Abstract
Euodiae Fructus, referred to as “Wuzhuyu” in Chinese, has been used as local and traditional herbal medicines in many regions, especially in China, Japan and Korea, for the treatment of gastrointestinal disorders, headache, emesis, aphtha, dermatophytosis, dysentery, etc. Substantial investigations into their chemical and pharmacological properties have been performed. Recently, interest in this plant has been focused on the different structural types of alkaloids like evodiamine, rutaecarpine, dehydroevodiamine and 1-methyl-2-undecyl-4(1H)-quinolone, which exhibit a wide range of pharmacological activities in preclinical models, such as anticancer, antibacterial, anti-inflammatory, anti-cardiovascular disease, etc. This review summarizes the up-to-date and comprehensive information concerning the botany, traditional uses, phytochemistry, pharmacology of Euodiae Fructus together with the toxicology and quality control, and discusses the possible direction and scope for future research on this plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Euodiae Fructus (EF), known as “Wuzhuyu” in China, “Goshuyu” in Japan and “Osuyu” in Korea, are the dried and nearly ripe fruits of Euodia rutaecarpa (Juss.) Benth. (ER), E. rutaecarpa (Juss.) Benth var. officinalis (Dode) Huang (ERO), and E. rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang (ERB). It has been used as traditional Chinese medicine (TCM) for more than 2000 years and is officially listed in multiple versions of Chinese Pharmacopoeia. At the same time, it is also traditionally and ethnically used in Japan and Korea. According to the records of TCM, Euodiae Fructus could be widely used either alone or in combination with other herbal medicines as remedies for gastrointestinal disorders (abdominal pain, dysentery), headache, emesis, aphtha, dermatophytosis, dysentery, amenorrhoea, menorrhalgia and postpartum haemorrhage. However, it is worth noting that irrational use of this herb could cause toxic symptoms such as stomach ache, vomiting, blurred vision, etc.
With the increasing interest paid to the pharmacologically phytochemicals from the Euodiae Fructus, a lot of investigations related to the phytochemical and pharmacological aspects of this plant have been carried out. To date, a variety of chemical constituents, including alkaloids, terpenoids and steroids, as well as phenols and volatile oils, have been isolated and identified from Euodiae Fructus. Pharmacological studies revealed that the crude extracts and purified compound possess a wide spectrum of biological activities, involving in anticancer, antibacterial, anti-inflammatory, insecticide, anti-cardiovascular, neuroprotective, anti-obesity and anti-diabetic activities, confirmed by various in vivo and in vitro experiments, as shown in Fig. 1. In recent years, several reviews have been published on the chemical and biological activities of ivodimine [1, 2], erythrartine [3, 4] and citrinin [5]. A review of Euodiae Fructus is essential for present and future study toward improving phytochemical and pharmacological investigation. Herein, we systematically described and summarized the study advances of Euodiae Fructus in recent decades, including phytochemical, pharmacological effects, toxicity, and quality control. We reviewed the literature up to February 2021.
2 Botanical descriptions
In Chinese Pharmacopoeia, the dried and nearly ripe fruits of three plants of the genus Euodia rutaecarpa (Juss.) Benth. (ER), E. rutaecarpa (Juss.) Benth var. officinalis (Dode) Huang (ERO), and E. rutaecarpa (Juss.) Benth. var. bodinieri (Dode) Huang (ERB) are commonly known as Euodiae Fructus.
The common botanical characteristics of the above three plants are small trees or shrubs, 3–5 m high. They often have opposite odd-pinnate leaves. Their inflorescences are terminal; the flowers of the male inflorescence are separated from each other, and the flowers of the female inflorescence are dense or separated. The dioecious flowers have 4 or 5 sepals, petals, stamens, and carpels. The fruits are oblate and split into 5 petals when mature, and they are follicle-like, purplish red, with large oil glands on the surface, and each fruit has one seed, black and shiny. The flowering period ranges from June to August, and the fruit period is typically from August to November. However, there are also some differences of them in botanical descriptions and distribution area, as shown in Table 1.
These three plants usually grow in mountains, roadsides, or sparse forests. It is mainly produced in the southern regions of China (such as Hunan, Guizhou, Sichuan, Yunnan), as well as in Japan, Korea, Bhutan, northeast India, Myanmar, and Nepal.
3 Traditional uses
Euodiae Fructus has a long history as a traditional remedy and has been widely used Chinese medicine as recorded in the ancient herbal books and Pharmacopoeia of the People’s Republic of China (Editorial Committee of Chinese Pharmacopoeia, 2020). According to the history of TCM, Euodiae Fructus, initially recorded in "Shen Nong’s Herbal Classic", is listed as a middle-grade herbal item and also described as being pungent and bitter in taste and can return to the liver, spleen, stomach and kidney meridians. According to the records of “Ri Hua Zi Ben Cao”, it could strengthen the spleen, treat abdominal pain, beriberi, edema, and postpartum haemorrhage. Furthermore, it was found to kill harmful insects and prevent tooth decay in “Ben Cao Shi Yi”. As it was recorded in “Compendium of Materia Medica”, the main function of Euodiae Fructus was to improve digestion, relieve headache, abdominal pain and treat hemorrhoids in throat, mouth and tongue [6]. According to the 2020 Edition of Chinese Pharmacopoeia, Euodiae Fructus is often used for external use and the recommended dosage is 2–5 g, the “standard” processing method of Euodiae Fructus is stir-frying with licorice water extract, other usual processing methods include washing with hot or cold water [7].
Since the compatibility of medicines is considered to improve effects, reduce toxicity, or achieve synergistic or balanced effects [7]. Euodiae Fructus was often combined with Jujubae Fructus, which has the effect of treating stomachache and pregnancy headache. If combined with Angelicae Sinensis Radix, it could promote blood circulation and relieve menstrual pain. When combined with Zingiberis Rhizoma Recens, it could promote yang and dispel cold. Moreover, Euodiae Fructus could be used in a combination with Codonopsis Radix or Foeniculi Fructus, thereby playing a significant role in tonifying and warming stomach, etc. Based on the above compatibility, Euodiae Fructus was typically used in polyherbal formulations in TCM (http://www.zysj.com.cn/zhongyaofang/index.html), and the composition and therapeutic effects of typical polyherbal formulations are summarized in Table 2. In recent years, numerous studies in vitro and vivo have indicated that “Zuo Jin Wan” (ZJW) possess good pharmacological effects, such as anti-inflammation, anti-ulcer [8], anti-acid [9], antidepressant-like [10], and anti-cancer properties [11]. Noteworthy, Li et al. conducted a systematic review and meta-analysis according to a total of 1736 patients in 18 studies, indicating “Wenjing Tang” was shown to be significantly superior to nonsteroidal anti-inflammatory drugs in improving primary dysmenorrhea in terms of clinical effective rate, the visual analogue scale, and the pain scale for dysmenorrhea [12].
Besides, Euodiae Fructus is also popular in Japan and South Korea. According to Dongui Bogam, a representative Korean Medicine book, Euodiae Fructus has been frequently used as a prescription for treating headache, abdominal pain, vomiting, cold, reducing blood circulation and gynecological diseases (amenorrhea), with a dose of 2–8 g. It is also one of the main components of traditional herbal prescriptions for the treatment of sterility caused by irregular menstruation such as Chokyungjongok-Tang, Nangungjongsa-whan, and Onkyung-Tang [13]. In addition, Euodiae Fructus was introduced in Japan as early as Edo, mainly applied for the treatment of cold and pain. For example, Goshuyuto, a representative traditional Japanese medicine, also known as “Wuzhuyu Tang” in China and “Osuyu-tang” in Korea, is composed of four medicinal herbs, Euodiae Fructus, Ginseng Radix Et Rhizoma, Zingiberis Rhizoma Recens, Jujubae Fructus, and it could be used to treat migraine headache, nausea, beriberi, and heart failure [14].
4 Phytochemistry
To date, more than 240 kinds of constituents have been isolated and identified from Euodiae Fructus, including 133 alkaloids, 36 terpenoids, 5 steroids, 51 phenols and 15 other compounds. Among them, alkaloids and terpenoids have been identified as the characteristic components. All compounds are summarized and compiled in Table 3.
4.1 Alkaloids
The alkaloids extracted from Euodiae Fructus have attracted wide attention from chemists and pharmacologists due to their various biological effects. Among these compounds, indole alkaloids and quinolone alkaloids are the main structural types.
Up to 53 indole alkaloids were isolated from Euodiae Fructus, and their structures are shown in Fig. 2. Evodiamine, rutaecarpine and dehydroevodiamine are regarded as the dominant chemical constituents with a wide range of pharmacological activities.
There are 73 quinolone alkaloids extracted from Euodiae Fructus and their structure are shown in Fig. 3. Among them, quinolinone with an alpha-substituted saturated or unsaturated aliphatic hydrocarbon group is the typical structures of these compounds [6]. 1-Methyl-2-undecyl-4(1H)-quinolone is a representative constituent of these compounds, which has been reported to exhibit anticancer activity [20, 47], anti-calcific aortic stenosis [88], and monoamine oxidase type B (MAO-B) inhibitory [51].
Other types of alkaloids have also been isolated from plants of Euodiae Fructus, including berberine, synephrine, caffeine, N-methylanthranylamide, N-(trans-p-coumaroyl)-tyramine, N-(cis-p-coumaroyl)-tyramine, etc. Their structures are shown in Fig. 4.
4.2 Terpenoids
There are 36 terpenoids (25 limonoids, 10 triterpenoids and a diterpenoid) also isolated from this plant, and their structures are presented in Figs. 5, 6. Limonoids are highly oxidized tetracyclic triterpenoids with furan ring, in which limonin is the most typical bioactive limonoids from Euodiae Fructus. In 1988, Tohru et al. isolated seven known limonoids, including limonin (134), rutaevin (138), rutaevin acetate (139), graucin A (140), evodol (141), jangomolide (145), obacunone (155), together with four new limonoids, 12α-hydroxylimonin (135), 12α-hydroxyevodol (142), 6α-acetoxy-5-epilimonin (146), 6β-acetoxy-5-epilimonin (147) [18]. In 1991, three limonoid glucosides, including limonin 17-β-D-glucopyranoside (137), limonin diosphenol 17-β-D-glucopyranoside (144) and 6β-hydroxy-5-epilimonin 17-β-D-glucopyranoside (148), were isolated from this plant [72]. In recent years, three new limonoids, such as evorubodinin (149), shihulimonin A (150) [73], and 6α-acetoxyl-12α-hydroxyevodol (143) [62], were first found from Euodiae Fructus, together with 12 known limonoids. Lately, an investigation of the 95% ethanol extract of Euodiae Fructus yielded two known limonoids (7-deacetylproceranone (156) and nomilin (157)), two novel nortriterpenoids (evoditrilones A (165) and B (166)), and three known triterpenoids (oleanic acid (164), ursolic acid (167), and 3β-hydroxyoleana-11,13(18)-diene (168)) [70]. Other triterpenoids mainly include 12-ursen-3-ol (159), 14-ursen-3-ol-1-one (160), glycyrrhizic acid (161), glycyrrhetinic (162) and taraxerone (163) [75].
4.3 Steroids
Phytosterols are a class of physiologically active constituents widely used in cosmetics, food and medicine. Steroids are relatively rare in Euodiae Fructus, and only five steroids were reported and characterized. In 2010, four steroids, namely, β-sitosterol (170), stigmasterol (171), β-hydroxystigmast-5-en-7-one (172) and 3β-hydroxystigmasta-5,22-dien-7-one (173), were found in methanol extract of the fruits of Euodiae Fructus [19]. In further studies, another steroid named daucosterol (174) was obtained from the 95% ethanol extract of Euodiae Fructus [81]. Their structures are presented in Fig. 7.
4.4 Phenols
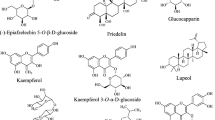
To date, 51 phenols are characterized in this plant (Figs. 8, 9). Among them, 27 flavonoids were classified into three structural types, including flavones, tricin-7-O-β-D-glucopyranoside (175), diosmetin-7-O-β-D-glucopyranoside (176), diosmin (177), chrysoeriol-7-O-rutinoside (178) and phellodensin F (195) [67, 76]; flavonols, isorhamnetin, quercetin and their derivatives, which have been confirmed to exhibit a wide spectrum of pharmacological activities [89, 90]; and dihydroflavones, such as flavaprin (197), evodioside B (198) and hesperidin (199) [16, 67, 81].
Moreover, several phenolic acids and their derivatives have also been found in Euodiae Fructus. In 2013, caffeic acid (208) was isolated from the genus Evodia for the first time [81]. In recent years, He et al. isolated a new caffeoylgluconic acid derivative, trans-caffeoyl-6-O-d-gluconic acid methyl ester (218), together with two known compounds named trans-caffeoyl-6-O-d-gluconog-lactone (219) and trans-caffeoyl-6-O-D-gluconic acid (217) from Euodiae Fructus. Moreover, four new caffeoylgluconic acids, including 2-O-trans-caffeoylgluconic acid (213), 3-O-trans-caffeoyl-gluconic acid (214), 4-O-trans-caffeoylgluconic acid (215), 5-O-trans-caffeoylgluconic acid (216), together with three known ones including neochlorogenic acid (205), chlorogenic acid (206) and 3-O-caffeoylquinic acid methyl ester (207) were obtained from Euodiae Fructus [83]. In addition, trans-caffeic acid methylate (209), ferulic acid (210), p-hydroxycinnamic acid (211), trans-feruloylgluconic acid (220), p-hydroxybenzoic acid ethyl ester (222), 3,4-dihydroxy-benzoic acid (224) [84], and a new phenylpropanoid glycoside, 9-O-feruloyl-4-O-β-d-glucopyanoside (221) [84], were characterized in Euodiae Fructus. Additionally, chrysophanol (202), emodin (203), physcion (204) [82], and isovanillin (223), were successfully extracted from Euodiae Fructus.
4.5 Volatile oil
The volatile oil is one of the main chemical compositions of Euodiae Fructus and its content is very high. Liu et al. identified 97 constituents by gas chromatography/mass spectrometer (GC/MS) analysis from 24 samples [91]. Another study showed that 97 constituents identified by SPME-GC–MS, accounted for 96.80% of volatile oil. Among the isolated volatile oil, the relative content of sesquiterpenes was more than 38%, monoterpenoids components was over 35%, ester components were above 13% [92]. It also indicated that the main constituents of the volatile oil from Euodiae Fructus were β-myrcene (17.7%), (Z)-β-ocimene (14.8%), α-phellandrene (14.7%), γ-terpinene (6.4%), linalool (5.7%) and β-thujene (5.1%) [93]. Moreover, several researches have been reported the volatile constituents obtained from Euodiae Fructus, such as caryophyllene oxide, linalool and γ-Elemene, have diverse functions, such as sedative, antiasthmatic, antibacterial, antitumor, antiviral and insect repellent, and its main components are caryophyllene oxide. It has been found that elemene is a new anticancer drug with great potential and has a broad clinical application prospect. Meanwhile, γ-Elemene can promote the immune function of erythrocytes [94]. However, modern toxicology studies showed that volatile oil could induce certain acute liver damage [95]. Taken together, the volatile oil may be efficacy material basis and toxicity material basis, but the research is isolated and lack of correlation, so further studies need be conducted to provide the experimental data and literature evidence for reasonable and safe development of the volatile oil from Evodia Fructus.
4.6 Other compounds
Besides the above chemical constituents, syringoside (231), coniferin (232), citric acid (233), 4-methoxybenzylalcohol (234), myo-inositol (235), phthalic acid dibutyl ester (236) [87], and some fatty acids, such as 2-pentadecanone (237), 1-octadecanol (238), n-heptacosanol (239), glycerol 1-octadecanoate (240) [74]; three new ester glycosides, such as ruticarpside A (226), ruticarpside B (227) and ruticarpside C (228) [85], and two new γ-lactone derivatives, evodinoids A (229) and B (230) [86], have also been reported in Euodiae Fructus. All the structures are shown in Fig. 10.
5 Pharmacology
As a well-known medicinal plant in TCM, Euodiae Fructus has been validated to possess a diverse set of pharmacological properties, such as anticancer activity [96, 97], antibacterial activity [98], anti-inflammatory activity [45, 99, 100], insecticide activity [31], antinociceptive activity [101], and anti-diarrheal effect [102]. Evodiamine [1], rutaecarpine [4], and limonin [5], which are major compounds of this plant and display a variety of biochemical and pharmacological properties in the cancer, cardiovascular, central nervous system and so on, and it is possible to be developed as a promising lead compound for drug discovery. All the detailed information is shown in Table 3.
5.1 Anticancer activity
In crude extracts, the methanol extract of Euodiae Fructus decreased the AP‐1 stimulator 12‐O‐tetradecanoylphorbol‐13‐acetate (TPA)‐induced AP‐1 transactivation in Chang/AP‐1 cells, with an EC50 value of 24.72 μg/mL [103]. Park et al. found that the 70% ethanol extract of Euodiae Fructus could induce apoptosis in HeLa cells with IC50 of about 45 μg/mL, which may be associated with a caspase-dependent cascade through activation of the intrinsic signaling pathway connected with AMP-activated protein kinase activation [96]. Another study showed that the 70% ethanol extract of Euodiae Fructus could inhibit the growth of benign prostatic hyperplasia-1 (BPH-1) epithelial cells by inhibiting proteins and antigens including 5α-reductase, proliferating cell nuclear antigen (PCNA), phosphor-ERK1/2, and cyclin D1 and by inhibiting cell viability dependently through the activation of caspase-3 and caspase-8 [97]. Additionally, ZJP aqueous extract exhibited its prominent therapeutic effects on hepatocellular carcinoma (HCC) mainly via the regulation of cell proliferation and survival though the EGFR/MAPK, PI3K/NF-κB, and CCND1 signaling pathways [104].
Numerous in vitro studies have reported that the isolated compounds of Euodiae Fructus display antitumor activities in several cancer cell lines, and the detailed information is presented in Table 3. Growing evidence demonstrates that evodiamine possesses anti-cancer activities both in vitro and in vivo by inhibiting proliferation, invasion and metastasis, inducing apoptosis of a variety of tumor cell lines, including colon cancer (HT-29, 26-L5, LoVo, COLO205 and HCT116), leukaemia (HL-60, CCRF-CEM, K562 and THP-1), hepatocellular carcinoma (Hep G2, Hepa1-6 and Hepa-1c1c7), lung cancer (H-460, A549), gastric cancer (N-87, AGS and SGC7901), renal carcinoma (Caki-1), breast cancer (MDA-MB-231), ovarian cancer cells (A2780/WT, A2780/PTXR, A2980, A2780CP, ES-2 and SKOV-3), prostate cancer (PC-3), melanoma (B16-F10, A375-S2), nasopharyngeal carcinoma (HONE1 and CNE1), glioblastoma (U87-MG, U87 and C6), urothelial cell carcinoma (5637 and HT1197), multiple myeloma (U266 and RPMI8226), cholangiocarcinoma (HuCCT-1 and TFK-1), cervical cancer (HeLa) cells etc. The related models are presented in Table 4.
5.2 Antibacterial and antifungal activity
Euodiae Fructus has been used to treat infection-related diseases including diarrhea, beriberi and oral ulcer for a long time due to its antibacterial and antifungal activities. The ethanol extract of Euodiae Fructus inhibited the growth of Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 6538, Streptococcus pyogenes Δ-68, Escherichia coli ATCC 11229, Proteus mirabilis ATCC 14159, P. aeruginosa ATCC 27853, and Candida albicans CBS 5982, with minimum inhibitory concentration (MIC) values of 1.0, 0.5–1.0, 0.25, 1.0, 1.0, 1.0, and 0.5 mg/mL, respectively after 24 h of incubation in Muller-Hinton broth [98]. Another study showed that the 95% methanol extract showed inhibitory activity against Helicobacter pylori ATCC 49503 with MIC value of 25 μg/mL, and inhibited the urease activity in H. pylori via inhibiting the ureB expression [216]. Moreover, Liu et al. found that essential oils of Euodiae Fructus show the most potent activities against Bacillus subtilis and Staphylococcus aureus, with the largest inhibition zone diameters of 17.9 and 12.2 mm, respectively, and the MIC values of 3.2–6.4 mg/mL [91].
In isolated compounds, the two novel alkyl methyl quinolone alkaloids (compounds 80–81) (AM quinolones) shown highly selective antimicrobial activity against H. pylori without harmful adverse effects against other intestinal flora [56], thereby being a candidate for use in eradication therapy for H. pylori in vitro and vivo [217]. In addition, evodiamine was able to augment the NLRP3 inflammasome activation by inducing acetylation at K40 residue of α-tubulin, thus conferring intensified innate immunity against bacterial infection [197].
5.3 Anti-inflammatory and analgesic activity
Euodiae Fructus has been used in TCM for the treatment of inflammation-related disorders such as gastrointestinal disorders (gastric ulceration, ulcerative colitis and dysentery), headache, postpartum hemorrhage, amenorrhea and dermatitis [121]. Numerous studies have demonstrated that dysregulation of nuclear factor-kappa B (NF-кB) pathways and inflammatory factors, such as TNF-α, IL-1β, IL-6 and NO, etc. play important roles in inflammatory responses [218].
5.3.1 Anti-inflammatory activity
The water extract of Euodiae Fructus could enhance the gastric mucosal barrier and promote the synthesis of NO in gastric mucosa, which has a significant protective effect toward ethanol-induced gastric injury in rats [219]. Ko et al. showed that the ethanol extract of Euodiae Fructus display potent antioxidative effects against both phorbol-12-myristate-13-acetate (PMA)- and N-formyl-methionyl-leucyl-phenylalanine (fMLP)-induced ROS production in neutrophils with respective IC50 values of 2.7 and 3.3 μg/mL and also inhibit lipopolysaccharide (LPS)-induced NO production with an IC50 of around 0.8 μg/mL, suggesting that the ethanol extract exhibited anti-inflammatory activities which could be partially explained by inhibiting NADPH oxidase-dependent ROS and/or iNOS-dependent NO production in activated inflammatory cells [118]. In another study, Euodiae Fructus and its active components may be useful in influenza virus infection-related inflammatory disorders by suppressing novel influenza A (H1N1)-induced chemokines (RANTES and MCP-1) production and blocking chemokine-attracted leukocytes recruitment [100].
In isolated compounds, results have showed that the anti-inflammatory effect of rutaecarpine is partly ascribed to the diminution of prostaglandin (PG) production through inhibition of arachidonic acid release in the RAW 264.7 [119]. In other studies, rutaecarpine ameliorated sepsis-induced peritoneal resident macrophages apoptosis and inflammation responses through inhibition of endoplasmic reticulum stress-mediated caspase-12 and NF-κB pathways [120], improved imiquimod-induced psoriasis-like dermatitis through effects on pDC- and Th17-associated cytokines via modulation of NF-κB and toll-like receptor 7 (TLR7) signaling [220], and ameliorated dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) via inhibiting KEAP1-NRF2 interaction to activate NRF2 [122]. Similarly, limonin was reported to improve the prognosis of DSS-induced UC mainly through downregulating p-STAT3/miR-214 levels [128]. Moreover, evodiamine could improve antioxidant and anti-inflammatory status through Rho/NF-κB pathway, which possibly exerted a gastro-protective effect against gastric ulceration [123]. In vitro and vivo, evodiamine was able to protect against zymosan-induced inflammation and DSS-induced murine experimental colitis by inactivating the expression of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), NF-κB signal pathway [124] and NLRP3 inflammasome [221], and the antiarthritic effect of evodiamine might be associated with its repression of synovial inflammation and regulation of Treg and Th17 differentiation [125].
5.3.2 Antinociceptive activity
Moreover, it has been reported that oral administration of 50 or 200 mg/kg 70% methanol extract of Euodiae Fructus has an antinociceptive effect on acetic acid induced-writhing responses, and the mode of action may be mediated by its anti-inflammatory action [101]. In vivo, limonin (30 or 100 mg/kg) possessed an antinociceptive effect and the effect may be accompanied by an anti-inflammatory action [69]. In other studies, evodiamine could reduce capsaicin-induced currents significantly in vitro and suppress capsaicin-induced thermal hyperalgesia in rats, which may be due to the activation and subsequent desensitization of TRPV1 in sensory neurons [212], and it could also inhibit the migraine-like pain response possibly due to the regulation of nNOS and suppression of the AMPA receptor GluA1 [126].
5.4 Anti-cardiovascular disease activity
Several studies have demonstrated that Euodiae Fructus has anti-cardiovascular activities, such as vasoconstrictive and vasodilator effects, anti-atherosclerosis, anti-platelet aggregation, anti-thrombus, anti-arrhythmia and cardioprotective effects [3].
5.4.1 Vasoconstrictive and vasodilator activity
It has been found that Euodiae Fructus (1 × 10−6–3 × 10–4 g/mL) has constrictive effects on rat aorta via adrenergic α1 receptors and serotonergic (5-HT1D and 5-HT2A) receptors [65], and the effect toward calcium channel on the membrane also played important roles [222]. In other investigations, rutaecarpine produced a fully (100%) NO-dependent vasodilatation in rat aorta, whereas dehydroevodiamine and evodiamine produced a partially endothelium-dependent effect, 10% and 50%, respectively. Furthermore, multiple-action mechanisms, including endothelium dependence, α1-adrenoceptor blockade, K+ channel activation, and Ca2+ channel blockade were probably involved in the vasorelaxant effects of dehydroevodiamine [223]. In vivo and vitro, the depressor and vasodilator effects of rutaecarpine were related to stimulation of endogenous CGRP release via activation of vanilloid receptors [181, 224].
5.4.2 Modulatory effects on VSMCs function and intimal hyperplasia
Results showed that evodiamine suppressed oxidative stress and inflammatory responses due to high free fatty acids and high glucose in human umbilical vein endothelial cells (HUVECs) via inhibiting the upregulated expression of P2X4R signaling pathway [179] and P2X7 receptor [180], respectively. Further investigations have shown that a promising anti-atherogenic effect of evodiamine through attenuation of vascular smooth muscle cells (VSMCs) migration by suppressing cell cycle progression, p38 MAPK and Erk1/2 activation, and ROS generation [178], and the activation of PPARγ also plays important role [225]. It was worth noting that rutaecarpine could modulate Cx (theroprotective Cx37 and atherogenic Cx43) expression through TRPV1/[Ca2+]i/CaM/NF-κB signal pathway [174] in monocytes to enhance its antiadhesive properties [171, 175], thereby preventing VSMCs dysfunction induced by ox-LDL [176]. Additionally, rutaecarpine inhibited Angiotensin II-induced proliferation in VSMCs partly through the modulation of NO signaling pathways and other related molecules (HRG-1 and c-myc) [173]. Moreover, rutaecarpine (10, 20, and 40 mg/kg) suppressed atherosclerosis in ApoE−/− mice through upregulating ABCA1 and SR-BI within reverse cholesterol transport (RCT) [177], and it could also promote NO production and inhibit ERK2 signal transduction pathways to inhibit the balloon injury-induced carotid intimal hyperplasia in rats [183].
5.4.3 Anti-platelet activity
“Goshuyuto” at the concentration of 1000 μg/mL inhibited collagen-induced platelet hyper-aggregation to the same degree as aspirin at the concentration of 100 μM [166]. Rutaecarpine was also able to display an anti-platelet effect in vivo [167], and the mechanism was investigated by inhibition of thromboxane formation and phosphoinositide breakdown [168]. Further investigation has shown that rutaecarpine inhibits agonists-induced platelet aggregation in human platelets, probably by inhibition of phospholipase C activity, leading to reduce phosphoinositide breakdown, followed by inhibition of thromboxane A2 formation and [Ca2+]i mobilization [169]. In another study, rutaecarpine has been seen to exert both antihypertensive and anti-platelet effects by stimulating the synthesis and release of CGRP in spontaneously hypertensive rats (SHR), and CGRP-mediated antiplatelet effect was related to inhibit the release of platelet-derived tissue factor [170].
5.4.4 Anti-arrhythmia activity
It has also been found that evodiamine and rutaecarpine induce the positive inotropic and chronotropic effects on the guinea-pig isolated right atria through their interaction with vanilloid receptors and the resultant release of CGRP [226, 227]. Additionally, dehydroevodiamine (0.1–0.3 μM) could depress trigger arrhythmias in Ca-overloaded guinea-pig cardiac myocytes through inhibiting INa, Iti and, to a smaller extent, ICa, while increasing the intracellular pH (pHi) and Na+–H+ exchanger (NHE) activity [228].
5.4.5 Regulatory effects on cardiac injury
Yi et al. found that the protective effects of rutaecarpine on cardiac anaphylactic injury or ischemia–reperfusion injury were related to inhibition of TNF-α production by stimulation of CGRP release [184], and the involvement of capsaicin-sensitive sensory nerves also played important roles [185], and the inhibition of Nox4‐ROS‐ADAM17 pathway and over‐activation of ERK1/2 might be associated with the beneficial role of rutaecarpine in hypertensive cardiac hypertrophy [182]. Moreover, evodiamine (0.3 and 3 μM) significantly attenuated Ang II-induced cardiomyocyte hypertrophy in vitro, and this effect is partly due to the promotion of NO production, the reduction of [Ca2+]i concentration, and the inhibition of CaN and ERK-2 signal transduction pathways [190], and it could also prevent cardiac fibroblasts from activation into myofibroblast and protect HUVEC against endothelial to mesenchymal transition (EndMT) probably by inhibition of canonical [189] and non-canonical TGFβ signaling [191].
5.5 Neuroprotective activity
A wide spectrum of pharmacological experiments indicated that Euodiae Fructus and its isolated compounds exerted a neuroprotective effect against ischemic injury, neuropathic pain, nerve inflammation, neurodegenerative disorders such as Alzheimer’s disease (AD), etc. The methanol extract of Euodiae Fructus (200 mg/kg) was able to have a protective effect against ischemia-induced neuronal and cognitive impairment [114]. In a MDCK-pHaMDR cell monolayer model, evodiamine and rutaecarpine entered the blood–brain barrier (BBB) by passive diffusion and promoted the absorption of dehydroevodiamine probably by inhibiting P-gp, while dehydroevodiamine showed moderate permeability through BBB by P-gp mediated efflux. Moreover, the above three alkaloids have been confirmed to exhibit neuroprotective effects on MPP+ or H2O2-injured PC12 cells [115]. In other studies, evodiamine (10 μM) and rutaecarpine (50 μM) reduced peripheral hypersensitivity and anxiety in mice with nerve injury or inflammation via TRPV1 [116]. Moreover, evodiamine could ameliorate paclitaxel-induced neuropathic pain by inhibiting inflammatory response and activating mitochondrial anti-oxidant functions [15], and induced JNK-mediated protective autophagy in astrocytes through TRPV1-dependent signaling and an influx of extracellular calcium, which may provide a possible option for ischemic stroke treatment [229]. Additionally, rutaecarpine improved neuronal injury, inhibited apoptosis, inflammation and oxidative stress in rats with cerebral ischemia–reperfusion (CI/R) by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway [117].
Besides the above functions on the nervous system, Euodiae Fructus and its isolated compounds could also potentially be developed as an alternative therapeutic agent for the management of AD. Cai et al. demonstrated that the water extract of Euodiae Fructus significantly ameliorated learning and memory deficits in Morris water maze tests, and in 3xTg AD mice, it could also decrease Aβ deposits and increase NeuN-positive cells by upregulating the expressions of Brain neurotrophic derived factor (BDNF) and tyrosine kinase B (TrkB) [105]. Evodiamine (100 mg/kg) significantly alleviated the impairments of learning ability and memory in transgenic mouse models [112], and inhibited glial cell activation and neuroinflammation (IL-1β, IL-6, TNF-α, and COX-2 levels) in the hippocampus by increasing the activity of AKT/GSK-3β signaling pathway and inhibiting the activity of NF-κB [111]. Further study has revealed that evodiamine exerts a protective effect against AD by modulating oxidative stress and reducing the apoptosis rate in vitro and vivo [113]. Additionally, dehydroevodiamine could inhibit acetylcholinesterase activity with IC50 value of 37.8 μM and show antiamnesic effect due to the combined effects of acetylcholinesterase inhibition and the known cerebral blood flow enhancement [107], and it could also suppress WT/GFX-induced overactivation of GSK-3 to improve spatial memory impairment and tau hyperphosphorylation in vivo [109], and its underlying mechanism might involve a decreased inhibitory phosphorylation of PP-2A at Tyr307 [108], and the protective effects on cognitive impairment might be related to its antioxidant activity, inhibition of neurotoxicity and intracellular calcium in memory-impaired rat models [110].
5.6 Anti-obesity and anti-diabetic activity
5.6.1 Anti-obesity activity
It has been reported that ruteacarpine and evodiamine [193] reduce food intake and bodyweight gain by improving orexigenic sensitivity through the inhibition of neuropeptide Y (NPY) and agouti-related protein (AgRP) mRNA expression and peptide expression [230]. Moreover, evodiamine, as a vanilloid receptor agonist, could simultaneously induce heat loss and heat production and dissipate food energy, preventing the accumulation of perivisceral fat and the body weight increase [231], and activate AMP-activated protein kinase (AMPK) and adiponectin multimerization in 3T3-L1 adipocytes, which was associated with the activation of Ca2+-dependent PI3K/Akt/ CaMKII-signaling pathway [192].
5.6.2 Anti-diabetic activity
Furthermore, rutaecarpine and evodiamine were able to suppress gluconeogenesis and lipogenesis through their activation of the constitutive androstane receptor (CAR) in vitro and vivo, thus having a therapeutic potential for the treatment of hyperglycemia and type 2 diabetes [195]. Evodiamine improved glucose tolerance and reduced insulin resistance in obese/diabetic mice, which was possibly related to inhibition of mammalian target of rapamycin (mTOR)- S6 protein kinase (S6K) signaling and insulin receptor substrate 1 (IRS1) serine phosphorylation in adipocytes [194]. An additional study demonstrated that rutaecarpine could regulate IRS-1/PI3K/Akt signaling pathway in liver and AMPK/ acetyl-CoA carboxylase2 (ACC2) signaling pathway in skeletal muscles to ameliorate hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats [196].
5.7 Insecticidal activity
In recent years, plant-based, environmentally friendly and biodegradable natural insecticides have received renewed attention as vector control agents, and some research have demonstrated that Euodiae Fructus exhibit insecticidal activity [232]. Lian et al. screened different extracts of Euodiae Fructus with anthelmintic activity against Gyrodactylus kobayashii (Monogenea) in goldfish, indicating that the ethyl acetate, the petroleum ether and methanol extracts had better anthelmintic efficacy, with EC50 values of 24.0, 71.9 and 40.9 mg/L, respectively, after a 48-h exposure, whereas the water extract of Euodiae Fructus had the weakest anthelmintic efficacy of 25.6% at 800.0 mg/L [198]. Moreover, the essential oil of Euodiae Fructus was found to possess insecticidal activity against maize weevils, Sitophilus zeamais and red flour beetles Tribolium castaneum with LC50 values of 36.89, 24.57 and 57.31 mg/L air, respectively [93]. Further study has shown that evodiamine and rutaecarpine showed insecticidal activity against larvae of D melanogaster with LC50 values of 0.30 and 0.28 μmol/mL diet respectively [199]. In another investigations, evodiamine, rutaecarpine, and wuchuyuamide I have been reported to exhibit strong larvicidal activity against the early fourth instar larvae of A. albopictus with LC50 values of 12.51, 17.02, and 26.16 μg/mL, respectively, and the ethanol extract, limonin and evodol also possessed larvicidal activity against the Asian tiger mosquitoes with LC50 values of 43.21, 32.43 and 52.22 μg/mL, respectively [31]. Liu et al. showed that evodiamine (LC50 = 73.55 μg/mL) and rutaecarpine (LC50 = 120.85 μg/mL) exhibit stronger nematocidal activity against M. incognita than the crude ethanol extract of Euodiae Fructus (LC50 = 131.54 μg/mL) [30]. Additionally, rhetsinine was found to show potential as a pesticide and exhibited excellent inhibition against Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and Xanthomonas campestris pv. campestris, with respective EC50 values of 3.13, 14.32, and 32.72 nmol in vitro [29]. Taken together, these results indicated that the ethanol extract of Euodiae Fructus and several isolated constituents have a good potential as a source for insecticidal activity, and further research is needed to determine its safety to human body and environment.
5.8 Hepatorenal protection
Consistent with traditional applications, Euodiae Fructus was reported to affect the liver and kidney [233]. Jin et al. reported that rutaecarpine augmented cellular antioxidant defense capacities through CaMKII-PI3K/Akt-dependent HO-1 induction via the Nrf2/ARE signaling pathway, thereby protecting cells from oxidative damage in hepatocytes [208]. It has been found that evodiamine (15 and 25 mg/kg) has an antifibrosis effect in CCl4-induced liver fibrosis and reduces hepatic stellate cells (HSCs) proliferation and collagen metabolism in vitro through downregulation of relative expression of TGF-β1, p-Smad 2/3, and α-SMA [205]. In other investigation, limonin alleviated acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative signals and inhibiting NF-κB inflammatory response via upregulating Sirt1 [210]. For the kidney, recent researches showed that a number of protective roles against I/R damage [206], LPS-induced acute kidney injury and cytotoxicity [207] due to the antioxidative, anti‐inflammatory and antiapoptotic properties of evodiamine. Additionally, Wang et al. showed that rutaecarpine be an effective compound for the prevention and treatment of renal ischemia–reperfusion injury (IRI), and its mechanism might be related to inhibition of JNK/p38MAPK signaling pathway and interference of oxidative stress response [209].
5.9 Anti-osteoporosis activity
Rutaecarpine significantly inhibited osteoclastogenesis and prevented bone resorption of bone marrow-derived macrophage (BMM)-derived osteoclasts through decreasing the protein level of nuclear factor of activated T cells cytoplasmic-1 (NFATc1) and the phosphorylation of other signaling pathways during the osteoclast differentiation [203]. Moreover, evodiamine was reported to inhibit the formation of osteoclasts via blocking the RANKL-induced activation of ERK and c-Fos as well as the induction of NFATc1[200], and the underlying mechanism might also be related to inhibit the activation of the NF‐κB and calcium signalling pathways [201], and in Zebrafish, evodiamine was found to prevent osteoporosis by reversing the imbalance of bone formation/bone resorption and activating MMP3-OPN-MAPK pathway signal [202]. Additionally, limonin stimulated alkaline phosphatase (ALP) activity and enhanced the expression of osteoblast differentiation gene markers by regulating ERK and P38 signals in osteoblastic MC3T3-E1 cells, and inhibited the reduction of bone mass and promote the increase of bone mineral density in ovariectomized rats [204].
5.10 Other activity
Apart from the summarized pharmacological activities mentioned above, the isolated constituents or crude extracts of Euodiae Fructus also involve other bioactivities including anti-diarrheal effect, antiallergic effect, antianoxic activity, antidepressant-like activity, antiviral activity, anti-ovotoxicity effect, etc. It has been reported that Euodiae Fructus has both anti-transit and anti-diarrheal effects with comparable ID50 (the dose for 50% inhibition) values of 54 ± 7 and 76 ± 17 mg/kg and the anti-diarrheal effect of Euodiae Fructus may be associated with its anti-transit [102]. In vitro and vivo, Euodiae Fructus and its constituents (evodiamine and rutaecarpine) might inhibit the biosynthesis of anaphylaxis-related cytokines (TNF-α and IL-4) in mast cells and basophils, suggesting that they might be effective for IgE-induced allergic diseases such as atopic dermatitis and rhinitis [214]. Other studies have demonstrated that the involvement of cholinergic mechanism plays important roles in the antianoxic potential of evodiamine in the KCN-induced anoxia model [213, 234]. Moreover, the antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats probably by modulating effects on the monoamine transmitters and brain-derived neurotrophic factor (BDNF)-tropomyosin-related kinase B receptor (TrkB) signaling in the hippocampus [211]. Dai et al. showed that evodiamine could significantly inhibit the replication of anti-influenza A virus (IAV), the accumulation of LC3-II, p62 and EGFP-LC3, the formation of the Atg5-Atg12/Atg16 heterotrimer, the expressions of Atg5, Atg7 and Atg12, and the cytokine release of TNF-α, IL-1β, IL-6 and IL-8 after IAV infection, meanwhile, the inhibition of IAV-induced autophagy by evodiamine was also dependent on its action on the AMPK/TSC2/mTOR signal pathway [215]. In addition, the water extract of Euodiae Fructus could activate Akt to protect ovary cells against 4-vinylcyclohexene diepoxide-induced ovotoxicity, which indicates that Euodiae Fructus may help prevent premature ovarian failure or unexplained infertility caused by environmental factors [13]. Interestingly, a recent study has shown that aqueous extract of Euodiae Fructus and evodiamine could improve caffeine-induced sleep and excitation behaviors, at least in part, through the γ-aminobutyric acid (GABA)A-ergic system, these results suggest a potential therapeutic agent to treat insomnia or sleep problems related to caffeine intake [235].
6 Toxicity
According to China’s most ancient herbal medicine book “Shen Nong’s Herbal Classic” and 2020 Edition of Chinese Pharmacopoeia, the mild toxicity of Euodiae Fructus has been noted. In recent years, it has been reported that the cases of patients with chronic esophagitis, excessive use of Euodiae Fructus could cause stomach pain, vomiting, blurred vision and other toxic symptoms [236, 237], and cause liver toxicity to the human body [238, 239]. Modern researches in vitro and in vivo have shown that the crude extract and several compounds isolated from Euodiae Fructus have been reported to exert hepatic injury, CYP inhibition, and to induce proarrhythmic cardiotoxicity when used in high doses as described in Table 5, and the details will be further discussed below.
In acute toxicity test, histopathological analysis revealed that Euodiae Fructus caused morphological changes in the liver, but no other main organs [240]. Cai et al. reported that oral gavaging of water decoction at dose of 6, 12 and 24 g/kg for 15 days in rats could increase malondialdehyde (MDA) level, and decrease the MnSOD activity and glutathione (GSH) levels reduction, followed by causing oxidative damage, finally resulting in adenosine triphosphate (ATP) depletion and cytochrome C (CytC) release, triggering cell death signaling pathways, which are all partial hepatotoxicity mechanisms of Euodiae Fructus [241], In another study, rutaecarpine might be a mechanism-based inhibitor of CYP1A2, and its potential hepatotoxicity might be related to reactive metabolites, and GSH trapping might be a detoxication route [242]. Furthermore, in vitro, rutaecarpine, evodiamine, and dehydroevodiamine significantly activated aryl hydrocarbon receptor (AHR), with an efficacy order of rutaecarpine > dehydroevodiamine > evodiamine, and ligand-docking analysis predicted that the methyl substitute at the N-14 atom was a key factor affecting AHR activation. The above three indole alkaloids were not hepatotoxic in vivo at the doses used. However, rutaecarpine and dehydroevodiamine disrupted bile acid homeostasis in an AHR-dependent manner, evodiamine failed to activate AHR due to its poor absorption in mice [243]. A recent study has revealed that rutaevin was shown to increase the activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in mice serum, suggesting the potential hepatotoxicity of rutaevin, and the potential mechanism was that rutaevin was converted into a electrophilic BDA intermediate by CYP3A4 [244]. Moreover, it has been reported that dihydrorutaecarpine (5), 6-acetoxy-5-epilimonin (146), goshuyuamide I (25), 1-methyl-2-[(Z)-5-undecenyl]-4(1H)-quinolone (65), 1-methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (83), evocarpine (73), and 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone (96)) [245], and another five quinoline alkaloid (1-methyl-2-undecyl-4(1H)-quinolone (62),1-methyl-2-[(6Z,9Z,12E)-pentadecatriene]-4(1H)-quinolone (99), 1-methyl-2-[(Z)-7-tridecenyl]-4(1H)-quinolone (80), dihydroevocarpine (72), and 1-methyl-2-tetradecy-4(1H)-quinolone (89)) [60], are speculated as possible hepatotoxic components based on spectrum-toxicity relationship and UPLC-Q-TOF-MS, whether these components were toxic as well still requires further exploring and researching. Therefore, attention should be given to monitoring bile acid metabolism in the clinical use of Euodiae Fructus.
It was worth noting that P450-mediated dehydrogenation reactions of evodiamine and rutaecarpine might cause toxicities through the generation of highly electrophilic intermediate and lead to drug-drug interactions mainly via the inactivation of CYP3A4 [246], Zhu et al. demonstrated that the induction of cytochrome P450 enzyme genes, hepatic transporters and phase-2 enzyme genes are involved in the interaction between rutaecarpine and drugs [247]. In addition, evodiamine could inhibit CYP1A2, CYP2C9 and CYP2D6 in rats, which might affect the disposition of drugs that rely on these pathways [248]. Therefore, it is necessary to pay attention to CYP3A4-, CYP1A2-, CYP2C9- and CYP2D6-mediated herb–drug interactions between Euodiae Fructus and western drugs to avoid undertreatment.
Additionally, dehydroevodiamine inhibited hERG channels with IC50 values of 253.2 ± 26.3 nM on human embryonic kidney cells, prolonged the action potential duration (APD) in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in a concentration-dependent manner from 0.01 to 1 μM and induced early afterdepolarizations (EADs) at 3 μM. Dehydroevodiamine (0.5 mg/kg) induced TdP arrhythmias in 2 out of 8 animals, and STV increased accordingly [249] in rabbits. In another study, evodiamine inhibited rat cardiomyocytes viability with IC50 value of 28.44 µg/mL at 24 h, increased LDH release and MDA levels, and reduced superoxide dismutase (SOD) activity on primary cultured neonatal rat cardiomyocytes. In zebrafish model, evodiamine also has a 10% lethal concentration of 354 ng/mL and induce cardiac malfunction, as evidenced by changes in heart rate and circulation, and pericardial malformations. These results indicated that evodiamine could cause cardiovascular side effects involving oxidative stress [250].
Since Euodiae Fructus contains potentially toxic compounds, reliable analytical methods are needed to control the quality of product development to ensure that the potential toxic components of Euodiae Fructus-related products are kept below allowable levels, and more attention should be given to herb–drug interactions and monitoring bile acid metabolism in the clinical use of Euodiae Fructus.
7 Quality control
As we all know, the intrinsic quality of TCM might vary greatly due to different geographic conditions and harvest periods [251]. Therefore, an efficient, rapid, sensitive and reproducible detection method was important to ensure the quality of each batch of medicinal materials [252]. According to the 2020 Edition of Chinese Pharmacopoeia, the concentration of evodiamine and rutaecarpine should exceed 0.15%, and the concentration of limonin should exceed 0.20% as determined by HPLC with the mobile phase making up of 0.02% phosphoric acid water and acetonitrile-tetrahydrofuran (25:15) at a ratio of 35: 65, and the detection wavelength should be at 225 nm. However, due to the pharmacological activity and toxicity of multiple ingredients mentioned above, the content of single or small amount of labeled compounds cannot accurately reflect the quality of TCM [253]. With the advancement of analytical tools, it is necessary to adopt more advanced detection methods to qualitatively and quantitatively analyze as many biologically active ingredients as possible. A total of 13 compounds: Wuchuyuamide-I, quercetin, limonin, evodiamine, rutaecarpine [254], dehydroevodiamine, evodine [26], evodiamide, 14-formyldihydrorutaecarpine [25], 1-methyl-2-undecyl-4(1H)quinolone, evocarpine, 1-methy-2-[(6Z,9Z)]-6,9pentadecadienyl-4-(1H)-quinolone, and dihydroevocarpine [255], were selected to ensure the quality of Euodiae Fructus by HPLC–DAD, HPLC–DAD-MS/MS, HPLC/UV/APCI-MS/MS, and CEC-MS, and the additional details are listed in Table 6. To evaluate the quality, the newly established fingerprint analysis was conducted on this kind of plants. The fingerprint analysis of Euodiae Fructus was carried out and the results suggest that the chemical components would vary greatly in different locations and vary a little in different years in the same site [64, 256]. In recent years, one study compared the differences of essential oils from three species of Euodiae Fructus cultured in China. The results showed that the differences in chemical composition and oil production within species are greater than the differences between species [91].
8 Conclusions
This review has summarized the multifaceted uses and recent findings regarding studies of the phytochemistry, traditional use, bioactive constituents, pharmacology, toxicity, and quality control of different extracts and compounds of Euodiae Fructus and provides a practical base for further scientific research and favorable clinical application on this plant. Extensive researches have been conducted on the phytochemistry of the Euodiae Fructus and approximately 240 compounds have been isolated and identified from this plant, including alkaloids, terpenoids, steroids, phenols, volatile oil and other compounds. As the literature has demonstrated, alkaloids and terpenoids are the main components of Euodiae Fructus, and alkaloids are mostly responsible for its pharmacological activities. Additionally, recent reports have primarily focused on evaluating anticancer, antibacterial, anti-inflammatory, insecticidal, anti-cardiovascular disease, neuroprotective, anti-obesity and anti-diabetic activities of the herbal medicines derived from this plant. In particular, the indole alkaloids (e.g., evodiamine, rutaecarpine and dehydroevodiamine) and limonin have been confirmed to has low toxicity and high medicinal value through various pharmacological activities in vivo and in vitro investigations.
Euodiae Fructus exhibits a diverse set of pharmacological properties and its chemistry is complex. For these reasons, it is of great importance to systematically and critically evaluate the future direction and application of this field. Although many efforts have been made to study these plants, there are also a number of points and aspects that need to be improved and researched further: (1) According to TCM, Euodiae Fructus is traditionally considered to have mild toxicity, and a few support studies have been linked to its toxicity, including the potential hepatotoxicity, CYP inhibition, and cardiotoxicity of this plant. Thus, it is necessary to investigate the potential toxic effects induced by Euodiae Fructus and clarify the toxic components, target-organs and mechanisms, so as to lay a foundation for future research. (2) Several traditional uses of these plants have been validated in recent pharmacological studies, but some of these were only tested in vitro. Therefore, the effectiveness of these compounds in vivo and comprehensive placebo-controlled and double-blind clinical trials need to be further studied, and more detailed pharmacology and mechanism of action may help to better understand TCM theory. (3) Alkaloids are traditionally considered as the major bioactive compounds in Euodiae Fructus. However, their mechanisms of action remain unclear, and further studies are required to understand the structure–activity relationships of these constituents and bioactivities. For isolated alkaloids, too many researches are focused on evodiamine and rutaecarpine, and there are other active ingredients like dehydroevodiamine, evocarpine and dihydroevocarpine, etc. that have been lacked of research or ignored. Further investigation should be encouraged to study these components or their analogues. (4) Numerous studies have demonstrated evodiamine process extensive activities, however, due to its poor water solubility and low oral bioavailability, thereby limiting its anticancer efficacy clinically. Future studies should aim to overcome these problems in the clinical application of TCM. (5) In view of the toxicity of some compounds, reliable analytical methods are required for proper quality control of drug development to ensure that potential toxic components remain below the tolerance level of Euodiae Fructus.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852–9.
Gavaraskar K, Dhulap S, Hirwani RR. Therapeutic and cosmetic applications of Evodiamine and its derivatives–a patent review. Fitoterapia. 2015;106:22–35.
Jia S, Hu C. Pharmacological effects of rutaecarpine as a cardiovascular protective agent. Molecules. 2010;15:1873–81.
Tian KM, Li JJ, Xu SW. Rutaecarpine: a promising cardiovascular protective alkaloid from Evodia rutaecarpa (Wu Zhu Yu). Pharmacol Res. 2019;141:541–50.
Fan SM, Zhang CL, Luo T, Wang JQ, Tang Y, Chen ZM, Yu LY. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules. 2019;24:22.
Zhao Z, He X, Han W, Chen X, Liu P, Zhao X, Wang X, Zhang L, Wu S, Zheng X. Genus Tetradium L.: a comprehensive review on traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol. 2019;231:337–54.
Shan QY, Sang XN, Hui H, Shou QY, Fu HY, Hao M, Liu KH, Zhang QY, Cao G, Qin LP. Processing and polyherbal formulation of Tetradium ruticarpum (A. Juss.) Hartley: phytochemistry, pharmacokinetics, and toxicity. Front Pharmacol. 2020;11:133.
Wang T, Yan YF, Yang L, Huang YZ, Duan XH, Su KH, Liu WL. Effects of Zuojin pill on depressive behavior and gastrointestinal function in rats with chronic unpredictable mild stress: role of the brain-gut axis. J Ethnopharmacol. 2020;254: 112713.
Wang QS, Cui YL, Dong TJ, Zhang XF, Lin KM. Ethanol extract from a Chinese herbal formula, “Zuojin Pill”, inhibit the expression of inflammatory mediators in lipopolysaccharide-stimulated RAW 264.7 mouse macrophages. J Ethnopharmacol. 2012;141:377–85.
Wang QS, Ding SL, Mao HP, Cui YL, Qi XJ. Antidepressant-like effect of ethanol extract from Zuojin Pill, containing two herbal drugs of Rhizoma Coptidis and Fructus Evodiae, is explained by modulating the monoaminergic neurotransmitter system in mice. J Ethnopharmacol. 2013;148:603–9.
Sun MY, Wang DD, Sun J, Zhao XH, Cai S, Wu QX, Jie T, Ni ZH, Sun JY, Tang QF. The Zuo Jin Wan Formula increases chemosensitivity of human primary gastric cancer cells by AKT mediated mitochondrial translocation of cofilin-1. Chin J Nat Med. 2019;17:198–208.
Gao L, Jia C, Zhang H, Ma C. Wenjing decoction (herbal medicine) for the treatment of primary dysmenorrhea: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017;296:679–89.
Nam EY, Kim SA, Kim H, Kim SH, Han JH, Lee JH, Kim DI. Akt activation by Evodiae Fructus extract protects ovary against 4-vinylcyclohexene diepoxide-induced ovotoxicity. J Ethnopharmacol. 2016;194:733–9.
Hibino T, Yuzurihara M, Kanno H, Kase Y, Takeda A. Goshuyuto, a traditional Japanese medicine, and aqueous extracts of Evodiae Fructus constrict isolated rat aorta via adrenergic and/or serotonergic receptors. Biol Pharm Bull. 2009;32:237–41.
Wu P, Chen Y. Evodiamine ameliorates paclitaxel-induced neuropathic pain by inhibiting inflammation and maintaining mitochondrial anti-oxidant functions. Hum Cell. 2019;32:251–9.
Liang X, Li B, Wu F, Li T, Wang Y, Ma Q, Liang S. Bitterness and antibacterial activities of constituents from Evodia rutaecarpa. BMC Complement Altern Med. 2017;17:180.
Zuo GY, He HP, Wang BG, Hong X, Hao XJ. New indoloquinazoline alkaloid from the fruit of Evodia rutaecarpa. Plant Diversity and Resources. 2003;25:103–6.
Sugimoto T, Miyase T, Kuroyanagi M, Ueno A. Limonoids and Quinolone Alkaloids from Evodia rutaecarpa BENTHAM. Chem Pharm Bull. 1988;36:4453–61.
Wang TY, Wu JB, Hwang TL, Kuo YH, Chen JJ. A new quinolone and other constituents from the fruits of Tetradium ruticarpum effects on neutrophil pro-inflammatory responses. Chem Biodiversity. 2010;7:1828–34.
Zhao N, Li ZL, Li DH, Sun YT, Shan DT, Bai J, Pei YH, Jing YK, Hua HM. Quinolone and indole alkaloids from the fruits of Euodia rutaecarpa and their cytotoxicity against two human cancer cell lines. Phytochemistry. 2015;109:133–9.
Huang X, Zhang YB, Yang XW. Indoloquinazoline alkaloids from Euodia rutaecarpa and their cytotoxic activities. J Asian Nat Prod Res. 2011;13:977–83.
Li DW, Zhang M, Feng L, Huang SS, Zhang BJ, Liu SS, Deng S, Wang C, Ma XC, Leng AJ. Alkaloids from the nearly ripe fruits of Evodia rutaecarpa and their bioactivities. Fitoterapia. 2020;146: 104668.
Zhang XL, Sun J, Wu HH, Jing YK, Chai X, Wang YF. A new indoloquinazoline alkaloidal glucoside from the nearly ripe fruits of Evodia rutaecarpa. Nat Prod Res. 2013;27:1917–21.
Yan Q, Shan Y, Yin M, Xu S, Ma C, Tong HY, Feng X, Wang QZ. Phytochemical and chemotaxonomic study on Evodia rutaecarpa var. officinalis. Biochem Syst Ecol. 2020;88.
Zhou Y, Li SH, Jiang RW, Cai M, Liu X, Ding LS, Xu HX, But PP, Shaw PC. Quantitative analyses of indoloquinazoline alkaloids in Fructus Evodiae by high-performance liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3111–8.
Xu HY, Zhang TJ, Xiao XF, Zhao P, Liu CX, Xu J. Simultaneous analysis of thirteen bioactive components in Evodia rutaecarpa and its varieties by HPLC-DAD-MS. Chin Herb Med. 2010;2:112–7.
Wang QZ, Liang JY, Feng X. Evodiagenine and dievodiamine, two new indole alkaloids from Evodia rutaecarpa. Chin Chem Lett. 2010;21:596–9.
Kato A, Yasuko H, Goto H, Hollinshead J, Nash RJ, Adachi I. Inhibitory effect of rhetsinine isolated from Evodia rutaecarpa on aldose reductase activity. Phytomedicine. 2009;16:258–61.
Su XL, Xu S, Shan Y, Yin M, Chen Y, Feng X, Wang QZ. Three new quinazolines from Evodia rutaecarpa and their biological activity. Fitoterapia. 2018;127:186–92.
Liu QZ, Li HQ, Liu ZL. Nematocidal Constituents from the Ethanol Extract ofEvodia rutaecarpaHort Unripe Fruits. J Chem. 2013;2013:1–5.
Liu ZL, Liu QZ, Du SS, Deng ZW. Mosquito larvicidal activity of alkaloids and limonoids derived from Evodia rutaecarpa unripe fruits against Aedes albopictus (Diptera: Culicidae). Parasitol Res. 2012;111:991–6.
Zuo GY, Yang XS, Hao XJ. Two New Indole Alkaloids from Evodia rutaecarpa. Chin Chem Lett. 2000;11:127–8.
Jin HZ, Du JL, Zhang WD, Chen HS, Lee JH, Lee JJ. A novel alkaloid from the fruits of Evodia officinalis. J Asian Nat Prod Res. 2007;9:685–8.
Jin HZ, Du JL, Zhang WD, Yan SK, Chen HS, Lee JH, Lee JJ. A new quinazolinedione alkaloid from the fruits of Evodia officinalis. Fitoterapia. 2008;79:317–8.
Teng J, Yang XW. Two new indoloquinazoline alkaloids from the unripe fruits of Evodia rutaecarpa. Heterocycles. 2006;68:1691–8.
Yang XW, Teng J, Wang Y, Xu W. The permeability and the efflux of alkaloids of the Evodiae fructus in the Caco-2 model. Phytother Res. 2009;23:56–60.
Li YH, He J, Li Y, Wu XD, Peng LY, Du RN, Cheng X, Zhao QS, Li RT. Evollionines A-C, three new alkaloids isolated from the fruits of Evodia rutaecarpa. Helv Chim Acta. 2014;97:1481–6.
Tang YQ, Feng XZ, Huang L. Studies on the chemical constituents of Evodia rutaecarpa [Juss] Benth. J Chin Pharm Sci. 1997;6:65–9.
Su XL, Yin M, Xu S, Shan Y, Feng X, Wang QZ. Analysis of chemical constituents in Evodia rutaecarpa by UPLC-Q-TOF-MS. Chin Tradit Patent Med. 2017;39:1223–7.
Li YH, Zhang Y, Peng LY, Li XN, Zhao QS, Li RT, Wu XD. (+/-)-Evodiakine, a pair of rearranged rutaecarpine-type alkaloids from Evodia rutaecarpa. Nat Prod Bioprospect. 2016;6:291–6.
Wang XX, Gao HY, Jiang Y, Zhao MB, Tu PF. Chemical constituents from fruits of Euodia rutaecarpa. Chin Tradit Herbal Drugs. 2013;44:1241–4.
Yu LL, Ho LK, Liao JF, Chen CF. Two 5-HT1A receptor-interactive tryptamine derivatives from the unripe Fruit of Evodia rutaecarpa. J Nat Prod. 1997;60:1196–8.
Wang XX, Zan K, Shi SP, Zeng KW, Jiang Y, Guan Y, Xiao CL, Gao HY, Wu LJ, Tu PF. Quinolone alkaloids with antibacterial and cytotoxic activities from the fruits of Evodia rutaecarpa. Fitoterapia. 2013;89:1–7.
Zhuang PY, Wang XX, Chen JJ, Zhang DY, Lin XY, Yang YK. Extraction of novel quinolones alkaloid in evodia rutaecarpa useful as neuroprotector patent CN106810495A. 2017.
Jin HZ, Lee JH, Lee D, Lee HS, Hong YS, Kim YH, Lee JJ. Quinolone alkaloids with inhibitory activity against nuclear factor of activated T cells from the fruits of Evodia rutaecarpa. Biol Pharm Bull. 2004;27:926–8.
Adams M, Kunert O, Haslinger E, Bauer R. Inhibition of leukotriene biosynthesis by quinolone alkaloids from the fruits of Evodia rutaecarpa. Planta Med. 2004;70:904–8.
Huang X, Li W, Yang XW. New cytotoxic quinolone alkaloids from fruits of Evodia rutaecarpa. Fitoterapia. 2012;83:709–14.
Han XH, Hong SS, Lee D, Lee JJ, Lee MS, Moon DC, Han K, Oh KW, Lee MK, Ro JS, Hwang BY. Quinolone alkaloids from evodiae fructus and their inhibitory effects on monoamine oxidase. Arch Pharm Res. 2007;30:397–401.
Pan X, Bligh SW, Smith E. Quinolone alkaloids from Fructus Euodiae show activity against methicillin-resistant Staphylococcus aureus. Phytother Res. 2014;28:305–7.
Yang XW, Zhang H, Li M, Du LJ, Yang Z, Xiao SY. Studies on the alkaloid constituents of Evodia rutaecarpa (Juss) Benth var. bodinaieri (Dode) Huang and their acute toxicity in mice. J Asian Nat Prod Res. 2006;8:697–703.
Lee MK, Hwang BY, Lee SA, Oh GJ, Choi WH, Hong SS, Lee KS, Ro JS. 1-methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase. Chem Pharm Bull. 2003;51:409–11.
Adams M, Mahringer A, Kunert O, Fricker G, Efferth T, Bauer R. Cytotoxicity and p-glycoprotein modulating effects of quinolones and indoloquinazolines from the Chinese herb Evodia rutaecarpa. Planta Med. 2007;73:1554–7.
Ma C, Liu X, Shan Y, Xu S, Su XL, Feng X, Wang QZ. A new quinolone alkaloid with cytotoxic activity from the fruits of Euodia Rutaecarpa. Nat Prod Commun. 2018;13:339–41.
Shin HK, Do JC, Son JK, Lee CS, Lee CH, Cheong CJ. Quinoline alkaloids from the fruits of Evodia officinalis. Planta Med. 1998;64:764–5.
Ko JS, Rho MC, Chung MY, Song HY, Kang JS, Kim K, Lee HS, Kim YK. Quinolone alkaloids, diacylglycerol acyltransferase inhibitors from the fruits of Evodia rutaecarpa. Planta Med. 2002;68:1131–3.
Hamasaki N, Ishii E, Tominaga K, Tezuka Y, Nagaoka T, Kadota S, Kuroki T, Yano I. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese Herbal Medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol Immunol. 2000;44:9–15.
Ling Y, Hu P, Zhang L, Jin H, Chen J, Tao Z, Huang L, Ren R. Identification and structural characterization of acylgluconic acids, flavonol glycosides, limonoids and alkaloids from the fruits of Evodia Rutaecarpa by high performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry. J Chromatogr Sci. 2016;54:1593–604.
Tang YQ, Feng XZ, Huang L. Quinolone alkaloids from Evodia Rutaecarpa. Phytochemistry. 1996;43:719–22.
Chen M, Liu X, Shan Y, Xu S, Feng X, Wang QZ. A new quinolone alkaloid from the fruits of Tetradium ruticarpum. Nat Prod Res. 2021;35:222–7.
Liang J, Chen Y, Ren G, Dong W, Shi M, Xiong L, Li J, Dong J, Li F, Yuan J. Screening hepatotoxic components in Euodia rutaecarpa by UHPLC-QTOF/MS based on the spectrum-toxicity relationship. Molecules. 2017;22:1264.
Varamini P, Javidnia K, Soltani M, Mehdipour A, Ghaderi A. Cytotoxic activity and cell cycle analysis of quinoline alkaloids isolated from haplophyllum canaliculatum boiss. Planta Med. 2009;75:1509–16.
Qiong LI, Tang C, Changqiang KE, Yang YE. A new limonoid from the fruits of Evodia rutaecarpa (Juss.) Benth. J Technol. 2018;18:14–8.
Li YH, Liu X, Yin M, Liu F, Wang B, Feng X, Wang QZ. Two new quinolone alkaloids from the nearly ripe fruits of Tetradium ruticarpum. Nat Prod Res. 2019;4:1–6.
Zhou X, Zhao Y, Lei P, Cai Z, Liu H. Chromatographic fingerprint study on Evodia rutaecarpa (Juss.) Benth by HPLC/DAD/ESI-MS(n) technique. J Sep Sci. 2010;33:2258–65.
Hibino T, Yuzurihara M, Kase Y, Takeda A. Synephrine, a component of Evodiae Fructus, constricts isolated rat aorta via adrenergic and serotonergic receptors. J Pharmacol Sci. 2009;111:73–81.
Zhang QH, Gao HY, Wu LJ, Zhang L. Chemical constituents of Evodia rutaecarpa (Juss.) Benth. J Shenyang Pharm Univ. 2005;22:12–4.
Zhao N, Li DH, Li ZL, Hua HM. Isolation and identification of the chemical constituents from the fruits of Euoida rutaecarpa. J Shenyang Pharm Univ. 2016;33:103–9.
Gong XJ, Zhou X, Cai ZW, Zhang JX, Zhou W. Studies on chemical constituents of Evodia rutaecarpa. China J Chin Mater Med. 2009;34:177–9.
Matsuda H, Yoshikawa M, Iinuma M, Kubo M. Antinociceptive and anti-inflammatory activities of limonin isolated from the fruits of Evodia rutaecarpa var. bodinieri. Planta Med. 1998;64:339–42.
Shi YS, Xia HM, Wu CH, Li CB, Duan CC, Che C, Zhang XJ, Li HT, Zhang Y, Zhang XF. Novel nortriterpenoids with new skeletons and limonoids from the fruits of Evodia rutaecarpa and their bioactivities. Fitoterapia. 2020;142: 104503.
Yang ZX, Meng YH, Wang QH, Yang BY, Kuang HX. Substance basis of bitter resolution and composition from Fructus Evodiae. Chin J Exp Tradit Med Formulae. 2011;17:74–7.
Ozaki Y, Miyake M, Maeda H, Ifuku Y, Bennett RD, Hasegawa S. Limonoid glucosides in Tetradium Rutaecarpa. Phytochemistry. 1991;30:2365–7.
Yang XB, Qian P, Yang XW, Liu JX, Gong NB, Lv Y. Limonoid constituents of Euodia rutaecarpa var. bodinieri and their inhibition on NO production in lipopolysaccharide-activated RAW264.7 macrophages. J Asian Nat Prod Res. 2013;15:1130–8.
Wang QZ, Liang JY, Chen J. Chemical constituents of Evodia rutaecarpa. J Chin Pharm Univ. 2005;36:520–2.
Hu J, Wu X, Cao G, Chen X. Analysis of the influence of processing of stir-baking with glycyrrhizae on the main components of Euodiae Fructus by high-performance liquid chromatography with diode array detector. Nat Prod Res. 2014;28:1853–8.
Hu CQ, Yang XB, Yang XW, Liu JX. Flavonoid glycosides from dried and nearly ripe fruits of Evodia rutaecarpa. China J Chin Mater Med. 2012;37:2571–5.
Liu SS, Dai YT, Sui F, Chen LM, Yan LH, Zhang QW, Wang ZM. Flavonol glycosides from the fruits of Evodia rutaecarpa. J Asian Nat Prod Res. 2018;20:867–74.
Xu ML, Li G, Moon DC, Lee CS, Woo MH, Lee ES, Jahng Y, Chang HW, Lee SH, Son JK. Cytotoxicity and DNA topoisomerase inhibitory activity of constituents isolated from the fruits of Evodia officinalis. Arch Pharm Res. 2006;29:541–7.
Liu AJ, Wang SH, Chen KC, Kuei HP, Shih YL, Hou SY, Chiu WT, Hsiao SH, Shih CM. Evodiamine, a plant alkaloid, induces calcium/JNK-mediated autophagy and calcium/mitochondria-mediated apoptosis in human glioblastoma cells. Chem Biol Interact. 2013;205:20–8.
Chuang WC, Cheng CM, Chang HC, Chen YP, Sheu SJ. Contents of constituents in mature and immature fruits of Evodia species. Planta Med. 1999;65:567–71.
Zhang XL, Jing YK, Peng SW, Li SS, Chai X, Wang YF. Chemical constituents from the nearly ripe fruits of Evodia rutaecarpa (Juss.) Benth. Nat Prod Res Dev. 2013;25:470–4.
Gai L, Rao GX, Song CQ, Hu ZB. Studies on the chemical constituents of Evodia rutaecarpa (Juss.) Benth. var. officinalis (Dode) Huang. Acta Pharm Sin. 2001;36:743–5.
Wang L, Wang DJ, Guo W, Sun KB, Huang NN, Sun R. Four new caffeoylgluconic acid positional isomers from the fruits of Evodia rutaecarpa. J Asian Nat Prod Res. 2019;21:1104–11.
Zhao N, Li ZL, Li DH, Hua HM. A new phenylpropanoid glycoside from Euodia rutaecarpa. Chin Tradit Herbal Drugs. 2015;46:15–8.
He W, Jiang Y, Zhao MB, Zeng KW, Tu PF. Ruticarpsides A-C, three new ester glycosides from the fruits of Tetradium ruticarpum. J Asian Nat Prod Res. 2017;19:659–65.
Xin X, Shao B, Li Y, Liu S, Li D, Wang C, Chen L, Jin L, Ma X, Wu G. New chemical constituents from the fruits of Tetradium ruticarpum. Nat Prod Res. 2022;36:1673–8.
Zhao MY, Yang XW. Two new acylgluconic acids from the nearly ripe fruits of Evodia rutaecarpa. J Asian Nat Prod Res. 2008;10:759–63.
Seya K, Furukawa K, Chiyoya M, Yu Z, Kikuchi H, Daitoku K, Motomura S, Murakami M, Oshima Y, Fukuda I. 1-Methyl-2-undecyl-4(1H)-quinolone, a derivative of quinolone alkaloid evocarpine, attenuates high phosphate-induced calcification of human aortic valve interstitial cells by inhibiting phosphate cotransporter PiT-1. J Pharmacol Sci. 2016;131:51–7.
Survay NS, Upadhyaya CP, Kumar B, Young KE, Yoon DY, Park SW. New genera of flavonols and flavonol derivatives as therapeutic molecules. J Korean Soc Appl Biol Chem. 2011;54:1–18.
Carullo G, Cappello AR, Frattaruolo L, Badolato M, Armentano B, Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med Chem. 2017;9:79–93.
Liu SS, Liu ZX, Wei H, Yin YY, Zhang QW, Yan LH, Wang ZM, Yang LX. Chemical compositions, yield variations and antimicrobial activities of essential oils from three species of Euodiae Fructus in China. Ind Crop Prod. 2019;138:7.
Lou FM, Li QF, Huang XN, Qiu WW. Analysis of the essential oil from Evodia rutaecarpa (Juss.) Benth. by SPME-GC-MS. Chin J Pharm Anal. 2010;30:1248–53.
Liu ZL, Du SS. Fumigant components from the essential oil of Evodia rutaecarpa Hort unripe fruits. E-J Chem. 2011;8:1937–43.
Fu J, Zhao H. Analysis of volatile oil constituents in Evodia rutaecarpa Benth. and E. lenticellata Huang by GC-MS. Lishizhen Med Mater Med Res. 2010;21:60–4.
Yin LS, Sun R. Research progress on pharmacology and toxicology of the Volatile Oil components from Evodia Fructus. Chin J Pharmacovigilance. 2016;13:162–4.
Park SY, Park C, Park SH, Hong SH, Kim GY, Hong SH, Choi YH. Induction of apoptosis by ethanol extract of Evodia rutaecarpa in HeLa human cervical cancer cells via activation of AMP-activated protein kinase. Biosci Trends. 2017;10:467–76.
Park E, Lee MY, Seo CS, Jang JH, Kim YU, Shin HK. Ethanol extract of Evodia rutaecarpa attenuates cell growth through caspase-dependent apoptosis in benign prostatic hyperplasia-1 cells. Nutrients. 2018;10:523.
Thuille N, Fille M, Nagl M. Bactericidal activity of herbal extracts. Int J Hyg Environ Health. 2003;206:217–21.
Chang CP, Chang JY, Wang FY, Tseng YM, Chang JG. The effect of Evodia rutaecarpa extract on cytokine secretion by human mononuclear cells in vitro. Am J Chin Med. 1995;23:173–80.
Chiou WF, Ko HC, Wei BL. Evodia rutaecarpa and three major alkaloids abrogate influenza A virus (H1N1)-induced chemokines production and cell migration. J Evid-Based Complement Altern Med. 2011;2011:1–10.
Matsuda H, Wu JX, Tanaka T, Iinuma M, Kubo M. Antinociceptive activities of 70% methanol extract of evodiae fructus (fruit of Evodia rutaecarpa var. bodinieri) and its alkaloidal components. Biol Pharm Bull. 1997;20:243–8.
Yu LL, Liao JF, Chen CE. Anti-diarrheal effect of water extract of Evodiae fructus in mice. J Ethnopharmacol. 2000;73:39–45.
Chao DC, Lin LJ, Hsiang CY, Li CC, Lo HY, Liang JA, Kao ST, Wu SL, Ho TY. Evodiamine inhibits 12-O-tetradecanoylphorbol-13-acetate-induced activator protein 1 transactivation and cell transformation in human hepatocytes. Phytother Res. 2011;25:1018–23.
Guo W, Huang JH, Wang N, Tan HY, Cheung F, Chen FY, Feng YB. Integrating network pharmacology and pharmacological evaluation for deciphering the action mechanism of herbal formula zuojin pill in suppressing hepatocellular carcinoma. Front Pharmacol. 2019;10:1185.
Cai A, Xiao L, Zhou YP, Zhang ZG, Yang QW. Effect of Evodia rutaecarpa (Juss) Benth extract on Alzheimer disease in mice. Trop J Pharm Res. 2020;19:823–8.
Lim DK, Lee YB, Kim HS. Effects of dehydroevodiamine exposure on glutamate release and uptake in the cultured cerebellar cells. Neurochem Res. 2004;29:407–11.
Park CH, Kim SH, Choi W, Lee YJ, Kim JS, Kang SS, Suh YH. Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa. Planta Med. 1996;62:405–9.
Fang J, Liu R, Tian Q, Hong XP, Wang SH, Cao FY, Pan XP, Wang JZ. Dehydroevodiamine attenuates calyculin A-induced tau hyperphosphorylation in rat brain slices. Acta Pharmacol Sin. 2007;28:1717–23.
Peng JH, Zhang CE, Wei W, Hong XP, Pan XP, Wang JZ. Dehydroevodiamine attenuates tau hyperphosphorylation and spatial memory deficit induced by activation of glycogen synthase kinase-3 in rats. Neuropharmacology. 2007;52:1521–7.
Shin KY, Kim KY, Suh YH. Dehydroevodiamine. HCl enhances cognitive function in memory-impaired rat models. Korean J Physiol Pharmacol. 2017;21:55–64.
Wang D, Wang C, Liu L, Li S. Protective effects of evodiamine in experimental paradigm of Alzheimer’s disease. Cogn Neurodyn. 2018;12:303–13.
Yuan SM, Gao K, Wang DM, Quan XZ, Liu JN, Ma CM, Qin C, Zhang LF. Evodiamine improves congnitive abilities in SAMP8 and APP(swe)/PS1(DeltaE9) transgenic mouse models of Alzheimer’s disease. Acta Pharmacol Sin. 2011;32:295–302.
Zhang Y, Wang J, Wang C, Li Z, Liu X, Zhang J, Lu J, Wang D. Pharmacological basis for the use of Evodiamine in Alzheimer’s Disease: antioxidation and antiapoptosis. Int J Mol Sci. 2018;19:1527.
Lee B, Choi EJ, Lee EJ, Han SM, Hahm DH, Lee HJ, Shim I. The neuroprotective effect of methanol extract of gagamjungjihwan and fructus euodiae on ischemia-induced neuronal and cognitive impairment in the rat. J Evid-Based Complement Altern Med. 2011;2011: 685254.
Zhang YN, Yang YF, Yang XW. Blood-brain barrier permeability and neuroprotective effects of three main alkaloids from the fruits of Euodia rutaecarpa with MDCK-pHaMDR cell monolayer and PC12 cell line. Biomed Pharmacother. 2018;98:82–7.
Zhang WD, Chen XY, Wu C, Lian YN, Wang YJ, Wang JH, Yang F, Liu CH, Li XY. Evodiamine reduced peripheral hypersensitivity on the mouse with nerve injury or inflammation. Mol Pain. 2020;16:1744806920902563.
Han M, Hu L, Chen Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des Devel Ther. 2019;13:2923–31.
Ko HC, Wang YH, Liou KT, Chen CM, Chen CH, Wang WY, Chang S, Hou YC, Chen KT, Chen CF, Shen YC. Anti-inflammatory effects and mechanisms of the ethanol extract of Evodia rutaecarpa and its bioactive components on neutrophils and microglial cells. Eur J Pharmacol. 2007;555:211–7.
Woo HG, Lee CH, Noh MS, Lee JJ, Jung YS, Baik EJ, Moon CH, Lee SH. Rutaecarpine, a quinazolinocarboline alkaloid, inhibits prostaglandin production in RAW264.7 macrophages. Planta Med. 2001;67:505–9.
Li Z, Yang M, Peng Y, Gao M, Yang B. Rutaecarpine ameliorated sepsis-induced peritoneal resident macrophages apoptosis and inflammation responses. Life Sci. 2019;228:11–20.
Moon TC, Murakami M, Kudo I, Son KH, Kim HP, Kang SS, Chang HW. A new class of COX-2 inhibitor, rutaecarpine from Evodia rutaecarpa. Inflamm Res. 1999;48:621–5.
Zhang YB, Yan TT, Sun DX, Xie C, Wang TX, Liu XY, Wang J, Wang Q, Luo YH, Wang P, Yagai T, Krausz KW, Yang XW, Gonzalez FJ. Rutaecarpine inhibits KEAP1-NRF2 interaction to activate NRF2 and ameliorate dextran sulfate sodium-induced colitis. Free Radic Bio Med. 2020;148:33–41.
Zhao Z, Gong S, Wang S, Ma C. Effect and mechanism of evodiamine against ethanol-induced gastric ulcer in mice by suppressing Rho/NF-small ka, CyrillicB pathway. Int Immunopharmacol. 2015;28:588–95.
Fan X, Zhu JY, Sun Y, Luo L, Yan J, Yang X, Yu J, Tang WQ, Ma W, Liang HP. Evodiamine inhibits zymosan-induced inflammation in vitro and in vivo: inactivation of NF-kappaB by inhibiting IkappaBalpha phosphorylation. Inflammation. 2017;40:1012–27.
Zhang H, Yin L, Lu M, Wang J, Li YT, Gao WL, Yin ZS. Evodiamine attenuates adjuvant-induced arthritis in rats by inhibiting synovial inflammation and restoring the Th17/Treg balance. J Pharm Pharmacol. 2020;72:798–806.
Lin J, Zhang X, Li C, Zhang Y, Lu H, Chen J, Li Z, Yang X, Wu Z. Evodiamine via targeting nNOS and AMPA receptor GluA1 inhibits nitroglycerin-induced migraine-like response. J Ethnopharmacol. 2020;254: 112727.
Kobayashi Y. The nociceptive and anti-nociceptive effects of Evodiamine from fruits of Evodia rutaecarpa in mice. Planta Med. 2003;69:425–8.
Liu S, Zhang S, Lv X, Lu J, Ren C, Zeng Z, Zheng L, Zhou X, Fu H, Zhou D, Chen Y. Limonin ameliorates ulcerative colitis by regulating STAT3/miR-214 signaling pathway. Int Immunopharmacol. 2019;75: 105768.
Du J, Wang XF, Zhou QM, Zhang TL, Lu YY, Zhang H, Su SB. Evodiamine induces apoptosis and inhibits metastasis in MDAMB-231 human breast cancer cells in vitro and in vivo. Oncol Rep. 2013;30:685–94.
Wang KL, Hsia SM, Yeh JY, Cheng SC, Wang PS, Wang SW. Anti-proliferative effects of evodiamine on human breast cancer cells. PLoS ONE. 2013;8: e67297.
Hong Z, Wang Z, Zhou B, Wang J, Tong H, Liao Y, Zheng P, Jamshed MB, Zhang Q, Chen H. Effects of evodiamine on PI3K/Akt and MAPK/ERK signaling pathways in pancreatic cancer cells. Int J Oncol. 2020;56:783–93.
Su Z, Wang C, Chang D, Zhu X, Sai C, Pei J. Limonin attenuates the stemness of breast cancer cells via suppressing MIR216A methylation. Biomed Pharmacother. 2019;112: 108699.
Yang ZG, Chen AQ, Liu B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205). Chem Biodivers. 2009;6:924–33.
Li FS, Huang J, Cui MZ, Zeng JR, Li PP, Li L, Deng Y, Hu Y, He BC, Shu DZ. BMP9 mediates the anticancer activity of evodiamine through HIF1alpha/p53 in human colon cancer cells. Oncol Rep. 2020;43:415–26.
Huang J, Chen ZH, Ren CM, Wang DX, Yuan SX, Wu QX, Chen QZ, Zeng YH, Shao Y, Li Y, Wu K, Yu Y, Sun WJ, He BC. Antiproliferation effect of evodiamine in human colon cancer cells is associated with IGF-1/HIF-1alpha downregulation. Oncol Rep. 2015;34:3203–11.
Ogasawara M, Matsunaga T, Takahashi S, Saiki I, Suzuki H. Anti-invasive and metastatic activities of evodiamine. Biol Pharm Bull. 2002;25:1491–3.
Zhou P, Li XP, Jiang R, Chen Y, Lv XT, Guo XX, Tian K, Yuan DZ, Lv YW, Ran JH, Li J, Chen DL. Evodiamine inhibits migration and invasion by Sirt1-mediated post-translational modulations in colorectal cancer. Anticancer Drugs. 2019;30:611–7.
Hong JY, Park SH, Min HY, Park HJ, Lee SK. Anti-proliferative effects of evodiamine in human lung cancer cells. J Cancer Prev. 2014;19:7–13.
Su T, Yang X, Deng JH, Huang QJ, Huang SC, Zhang YM, Zheng HM, Wang Y, Lu LL, Liu ZQ. Evodiamine, a novel NOTCH3 methylation stimulator, significantly suppresses lung carcinogenesis in vitro and in vivo. Front Pharmacol. 2018;9:434.
Yang X, Zhang Y, Huang Y, Wang Y, Qi X, Su T, Lu L. Evodiamine suppresses Notch3 signaling in lung tumorigenesis via direct binding to gamma-secretases. Phytomedicine. 2020;68: 153176.
Gong C, Qi L, Huo Y, Zhang S, Ning X, Bai L, Wang Z. Anticancer effect of Limonin against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice and the inhibition of A549 cell proliferation through apoptotic pathway. J Biochem Mol Toxicol. 2019;33: e22374.
Hu CY, Wu HT, Su YC, Lin CH, Chang CJ, Wu CL. Evodiamine exerts an anti-hepatocellular carcinoma activity through a WWOX-dependent pathway. Molecules. 2017;22:1175.
Yang F, Shi L, Liang T, Ji L, Zhang G, Shen Y, Zhu F, Xu L. Anti-tumor effect of evodiamine by inducing Akt-mediated apoptosis in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017;485:54–61.
Guo XX, Li XP, Zhou P, Li DY, Lyu XT, Chen Y, Lyu YW, Tian K, Yuan DZ, Ran JH, Chen DL, Jiang R, Li J. Evodiamine induces apoptosis in SMMC-7721 and HepG2 cells by suppressing NOD1 signal pathway. Int J Mol Sci. 2018;19:3419.
Zhao S, Xu K, Jiang R, Li DY, Guo XX, Zhou P, Tang JF, Li LS, Zeng D, Hu L, Ran JH, Li J, Chen DL. Evodiamine inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells via the Hippo-Yes-Associated Protein signaling pathway. Life Sci. 2020;251: 117424.
Tang Z, Tang Y, Li L, Liu T, Yang J. Limonin provokes hepatocellular carcinoma cells with stemness entry into cycle via activating PI3K/Akt signaling. Biomed Pharmacother. 2019;117: 109051.
Wen Z, Feng S, Wei L, Wang Z, Hong D, Wang Q. Evodiamine, a novel inhibitor of the Wnt pathway, inhibits the self-renewal of gastric cancer stem cells. Int J Mol Med. 2015;36:1657–63.
Hu C, Gao X, Han Y, Guo Q, Zhang K, Liu M, Wang Y, Wang J. Evodiamine sensitizes BGC-823 gastric cancer cells to radiotherapy in vitro and in vivo. Mol Med Rep. 2016;14:413–9.
Wu WS, Chien CC, Liu KH, Chen YC, Chiu WT. Evodiamine prevents glioma growth, induces glioblastoma cell apoptosis and cell cycle arrest through JNK activation. Am J Chin Med. 2017;45:879–99.
Zhang T, Qu S, Shi Q, He D, Jin X. Evodiamine induces apoptosis and enhances TRAIL-induced apoptosis in human bladder cancer cells through mTOR/S6K1-mediated downregulation of Mcl-1. Int J Mol Sci. 2014;15:3154–71.
Shi CS, Li JM, Chin CC, Kuo YH, Lee YR, Huang YC. Evodiamine induces cell growth arrest, apoptosis and suppresses tumorigenesis in human urothelial cell carcinoma cells. Anticancer Res. 2017;37:1149–59.
Bae JR, Park WH, Suh DH, No JH, Kim YB, Kim K. Role of limonin in anticancer effects of Evodia rutaecarpa on ovarian cancer cells. BMC Complement Med Ther. 2020;20:94.
Chen TC, Chien CC, Wu MS, Chen YC. Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine. 2016;23:68–78.
Wei LJ, Jin XY, Cao ZP, Li WL. Evodiamine induces extrinsic and intrinsic apoptosis of ovarian cancer cells via the mitogen-activated protein kinase phosphatidylinositol-3-kinaseprotein kinase B signaling pathways. J Tradit Chin Med. 2016;36:353–9.
Yuan XL, Zhang P, Liu XM, Du YM, Hou XD, Cheng S, Zhang ZF. Cytological assessments and transcriptome profiling demonstrate that evodiamine inhibits growth and induces apoptosis in a renal carcinoma cell line. Sci Rep. 2017;7:12572.
Wu WS, Chien CC, Chen YC, Chiu WT. Protein kinase RNA-like endoplasmic reticulum kinase-mediated Bcl-2 protein phosphorylation contributes to evodiamine-induced apoptosis of human renal cell carcinoma cells. PLoS ONE. 2016;11: e0160484.
Wang C, Li S, Wang MW. Evodiamine-induced human melanoma A375–S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro. 2010;24:898–904.
Zhang Y, Zhang QH, Wu LJ, Tashiro S, Onodera S, Ikejima T. Atypical apoptosis in L929 cells induced by evodiamine isolated from Evodia rutaecarpa. J Asian Nat Prod Res. 2004;6:19–27.
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang RX, Pi CJ, He BC, Ke ZY, Chen L, Deng ZL, Yin LJ. Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol Rep. 2015;34:1388–96.
Zhou Y, Hu J. Evodiamine induces apoptosis, G2/M cell cycle arrest, and inhibition of cell migration and invasion in Human Osteosarcoma Cells via Raf/MEK/ERK signalling pathway. Med Sci Monit. 2018;24:5874–80.
Zhu B, Zhao L, Liu Y, Jin Y, Feng J, Zhao F, Sun J, Geng R, Wei Y. Induction of phosphatase shatterproof 2 by evodiamine suppresses the proliferation and invasion of human cholangiocarcinoma. Int J Biochem Cell Biol. 2019;108:98–110.
Zhu LH, Bi W, Liu XD, Li JF, Wu YY, Du BY, Tan YH. Induction of apoptosis by evodiamine involves both activation of mitotic arrest and mitotic slippage. Oncol Rep. 2011;26:1447–55.
Fang Q, Jiang S, Li C. Evodiamine selectively inhibits multiple myeloma cell growth by triggering activation of intrinsic apoptosis pathway. Onco Targets Ther. 2019;12:11383–91.
Pan X, Hartley JM, Hartley JA, White KN, Wang Z, Bligh SW. Evodiamine, a dual catalytic inhibitor of type I and II topoisomerases, exhibits enhanced inhibition against camptothecin resistant cells. Phytomedicine. 2012;19:618–24.
Zhang SL, Xiong YH, Zhang YX, Zhao HM. Targeting of mTORC by dihydroevocarpine induces cytotoxicity in acute myeloid leukemia. J Cell Physiol. 2019;234:13032–41.
Hibino T, Yuzurihara M, Terawaki K, Kanno H, Kase Y, Takeda A. Goshuyuto, a traditional Japanese medicine for migraine, inhibits platelet aggregation in guinea-pig whole blood. J Pharmacol Sci. 2008;108:89–94.
Sheu JR, Huang WC, Wu CH, Lee YM, Yen MH. Antithrombotic effect of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, on platelet plug formation in in vivo experiments. Br J Haematol. 2000;110:110–5.
Sheu JR, Hung WC, Lee YM, Yen MH. Mechanism of inhibition of platelet aggregation by rutaecarpine, an alkaloid isolated from Evodia rutaecarpa. Eur J Pharmacol. 1996;318:469–75.
Sheu JR, Kan YC, Hung WC, Su CH, Lin CH, Lee YM, Yen MH. The antiplatelet activity of rutaecarpine, an alkaloid isolated from Evodia rutaecarpa, is mediated through inhibition of phospholipase C. Thromb Res. 1998;92:53–64.
Li D, Peng J, Xin HY, Luo D, Zhang YS, Zhou Z, Jiang DJ, Deng HW, Li YJ. Calcitonin gene-related peptide-mediated antihypertensive and anti-platelet effects by rutaecarpine in spontaneously hypertensive rats. Peptides. 2008;29:1781–8.
Luo D, He H, Yan H, Zhao Y, Yu YR, Kuang HB, Huang QR, He M, Peng WJ. Rutaecarpine prevents high glucose-induced Cx37 gap junction dysfunction in human umbilical vein endothelial cells. Int J Pharmacol. 2018;14:698–706.
Deng J, Qin J, Cai Y, Zhong X, Zhang X, Yu S. Rutaecarpine suppresses proliferation and promotes apoptosis of human pulmonary artery smooth muscle cells in hypoxia possibly through HIF-1alpha-dependent pathways. J Cardiovasc Pharmacol. 2018;71:293–302.
Li YJ, Zhang F, Gong QH, Wu Q, Yu LM, Sun AS. Rutaecarpine inhibits angiotensin II-induced proliferation in rat vascular smooth muscle cells. Chin J Integr Med. 2014;20:682–7.
Wang M, Wu Y, Yu Y, Fu Y, Yan H, Wang X, Li T, Peng W, Luo D. Rutaecarpine prevented ox-LDL-induced VSMCs dysfunction through inhibiting overexpression of connexin 43. Eur J Pharmacol. 2019;853:84–92.
Liu Y, Fu YQ, Peng WJ, Yu YR, Wu YS, Yan H, Huang QR, He M, Luo D. Rutaecarpine reverses the altered connexin expression pattern induced by oxidized low-density lipoprotein in monocytes. J Cardiovasc Pharmacol. 2016;67:519–25.
Peng WJ, Liu Y, Yu YR, Fu YQ, Zhao Y, Kuang HB, Huang QR, He M, Luo D. Rutaecarpine prevented dysfunction of endothelial gap junction induced by Ox-LDL via activation of TRPV1. Eur J Pharmacol. 2015;756:8–14.
Xu Y, Liu Q, Xu Y, Liu C, Wang X, He X, Zhu N, Liu J, Wu Y, Li Y, Li N, Feng T, Lai F, Zhang M, Hong B, Jiang JD, Si S. Rutaecarpine suppresses atherosclerosis in ApoE-/- mice through upregulating ABCA1 and SR-BI within RCT. J Lipid Res. 2014;55:1634–47.
Ge X, Chen SY, Liu M, Liang TM, Liu C. Evodiamine inhibits PDGF-BB-induced proliferation of rat vascular smooth muscle cells through the suppression of cell cycle progression and oxidative stress. Mol Med Rep. 2016;14:4551–8.
Lv Q, Xue Y, Li G, Zou L, Zhang X, Ying M, Wang S, Guo L, Gao Y, Li G, Xu H, Liu S, Xie J, Liang S. Beneficial effects of evodiamine on P2X(4)-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int Immunopharmacol. 2015;28:1044–9.
Xue Y, Guo T, Zou L, Gong Y, Wu B, Yi Z, Jia T, Zhao S, Shi L, Li L, Yuan H, Liu H, Gao Y, Li G, Liu S, Xu H, Zhang C, Liang S, Li G. Evodiamine attenuates P2X7-mediated inflammatory injury of Human Umbilical Vein Endothelial Cells exposed to high free fatty acids. Oxid Med Cell Longev. 2018;2018:5082817.
Hu CP, Xiao L, Deng HW, Li YJ. The depressor and vasodilator effects of rutaecarpine are mediated by calcitonin gene-related peptide. Planta Med. 2003;69:125–9.
Zeng SY, Yang L, Lu HQ, Yan QJ, Gao L, Qin XP. Rutaecarpine prevents hypertensive cardiac hypertrophy involving the inhibition of Nox4-ROS-ADAM17 pathway. J Cell Mol Med. 2019;23:4196–207.
Xu Y, Chen XP, Zhang F, Hou HH, Zhang JY, Lin SX, Sun AS. Rutaecarpine inhibits intimal hyperplasia in a balloon-injured rat artery model. Chin J Integr Med. 2018;24:429–35.
Yi HH, Rang WQ, Deng PY, Hu CP, Liu GZ, Tan GS, Xu KP, Li YJ. Protective effects of rutaecarpine in cardiac anaphylactic injury is mediated by CGRP. Planta Med. 2004;70:1135–9.
Hu CP, Li NS, Xiao L, Deng HW, Li YJ. Involvement of capsaicin-sensitive sensory nerves in cardioprotection of rutaecarpine in rats. Regul Peptides. 2003;114:45–9.
Xue H, Cheng Y, Wang X, Yue Y, Zhang W, Li X. Rutaecarpine and evodiamine selected as beta1-AR inhibitor candidates using beta1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J Pharm Biomed Anal. 2015;115:307–14.
Rang WQ, Du YH, Hu CP, Ye F, Tan GS, Deng HW, Li YJ. Protective effects of calcitonin gene-related peptide-mediated evodiamine on guinea-pig cardiac anaphylaxis. Naunyn-Schmiedeberg’s Arch Pharmacol. 2003;367:306–11.
Rang WQ, Du YH, Hu CP, Ye F, Xu KP, Peng J, Deng HW, Li YJ. Protective effects of evodiamine on myocardial ischemia-reperfusion injury in rats. Planta Med. 2004;70:1140–3.
Wu QQ, Xiao Y, Jiang XH, Yuan Y, Yang Z, Chang W, Bian ZY, Tang QZ. Evodiamine attenuates TGF-beta1-induced fibroblast activation and endothelial to mesenchymal transition. Mol Cell Biochem. 2017;430:81–90.
He N, Gong QH, Zhang F, Zhang JY, Lin SX, Hou HH, Wu Q, Sun AS. Evodiamine inhibits angiotensin II-induced rat cardiomyocyte hypertrophy. Chin J Integr Med. 2018;24:359–65.
Jiang XH, Wu QQ, Xiao Y, Yuan Y, Yang Z, Bian ZY, Chang W, Tang QZ. Evodiamine prevents isoproterenol-induced cardiac fibrosis by regulating endothelial-to-mesenchymal transition. Planta Med. 2017;83:761–9.
Liu LH, Xie JY, Guo WW, Wu GY, Chen ZF, Yi JY, Zhang L, Zhang ZJ, Li Z. Evodiamine activates AMPK and promotes adiponectin multimerization in 3T3-L1 adipocytes. J Asian Nat Prod Res. 2014;16:1074–83.
Shi J, Yan J, Lei Q, Zhao J, Chen K, Yang D, Zhao X, Zhang Y. Intragastric administration of evodiamine suppresses NPY and AgRP gene expression in the hypothalamus and decreases food intake in rats. Brain Res. 2009;1247:71–8.
Wang T, Kusudo T, Takeuchi T, Yamashita Y, Kontani Y, Okamatsu Y, Saito M, Mori N, Yamashita H. Evodiamine inhibits insulin-stimulated mTOR-S6K activation and IRS1 serine phosphorylation in adipocytes and improves glucose tolerance in obese/diabetic mice. PLoS ONE. 2013;8: e83264.
Yu L, Wang Z, Huang M, Li Y, Zeng K, Lei J, Hu H, Chen B, Lu J, Xie W, Zeng S. Evodia alkaloids suppress gluconeogenesis and lipogenesis by activating the constitutive androstane receptor. Biochim Biophys Acta. 2016;1859:1100–11.
Nie XQ, Chen HH, Zhang JY, Zhang YJ, Yang JW, Pan HJ, Song WX, Murad F, He YQ, Bian K. Rutaecarpine ameliorates hyperlipidemia and hyperglycemia in fat-fed, streptozotocin-treated rats via regulating the IRS-1/PI3K/Akt and AMPK/ACC2 signaling pathways. Acta Pharmacol Sin. 2016;37:483–96.
Li CG, Zeng QZ, Chen MY, Xu LH, Zhang CC, Mai FY, Zeng CY, He XH, Ouyang DY. Evodiamine augments NLRP3 inflammasome activation and anti-bacterial responses through inducing alpha-Tubulin acetylation. Front Pharmacol. 2019;10:290.
Guan X, Song Y, Zhou L, Zhang M, Lian K. Japon Balıklarında (Carassius auratus) Gyrodactylus kobayashii’ye (Monogenea) Karşı Çin Şifalı Otlarının Anthelmintik Etkinliklerinin İncelenmesi. Kafkas Univ Vet Fak. 2019.
Miyazawa M, Fujioka J, Ishikawa Y. Insecticidal compounds from Evodia rutaecarpa against Drosophila melanogaster. J Sci Food Agr. 2002;82:1574–8.
Jiang L, Zhao XY, Pei JJ, Mei LJ, Cui YL, Wang S, Shao Y, Zhang XL, Tao YD. Daily chemical evodiamine from Chinese prickly ash attenuates osteoclast differentiation through RANKL induced NFAT pathways. J Funct Foods. 2017;37:594–602.
Jin H, Yao L, Chen K, Liu Y, Wang Q, Wang Z, Liu Q, Cao Z, Kenny J, Tickner J, Wang X, Xu J. Evodiamine inhibits RANKL-induced osteoclastogenesis and prevents ovariectomy-induced bone loss in mice. J Cell Mol Med. 2019;23:522–34.
Yin H, Wang JW, Wu M, Ma Y, Wang SF, Su QJ. Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. Biomed Res Int. 2019;2019:5859641.
Fukuma Y, Sakai E, Komaki S, Nishishita K, Okamoto K, Tsukuba T. Rutaecarpine attenuates osteoclastogenesis by impairing M-CSF and RANKL-stimulated signaling pathways. Clin Exp Pharmacol Physiol. 2018;45:863–5.
Lee DH, Jeon EJ, Ahn J, Hwang JT, Hur J, Ha TY, Jung CH, Sung MJ. Limonin enhances osteoblastogenesis and prevents ovariectomy-induced bone loss. J Funct Foods. 2016;23:105–14.
Yang D, Li L, Qian S, Liu L. Evodiamine ameliorates liver fibrosis in rats via TGF-beta1/Smad signaling pathway. J Nat Med. 2018;72:145–54.
Eraslan E, Tanyeli A, Polat E, Yetim Z. Evodiamine alleviates kidney ischemia reperfusion injury in rats: a biochemical and histopathological study. J Cell Biochem. 2019;120:17159–66.
Shi Y, Hua Q, Li N, Zhao M, Cui Y. Protective effects of evodiamine against LPS-induced acute kidney injury through regulation of ROS-NF-kappaB-mediated inflammation. J Evid-Based Complement Altern Med. 2019;2019:2190847.
Jin SW, Hwang YP, Choi CY, Kim HG, Kim SJ, Kim Y, Chung YC, Lee KJ, Jeong TC, Jeong HG. Protective effect of rutaecarpine against t-BHP-induced hepatotoxicity by upregulating antioxidant enzymes via the CaMKII-Akt and Nrf2/ARE pathways. Food Chem Toxicol. 2017;100:138–48.
Wang C, Hao Z, Zhou J, Zhang L, Sun Y, Liang C. Rutaecarpine alleviates renal ischemia reperfusion injury in rats by suppressing the JNK/p38 MAPK signaling pathway and interfering with the oxidative stress response. Mol Med Rep. 2017;16:922–8.
Yang R, Song C, Chen J, Zhou L, Jiang X, Cao X, Sun Y, Zhang Q. Limonin ameliorates acetaminophen-induced hepatotoxicity by activating Nrf2 antioxidative pathway and inhibiting NF-kappaB inflammatory response via upregulating Sirt1. Phytomedicine. 2020;69: 153211.
Jiang ML, Zhang ZX, Li YZ, Wang XH, Yan W, Gong GQ. Antidepressant-like effect of evodiamine on chronic unpredictable mild stress rats. Neurosci Lett. 2015;588:154–8.
Iwaoka E, Wang S, Matsuyoshi N, Kogure Y, Aoki S, Yamamoto S, Noguchi K, Dai Y. Evodiamine suppresses capsaicin-induced thermal hyperalgesia through activation and subsequent desensitization of the transient receptor potential V1 channels. J Nat Med. 2016;70:1–7.
Yamanara J, Yamada T, Kitani T, Naitoh Y, Fujimura H. Antianoxic action of evodiamine, an alkaloid in evodia rutaecarpa fruit. J Ethnopharmacol. 1989;27:185–92.
Shin YW, Bae EA, Cai XF, Lee JJ, Kim DH. In vitro and in vivo antiallergic effect of the fructus of Evodia rutaecarpa and its constituents. Biol Pharm Bull. 2007;30:197–9.
Dai JP, Li WZ, Zhao XF, Wang GF, Yang JC, Zhang L, Chen XX, Xu YX, Li KS. A drug screening method based on the autophagy pathway and studies of the mechanism of evodiamine against influenza A virus. PLoS ONE. 2012;7: e42706.
Yang JY, Lee P, Kim BJ. Effect of evodiae fructus methanol extract on virulence-related genes’ expression of Helicobacter pylori. Korean J Clin Lab Sci. 2019;51:316–22.
Tominaga K, Higuchi K, Hamasaki N, Hamaguchi M, Takashima T, Tanigawa T, Watanabe T, Fujiwara Y, Tezuka Y, Nagaoka T, Kadota S, Ishii E, Kobayashi K, Arakawa T. In vivo action of novel alkyl methyl quinolone alkaloids against Helicobacter pylori. J Antimicrob Chemother. 2002;50:547–52.
Liao JF, Chiou WF, Shen YC, Wang GJ, Chen CF. Anti-inflammatory and anti-infectious effects of Evodia rutaecarpa (Wuzhuyu) and its major bioactive components. Chin Med. 2011;6:6.
Yu X, Wu DZ, Yuan JY, Zhang RR, Hu ZB. Gastroprotective effect of Fructus Evodiae water extract on ethanol-induced gastric lesions in rats. Am J Chin Med. 2006;34:1027–35.
Li Y, Zhang G, Chen M, Tong M, Zhao M, Tang F, Xiao R, Wen H. Rutaecarpine inhibited imiquimod-induced psoriasis-like dermatitis via inhibiting the NF-kappaB and TLR7 pathways in mice. Biomed Pharmacother. 2019;109:1876–83.
Shen P, Zhang ZC, Zhu KP, Cao HY, Liu JX, Lu XJ, Li YX, Jing Y, Yuan X, Fu YH, Cao YG, Zhang NS. Evodiamine prevents dextran sulfate sodium-induced murine experimental colitis via the regulation of NF-kappa B and NLRP3 inflammasome. Biomed Pharmacother. 2019;110:786–95.
Wang XK, Wang YG, Zhan HL, Chai YS, Hu J, Xing DM, You XF, Lei F, Du LJ. Comprehensive study of Evodia rutaecarpa-induced contraction on blood vascular in vivo and in vitro. Chin J Nat Med. 2011;9:65–73.
Chiou WF, Liao JF, Shum AYC, Chen CF. Mechanisms of vasorelaxant effect of Dehydroevodiamine: a bioactive isoquinazolinocarboline alkaloid of plant origin. J Cardiovasc Pharm. 1996;27:845–53.
Ma J, Chen L, Fan J, Cao W, Zeng G, Wang Y, Li Y, Zhou Y, Deng X. Dual-targeting Rutaecarpine-NO donor hybrids as novel anti-hypertensive agents by promoting release of CGRP. Eur J Med Chem. 2019;168:146–53.
Ge X, Chen S, Liu M, Liang T, Liu C. Evodiamine attenuates PDGF-BB-induced migration of rat vascular smooth muscle cells through activating PPARgamma. Int J Mol Sci. 2015;16:28180–93.
Kobayashi Y, Hoshikuma K, Nakano Y, Yokoo Y, Kamiya T. The positive inotropic and chronotropic effects of evodiamine and rutaecarpine, indoloquinazoline alkaloids isolated from the fruits of Evodia rutaecarpa, on the guinea-pig isolated right atria: possible involvement of vanilloid receptors. Planta Med. 2001;67:244–8.
Hu CP, Xiao L, Deng HW, Li YJ. The cardioprotection of rutaecarpine is mediated by endogenous calcitonin related-gene peptide through activation of vanilloid receptors in guinea-pig hearts. Planta Med. 2002;68:705–9.
Loh SH, Tsai YT, Lee CY, Chang CY, Tsai CS, Cheng TH, Lin CI. Antiarrhythmic effects of dehydroevodiamine in isolated human myocardium and cardiomyocytes. J Ethnopharmacol. 2014;153:753–62.
Liu AJ, Wang SH, Hou SY, Lin CJ, Chiu WT, Hsiao SH, Chen TH, Shih CM. Evodiamine induces transient receptor potential vanilloid-1-mediated protective autophagy in U87-MG astrocytes. J Evid-Based Complement Altern Med. 2013;2013: 354840.
Kim SJ, Lee SJ, Lee S, Chae S, Han MD, Mar W, Nam KW. Rutecarpine ameliorates bodyweight gain through the inhibition of orexigenic neuropeptides NPY and AgRP in mice. Biochem Biophys Res Commun. 2009;389:437–42.
Kobayashi Y, Nakano Y, Kizaki M, Hoshikuma K, Yokoo Y, Kamiya T. Capsaicin-like anti-obese activities of evodiamine from fruits of Evodia rutaecarpa, a vanilloid receptor agonist. Planta Med. 2001;67:628–33.
Yang RZ, Tang CS. Plants used for pest control in China: a literature review. Econ Bot. 1988;42:376–406.
Li X, Ge J, Zheng Q, Zhang J, Sun R, Liu R. Evodiamine and rutaecarpine from Tetradium ruticarpum in the treatment of liver diseases. Phytomedicine. 2020;68: 153180.
Yamahara J, Yamada T, Kitani T, Naitoh Y, Fujimura H. Antianoxic action and active constituents of evodiae fructus. Chem Pharm Bull. 1989;37:1820–2.
Ko YH, Shim KY, Lee SY, Jang CG. Evodiamine reduces caffeine-induced sleep disturbances and excitation in mice. Biomol Ther. 2018;26:432–8.
Ma R, Chen Y, Zhao YR, Tan XL, Zhou XD. Case report of liver damage by Traditional Chinese Medicine drug granules. Clin Misdiagn Misther. 2018;31:84–6.
Cai XY, Meng N, Yang B. Analysis of one poisoning case caused by excessive Evodiae fructus. J Beijing Tradit Chin Med. 2006;25:171–2.
Teschke R. Traditional Chinese medicine induced liver injury. J Clin Transl Hepatol. 2014;2:80–94.
Teschke R, Wolff A, Frenzel C, Schulze J. Review article: Herbal hepatotoxicity–an update on traditional Chinese medicine preparations. Aliment Pharmacol Ther. 2014;40:32–50.
Shan QY, Tian G, Wang JL, Hui H, Shou QY, Fu HY, Hao M, Wang KL, Wu X, Cao G, Chen GQ, Qin LP. Change in the active component of processed Tetradium ruticarpum extracts leads to improvement in efficacy and toxicity attenuation. J Ethnopharmacol. 2021;264:11.
Cai Q, Wei J, Zhao W, Shi S, Zhang Y, Wei R, Zhang Y, Li W, Wang Q. Toxicity of Evodiae fructus on rat liver mitochondria: the role of oxidative stress and mitochondrial permeability transition. Molecules. 2014;19:21168–82.
Zhang FL, He X, Zhai YR, He LN, Zhang SC, Wang LL, Yang AH, An LJ. Mechanism-based inhibition of CYPs and RMs-induced hepatoxicity by rutaecarpine. Xenobiotica. 2015;45:978–89.
Zhang Y, Yan T, Sun D, Xie C, Zheng Y, Zhang L, Yagai T, Krausz KW, Bisson WH, Yang X, Gonzalez FJ. Structure-activity relationships of the main bioactive constituents of euodia rutaecarpa on aryl hydrocarbon receptor activation and associated bile acid homeostasis. Drug Metab Dispos. 2018;46:1030–40.
Liu YT, Liu C, Liu YM, Ge QL, Sun C. Cytochrome P450 mediated bioactivation of rutaevin, a bioactive and potentially hepatotoxic component of evodia rutaecarpa. Chem Res Toxicol. 2020;33:3054–64.
Li W, Sun X, Liu B, Zhang L, Fan Z, Ji Y. Screening and identification of hepatotoxic component in Evodia rutaecarpa based on spectrum-effect relationship and UPLC-Q-TOFMS. Biomed Chromatogr. 2016;30:1975–83.
Wen B, Roongta V, Liu L, Moore DJ. Metabolic activation of the indoloquinazoline alkaloids evodiamine and rutaecarpine by human liver microsomes: dehydrogenation and inactivation of cytochrome P450 3A4. Drug Metab Dispos. 2014;42:1044–54.
Zhu QN, Zhang D, Jin T, Wu Q, Liu J, Lu YF. Rutaecarpine effects on expression of hepatic phase-1, phase-2 metabolism and transporter genes as a basis of herb-drug interactions. J Ethnopharmacol. 2013;147:215–9.
Zhang YT, Zhang DF, Ge NY, Zhu GH, Hao C, Zhang Y, Chen RJ. Effect of evodiamine on CYP enzymes in rats by a cocktail method. Pharmacology. 2016;97:218–23.
Baburin I, Varkevisser R, Schramm A, Saxena P, Beyl S, Szkokan P, Linder T, Stary-Weinzinger A, van der Heyden MAG, Houtman M, Takanari H, Jonsson M, Beekman JHD, Hamburger M, Vos MA, Hering S. Dehydroevodiamine and hortiamine, alkaloids from the traditional Chinese herbal drug Evodia rutaecarpa, are IKr blockers with proarrhythmic effects in vitro and in vivo. Pharmacol Res. 2018;131:150–63.
Yang W, Ma L, Li S, Cui K, Lei L, Ye Z. Evaluation of the cardiotoxicity of evodiamine in vitro and in vivo. Molecules. 2017;22:943.
Yu G, Luo Z, Wang W, Li Y, Zhou Y, Shi Y. Rubus chingii Hu: a review of the phytochemistry and pharmacology. Front Pharmacol. 2019;10:799.
Zhang X, Zhan G, Jin M, Zhang H, Dang J, Zhang Y, Guo Z, Ito Y. Botany, traditional use, phytochemistry, pharmacology, quality control, and authentication of Radix Gentianae Macrophyllae-A traditional medicine: a review. Phytomedicine. 2018;46:142–63.
Xu H, Zhang T, Xiao X, Zhao P, Liu C, Xu J. Simultaneous analysis of thirteen bioactive components in evodia rutaecarpa and its varieties by HPLC-DAD-MS. Chin Herb Med. 2010;2:112–7.
Zhao Y, Li Z, Zhou X, Cai Z, Gong X, Zhou C. Quality evaluation of Evodia rutaecarpa (Juss.) Benth by high performance liquid chromatography with photodiode-array detection. J Pharmaceut Biomed. 2008;48:1230–6.
Zhang PT, Pan BY, Liao QF, Yao MC, Xu XJ, Wan JZ, Liu D, Xie ZY. Simultaneous quantification of limonin, two indolequinazoline alkaloids, and four quinolone alkaloids in Evodia rutaecarpa (Juss.) Benth by HPLC-DAD method. J Anal Methods Chem. 2013;2013:827361.
Huang D, Li SX, Cai GX, Yue CH, Jun WL, Zhang P. Molecular authentication and quality control using a High Performance Liquid Chromatography Technique of Fructus Evodiae. Biol Pharm Bull. 2008;31:312–5.
Liu Y, Zhou W, Mao Z, Chen Z. Analysis of Evodiae Fructus by capillary electrochromatography-mass spectrometry with methyl-vinylimidazole functionalized organic polymer monolilth as stationary phases. J Chromatogr. 2019;1602:474–80.
Acknowledgements
The authors are grateful to the staff of researchers at the Department of Natural Medicinal Chemistry, School of Pharmacy, Naval Medical University. The authors acknowledge the support of the National Natural Science Foundation of China, the Key Research and Development Program of China, the National Major Project of China, Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products, Shanghai Municipal Health Commission Project, Science and Technology Commission of Shanghai Municipality and Sailing Program of Naval Medical University.
Funding
The study was funded by the National Natural Science Foundation of China (82004215, 82173704, 31870327, 82003624, 82004003), the Key Research and Development Program of China (2019YFC1711006, 2017YFC1702002), the National Major Project of China (2018ZX09731016-005), Shanghai Engineering Research Center for the Preparation of Bioactive Natural Products (16DZ2280200), Shanghai Municipal Health Commission Project (20204Y0326), Science and Technology Commission of Shanghai Municipality (20YF1459000, 20YF1458700) and Sailing Program of Naval Medical University.
Author information
Authors and Affiliations
Contributions
The manuscript was prepared by S-JX, X-KX. S-JX, WC, and X-PZ completed the writing of this review. The research work was supported by the projects of X-PZ and Y-HS. All the authors reviewed the final version of the manuscript and approve it for publication. To the best of our knowledge and belief, this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. All authors have seen the manuscript and approved to submit to your journal. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, SJ., Xu, XK., Chen, W. et al. Traditional Chinese medicine Euodiae Fructus: botany, traditional use, phytochemistry, pharmacology, toxicity and quality control. Nat. Prod. Bioprospect. 13, 6 (2023). https://doi.org/10.1007/s13659-023-00369-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13659-023-00369-0