, Caudill MA, Jadavji NM. Maternal dietary deficiencies of folic acid or choline worsen stroke outcomes in adult male and female mouse offspring. Neural Regen Res. 2023;18(11):2443–8. https://doi.org/10.4103/1673-5374.371375.
Pull K, Folk R, Kang J, Jackson S, Gusek B, Esfandiarei M, Jadavji NM. Impact of maternal dietary folic acid or choline dietary deficiencies on vascular function in young and middle aged female mouse offspring after ischemic stroke. Am J Physiol Heart Circ Physiol. 2023;325(6):H1354–9. https://doi.org/10.1152/ajpheart.00502.2023.
Boeke CE, Gillman MW, Hughes MD, Rifas-Shiman SL, Villamor E, Oken E. Choline intake during pregnancy and child cognition at age 7 years. Am J Epidemiol. 2013;177(12):1338–47. https://doi.org/10.1093/aje/kws395.
Lavery AM, Brender JD, Zhao H, Sweeney A, Felkner M, Suarez L, Canfield MA. Dietary intake of choline and neural tube defects in Mexican-Americans. Birth Defects Res A Clin Mol Teratol. 2014;100(6):463–471. https://doi.org/10.1002/bdra.23236.
Dalla Via A, Gargari G, Taverniti V, Rondini G, Velardi I, Gambaro V, Visconti GL, De Vitis V, Gardana C, Ragg E, Pinto A, Riso P, Gugliemetti S. Urinary TMAO levels are associated with the taxonomic composition of the gut microbiota and with the choline TMA-lyase gene (cutC) harbored by enterobacteriaceae. Nutrients. 2020;12:62. https://doi.org/10.3390/nu12010062.
Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127(4):553–70. https://doi.org/10.1161/CIRCRESAHA.120.316242.
Kang JW, Zivkovic AM. Are eggs good again? A precision nutrition perspective of the effects of eggs on cardiovascular risk, taking into account plasma lipid profiles and TMAO. J Nutr Biochem. 2022;100:108906. https://doi.org/10.1016/j.jnutbio.2021.108906.
Guasch-Ferré M, Hu FB, Ruiz-Canela M, Bulló M, Toledo E, Wang DD, Corella D, Gómez-Gracia E, Fiol M, Estruch R, Lapetra J, Fitó M, Arós F, Serra-Majem L, Ros E, Dennis C, Liang L, Clish CB, Martínez-González MA, Salas-Salvadó J. Plasma metabolites from choline pathway and the risk of cardiovascular disease in the MREDIMED study. J Am Heart Assoc. 2017;6(11):e006524. https://doi.org/10.1161/JAHA.117.006524.
Fadhlaoui K, Arnal ME, Martineau M, Camponova P, Ollivier B, O’Toole P, Brugère JF. Archaea, specific genetic traits, and development of improved bacterial live biotherapeutic products: another face of next-generation probiotics. Appl Microbiol Biotechnol. 2020;104:4705–16. https://doi.org/10.1007/s00253-020-10599-8.
DiStefano JK. The role of choline, soy isoflavones and probiotics as adjuvant treatments in the prevention and management of NAFLD in postmenopausal women. Nutrients. 2023;15:2670. https://doi.org/10.3390/nu15122670.
Papadavid E, Vlami K, Dalamaga M, Giatrakou S, Theodoropoulos K, Gyftopoulos S, Stavrianeas N, Papiris S, Rigopoulos D. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol. 2013;27:820–6. https://doi.org/10.1111/j.1468-3083.2012.04580.x.
Spyrou N, Vallianou N, Kadillari J, Dalamaga M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin Cancer Biol. 2021;73:356–76. https://doi.org/10.1016/j.semcancer.2021.05.008.
Vallianou NG, Kounatidis D, Tsilingiris D, Panagopoulos F, Christodoulatos GS, Evangelopoulos A, Karampela I, Dalamaga M. The role of next-generation probiotics in obesity and obesity-associated disorders: current knowledge and future perspectives. Int J Mol Sci. 2023;24:6755. https://doi.org/10.3390/ijms24076755.
Funding
Open access funding provided by HEAL-Link Greece.
Author information
Authors and Affiliations
Contributions
NV wrote the manuscript. DK, FP, EG, and TS were responsible for literature search and references. SP, IK, and DT made the figures and tables. MD reviewed and supervised the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
Not applicable
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vallianou, N.G., Kounatidis, D., Psallida, S. et al. The Interplay Between Dietary Choline and Cardiometabolic Disorders: A Review of Current Evidence. Curr Nutr Rep 13, 152–165 (2024). https://doi.org/10.1007/s13668-024-00521-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-024-00521-3