Abstract
Tinospora crispa (L.) Hook. f. & Thomson (Menispermaceae) is a plant indigenous to Africa and South-East Asia. It is widely used in ethnomedicine to alleviate various diseases including hypertension, diabetes, rheumatism, jaundice, inflammation, fever, fractures, scabies, and urinary disorders. A total of 167 phytoconstituents, belonging to 12 different chemical categories, including alkaloids, flavonoids, terpenoids, and phenolic compounds have thus far been isolated from various parts of T. crispa. Numerous in vitro and in vivo investigations have already established the antidiabetic, anticancer, antiparasitic, antimicrobial, immunomodulatory, hepatoprotective, analgesic, antipyretic, antihyperuricemic, and pesticidal activity of this plant, as well as its effects on the cardiac and the central nervous system. Most pharmacological investigations to date have been carried out on plant extracts and fractions. The exact identity of the phytoconstituents responsible for the observed biological effects and their mode of action at the molecular level are yet to be ascertained. Toxicological studies have demonstrated that T. crispa is relatively safe, although dose-dependent hepatotoxicity is a concern at high doses. This review presents a comprehensive update and analysis on studies related to the ethnomedicinal uses, phytochemistry, pharmacological activity and toxicological profile of T. crispa. It provides some critical insights into the current scientific knowledge on this plant and its future potential in pharmaceutical research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinospora crispa (L.) Hook. f. & Thomson is a deciduous climbing plant belonging to the Menispermaceae family. It is native to the tropical rainforests and mixed deciduous forests of Africa and South-East Asia (Pathak et al. 1995). The plant is used ethnomedicinally in several countries, including Bangladesh, Malaysia, China, Philippines, Brunei, Vietnam, Laos, Thailand, Cambodia, Indonesia, Martinique, and Nepal (Quisumbing 1951; Forman 1981; Noor et al. 1989; Longuefosse and Nossin 1996; Ahmad and Ismail 2003; Grenand et al. 2004; Dweck and Cavin 2006; Hout et al. 2006; Li et al. 2006; Roosita et al. 2008; Islam et al. 2011; Rahmatullah et al. 2011; Koay and Koay 2013; Haque et al. 2017; Dapar 2020; Dapar et al. 2020; Paudel et al. 2020). Its leaves, stems, seeds, rhizomes and roots are used in the formulation of various preparations that are employed to treat a range of conditions such as hypertension, diabetes, rheumatism, jaundice, inflammation, fever, malaria, loss of appetite, fractures, scabies, and urinary disorders (Gimlette and Burkill 1930; Quisumbing 1951; Kongsaktrakoon et al. 1984; Noor et al. 1989; Longuefosse and Nossin 1996; Ahmad and Ismail 2003; Hout et al. 2006; Li et al. 2006; Roosita et al. 2008; Rahmatullah et al. 2009; Srithi et al. 2009; Haque et al. 2011; Islam et al. 2011; Koay and Koay 2013; Kadir et al. 2014). The use of T. crispa in several of these conditions has already been validated scientifically in in vitro and in vivo studies which have demonstrated the biological (e.g. cardiovascular, hypoglycemic, cytotoxic, immunomodulatory, anti-inflammatory, antimalarial) activity of extracts, fractions, and some phytoconstituents (Noor et al. 1989; Anulukanapakorn et al. 1999; Amom et al. 2011; Ibahim et al. 2011; Praman et al. 2011, 2013; Hipol et al. 2012; Kamarazaman et al. 2012; Lam et al. 2012; Lokman et al. 2013; Abood et al. 2014). The phytoconstituents in T. crispa are diverse and Clerodane-type furanoditerpenoids are characteristic for the species (Bisset and Nwaiwu 1983; Pachaly et al. 1992; Umi Kalsom and Noor 1995; Cavin et al. 1998; Kongkathip et al. 2002; Choudhary et al. 2010a, b; Chung 2011; Lam et al. 2012; Praman et al. 2012; Yusoff et al. 2014; Ahmad et al. 2016b). Many studies have focused on the bioactivity of T. crispa extracts. Relatively few studies have been carried out on T. crispa phytoconstituents. Toxicity and biosafety studies on T. crispa phytoconstituents are also scarce. Given the potential of T. crispa as a possible source of new drug leads for various pathological conditions, further pharmacodynamic and pharmacokinetic investigations of its phytoconstituents are warranted.
This study aims to provide a detailed account of the taxonomy, phytochemistry, pharmacology, and toxicology relevant to T. crispa, so that it may serve as a valuable resource providing future direction for researchers. Electronic versions of tertiary literature sources (e.g. Google Scholar, PubMed, ScienceDirect, Scopus, Wiley Online Library, SpringerLink, Semantic Scholar, Web of Science and MEDLINE) were used to retrieve data on the ethnopharmacology, phytochemistry, pharmacology, and toxicology of T. crispa published within 1930–2021.
Vernacular names
The following vernacular names for T. crispa have been reported (Quisumbing 1951; Forman 1981; Noor et al. 1989; Longuefosse and Nossin 1996; Ahmad and Ismail 2003; Grenand et al. 2004; Dweck and Cavin 2006; Hout et al. 2006; Li et al. 2006; Roosita et al. 2008; Islam et al. 2011; Rahmatullah et al. 2011; Koay and Koay 2013; Haque et al. 2017; Dapar 2020; Dapar et al. 2020):
-
Bangladesh: Guloncho-ban, Golonchi, Khorosh, Guntai
-
India: Dier, Faridbuti, Dagadi, Chipuru-tige, Kattle-ti, Giloya
-
Malaysia: Brotowali, Akar Patawali, Patawali, Akar Seruntum, Seruntum, Sapai, Daun akar walli
-
China: Da ye ruan jin teng, Bo ye qing, Niu dan, Ye qing niu dan, Fa leng teng
-
Philippines: Makabuhay, Panyawan, Meliburigan, Manunggal
-
Thailand: Boraphet, Ho-Boraphet, Khruea khao, Pae jae, Wan kab hoi yai, Chung ching, Kuakhohoo, Ching cha li
-
Indonesia: Bratawali, Brotowali, Antawali, Andawali, Putrawali, Daun gade
-
Cambodia: Banndol Pech
-
Vietnam: Day coc
-
Laos: Hmab Iab, Kheuah khao, Ho
-
Brunei: Ratnawali, Akar nawali, Nawali
-
Republic of Guinea (French Guinea): Liane-quinine
-
Guyana: Liane amère
-
Martinique Island: Lyann span, Zeb kayenn
-
Indochina: Day than thong, Bandaul pich, Day ki nin, Thuoc sot ret
-
Java: Brotowali, Andawali, Putrowali, Akar pahat
Taxonomy
Tinospora crispa is one of the 34 species that belong to the genus Tinospora. All species of this genus are found in tropical and subtropical regions of Asia, Africa and Australia. Most species produce bioactive constituents (especially diterpenoids and alkaloids) and are used widely in ethnomedicine (Chi et al. 2016). Tinospora crispa is also known as Chasmanthera crispa Baill., Cocculus crispus DC., Cocculus verrucosus Wall., Menispermum crispum L., Menispermum rimosum Blanco, Menispermum tuberculatum Lam., Menispermum verrucosum Roxb., Menispermum verrucosum Roxb. ex Fleming, Tinospora crispa Diels, Tinospora gibbericaulis Hand.-Mazz., Tinospora mastersii Diels, Tinospora rumphii Boerl., Tinospora thorelii Gagnep. and Tinospora tuberculata Beumée ex K. Heyne. (The Plant List 2013; Global Biodiversity Information Facility 2021; World Flora Online 2021). This species has a generally fleshy, with older stems being fleshier than younger ones. Younger stems present a thin membranous and glabrous epidermis is characteristic of younger stems, while tubercles are observed on older ones. The stem contains a bitter, milky sap. Tinospora crispa has long, filamentous, aerial roots. The leaves are cordiform in shape and are usually 6–12 cm long and 7–12 cm wide. They are marginally fleshy with chartaceous leaf-blades. The dried leaves are quite delicate. Domatia are not usually observed, but a flat pocket appears intermittently in the axis of the basal nerves on the ventral surface. The leaf petioles are 5–15 cm long and glabrous. The flowers are fascicled and greenish-yellow or yellow. The male inflorescences are taller and thinner compared to the female counterparts, 5–10 cm versus 2–6 cm respectively. Both male and female flowers share morphological similarities in terms of sepals and petals with six green sepals in two verticils. The inner three sepals are obovate while the rest are ovate. Both male and female flowers have 3–6 yellow petals. The fruits are vermillion or scarlet, with a pale white endocarp. They are ellipsoidal, 7–8 mm long, and feature a distinctive dorsal ridge with a small ventral aperture and a deeply seed-cavity intrusive condyle. The seeds are curved, bean-shaped, and white. The root is succulent (Forman 1981; Patel et al. 2013; Haque et al. 2017). Tinospora crispa and its various parts are illustrated for identification in Fig. 1.
The complete taxonomic classification of T. crispa is provided below (Global Biodiversity Information Facility 2021):
Kingdom: Plantae
Division: Magnoliophyta
Class: Magnoliopsida
Order: Ranunculales
Family: Menispermaceae
Genus: Tinospora
Species: Tinospora crispa
Ethnomedicinal uses
Tinospora crispa is used in ethnomedicine predominantly in South-East Asia. Some of its uses are common across multiple ethnicities (e.g. diabetes) while others are reserved to certain regions only. In Bangladesh, various preparations are used for fever, body pain, rheumatism, skin diseases, paralysis, abdominal pain, intestinal disorders and leprosy (Rahmatullah et al. 2009, 2011; Islam et al. 2011; Kadir et al. 2014). In Malaysia, infusions of the stems and of the whole plant are used as a postpartum remedy and to treat type-2 diabetes mellitus, tuberculosis, cholera, malaria, hypertension, lumbago, muscle pain and intestinal parasites (Forman 1981; Noor et al. 1989; Ahmad and Ismail 2003; Mohamad et al. 2011; Dapar 2020). In the Philippines, the stems and leaves are employed for fever, indigestion, flatulence, intestinal disorders, diarrhea, vomiting, ulcer, body ache, rheumatism, toothache, ocular soreness, scabies, lacerations and boils (Quisumbing 1951; Dapar 2020; Dapar et al. 2020). In Thailand, the leaves, stems, roots and seeds are prepared into powders, infusions and decoctions to treat wounds, itches, cholera, diabetes, fever, rheumatism, intestinal parasites, snake-bites, syphilitic sores, sore eyes, and alcohol or drug-induced poisoning (Dweck and Cavin 2006). People in the Yao community in China use hot infusions of the stems as bath water to treat fractures, contusions, furuncles, carbuncles and viper-bites (Li et al. 2006). In China again, the plant is used for fever, septicemia, scabies and ulcers (Koay and Koay 2013). In the South Kerala region of India, locals use the plant as an antidiabetic (Thomas et al. 2016). The use of T. crispa as an antimalarial agent is widespread in Malaysia, the Philippines, Indonesia, Vietnam, Southern Laos and the Republic of Guinea (Forman 1981; Ahmad and Ismail 2003; Bertani et al. 2005; Elkington et al. 2014; Ramadani et al. 2018; Dapar 2020; Dapar et al. 2020). Indonesians also employ the plant for hyperglycemia, inflammation, fever and rheumatism. The last two uses are also reported in Cambodia (Hout et al. 2006; Adnan et al. 2016; Ramadani et al. 2018). Apart from the aforementioned uses, T. crispa stems are also employed to treat jaundice and fever in Vietnam (Forman 1981). The Kadayan Malay community in the Sengkurong mukim region of Brunei use the stems for hypertension and abdominal ache (Dapar 2020). In Guyana, a bitter beverage produced from T. crispa macerated stems, combined with Quassia amara bark, is taken for albuminuria and diabetes (Grenand et al. 2004; Thomas et al. 2016). In Martinique, the leaves and stems are used in decoctions and tinctures to treat diabetes (Longuefosse and Nossin 1996). The ethnomedicinal uses of T. crispa are listed in Table 1.
Phytoconstituents
Extensive phytochemical investigations on the aerial parts of T. crispa, both as a whole and as individual parts (stems, leaves, and vines), led to the identification of 167 phytoconstituents belonging to diverse chemical classes. Clerodane-type furanoditerpenoids are the most abundant phytoconstituents in this species. A considerable number of alkaloids, flavonoids, and steroidal compounds have also been reported. Other classes of secondary metabolites, present to a lesser extent, include triterpenes, phenolic compounds, nucleosides, aromatic constituents, volatile terpenoids and long-chain fatty acid derivatives. All compounds reported from T. crispa to date are listed in Table 2 and their structures are illustrated in Figs. 2, 3, 4, 5, 6, 7 and 8.
Clerodane-type furanoditerpenoids
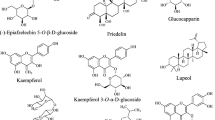
Furanoditerpenoids are a class of compounds that features at least one furan ring as part of their core skeleton. The outstanding significance of this class lies in its pharmacological potential, which is primarily be attributed to the biologically-interactive furan ring. The clerodane-type furanoditerpenoids are based on a decahydronaphthalene skeleton with a furan ring attached to it via a two-carbon bridge. Based on the number of lactone rings present, these compounds have been further categorized into three major subgroups viz. A, B and C, featuring zero, one and two lactone rings, respectively (Bao et al. 2017). A total of 38 clerodane-type furanoditerpenoids have been identified in T. crispa (1–38) (Fig. 2). Among them, only two (1, 2) were of type A (Hossen et al. 2016; Noman et al. 2018), while 28 compounds (3–30) featured one lactone ring in their structures and belonged to type B (Ruan et al. 2012; Lokman et al. 2013; Abood et al. 2014; Langrand et al. 2014; Hamid et al. 2015; Mantaj et al. 2015; Adnan et al. 2016; Gao et al. 2016; Xu et al. 2017; Rahman et al. 2020). Five of the furanoditerpenoids (31–35) were of type C with two lactone rings (Ahmed et al. 2006; Choudhary et al. 2010b; Lam et al. 2012; Praman et al. 2012). Compounds from both type B and C exhibited further structural diversification in terms of the position of the lactone ring(s), extent of hydroxylation and presence of monosaccharides at different positions. A total of 21 furanoditerpenoids (4–6, 8, 13–15, 17–27, 30, 32, 35) were characterized as glycosides. While most of the glycosidic constituents contained a single β-D-glucose moiety in their structure, two of them (6, 21) featured two saccharide moieties (Gao et al. 2016), and one of them (21) included an α-D-xylose moiety (Choudhary et al. 2010b). The furanoditerpenoids isolated from T. crispa also included three re-arranged derivatives, including compound (36) with a saturated furan ring and extensive hydroxylation on all side chains (Choudhary et al. 2010b) and compounds (37, 38) with a shortened first ring in the basic skeleton along with a fusion of a five-membered lactone ring (Parveen et al. 2019).
Alkaloids
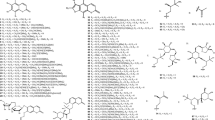
Alkaloids reported from T. crispa mostly originated from the structural extension of the basic isoquinoline skeleton. Thirteen aporphine alkaloids (39–51) have been isolated from different parts of T. crispa (Fig. 3) (Pachaly et al. 1992; Bakhari et al. 2005, 2013; Sunthikawinsakul 2005; Imphanban et al. 2009; Choudhary et al. 2010a; Hamid 2013; Yusoff et al. 2014; Hamid et al. 2015; Ahmad et al. 2018; Parveen et al. 2019). Five protoberberine-type alkaloids (52–56) have also been reported (Yusoff et al. 2014; Hamid et al. 2015, 2021; Syarifah et al. 2017; Rahman et al. 2020). Both aporphine and protoberberine alkaloids feature a tetracyclic skeleton based on the benzylisoquinoline moiety, originating from the oxidative fusion of phenol and isoquinoline rings, with partial or complete aromatization. However, these alkaloids differ in the orientation of their fusion. The bridging in aporphine-based structures takes place along the middle of the isoquinoline skeleton without incorporating the nitrogen atom into the extended ring (Ge and Wang 2018). On the other hand, in protoberberine alkaloids, the incoming phenol fuses along the N-methyl group and incorporates nitrogen into the new ring (Da-Cunha et al. 2005). Two similarly-fused isoquinoline alkaloids (57, 58) and one simple isoquinoline alkaloid (59) have also been isolated from the stems of T. crispa (Praman et al. 2011, 2012, 2013; Parveen et al. 2019). Eight other alkaloids (60–67), including four hydroxycinnamoyl tyramine derivatives (60–63) along with tyramine itself (67), have also been reported (Cavin et al. 1998; Choudhary et al. 2010a; Praman et al. 2012, 2013; Hamid 2013; Langrand et al. 2014; Yusoff et al. 2014; Noman et al. 2018; Parveen et al. 2019; Rakib et al. 2020c).
Flavonoids
Different parts of T. crispa have been characterized with the presence of 24 flavones (68–91) and one flavanol (92) (Fig. 4) (Umi Kalsom and Noor 1995; Amom et al. 2009; Abood et al. 2014; Chang et al. 2015). Among the flavones, 16 compounds (69, 70, 72, 75, 76, 78–80, 84–91) were identified as glucosides while (83) was identified as a rutinoside. Eight of these flavones (84–91) were further conjugated with hydroxycinnamoyl moieties.
Steroidal compounds
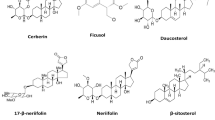
Thirty-two steroidal constituents (93–124) have been isolated from T. crispa (Fig. 5) (Ahmed et al. 2006; Lin 2009; Hamid et al. 2015; Ismail and Choudhary 2016; Marlina et al. 2017; Noman et al. 2018; Musa et al. 2019; Rahman et al. 2020; Rakib et al. 2020c). All compounds displayed the characteristic steroidal backbone and showed diversity in their unsaturation, oxidation and cyclization in different parts of this backbone.
Triterpenes
Four lupane-based (125–128) and one oleanane-based (129) pentacyclic triterpenes have been isolated from the aerial parts and stems of T. crispa (Fig. 6) (Noman et al. 2018; Rakib et al. 2020c).
Phenolic compounds
Ten phenolic constituents (130–139) have been identified in T. crispa (Fig. 7), including one (134) identified as a glucoside (Cavin et al. 1998; Praman et al. 2012, 2013; Hamid et al. 2015; Ismail and Choudhary 2016; Ahmad et al. 2018; Rakib et al. 2020c). One of the phenolics (136) was the ester product of a hydroxycinnamoyl derivative (Bakhari et al. 2013), whereas three of them (137–139) were polyphenolic lignans (Chung 2011; Parveen et al. 2019; Rakib et al. 2020c). Although hydroxycinnamoyl conjugations are common within the alkaloidal and flavonoid pool of T. crispa, the presence of hydroxycinnamic acids has never been reported and warrants future attention.
Other constituents
Less prominent secondary metabolites, including three nucleosides (140–142) (Choudhary et al. 2010a; Praman et al. 2012, 2013), three aromatic compounds (143–145) (Nor Aziyah et al. 2014; Rakib et al. 2020c), three volatile monoterpenes (146–148), six volatile sesquiterpenes (149–154), three volatile diterpenes (155–157) (Rakib et al. 2020c) and ten long chain alcohols and fatty acid derivatives (158–167) (Fig. 8) (Bakhari et al. 2013; Abood et al. 2014; Nor Aziyah et al. 2014; Ahmad et al. 2018; Lee et al. 2020; Rakib et al. 2020c) have also been reported in T. crispa.
Pharmacological activity
Tinospora crispa has been extensively studied in vitro, in vivo and in silico to scientifically validate its use in ethnomedicine. Most studies have focussed on the antidiabetic and cardiac activity, including the mechanisms of action at the molecular level, of T. crispa extracts and phytoconstituents. Significant evidence to support the anticancer, antiparasitic, antimicrobial, antioxidant and immunomodulatory potential of this plant has also been obtained. Preliminary evidence of its hepatoprotective, analgesic, antipyretic, anticholinesterase, central nervous system, antihyperuricemic and pesticidal activity has been reported. Such effects, however, remain comparatively unexplored and require further exhaustive investigations. A concise summary of the pharmacological activities of the plant is presented in Table 3.
Antidiabetic activity
The aqueous extract of T. crispa has been evaluated for its activity on diabetic male Wistar albino rats, on rat and human islets of Langerhans, and on HIT-T15 cells. A week after administration of the extract (4 mg/mL), lowered blood glucose levels (10.4 ± 1.0 mmol/L) were observed compared to the control group (17.4 ± 1.7 mmol/L). Additionally, insulinotropic activity was also evident with comparatively greater insulin levels in the test group than in the control (12.8 ± 1.1 µU/mL and 8.0 ± 0.7 µU/mL, respectively). In the rat islets, the extract (0.01–1 mg/mL) led to a dose-dependent enhancement of basal insulin secretion up to a maximum of fivefold. The extract also potentiated (1.5-fold) the glucose-mediated induction of basal insulin secretion. Similar results were obtained in the human islet system as the extract (1 mg/mL) induced insulin release similar to that of a high dose of glucose (20 mmol/L). The extract also further potentiated glucose-mediated insulin release. In HIT-T15 cells, the extract (0.01–4.00 mg/mL) boosted the basal insulin release sevenfold, along with a 1.5-fold enhancement of glucose-induced insulin secretion. This was the first evidence of the plant acting as an oral hypoglycemic and insulinotropic agent (Noor et al. 1989). The in vivo antidiabetic effect was further confirmed by multiple subsequent studies in other animal models (Arcueno et al. 2015; Hassani et al. 2016; Arundina et al. 2017; Firdausa et al. 2020).
Antidiabetic mechanisms other than an insulinotropic effect were evaluated in another study using the aqueous extract. It was found that the extract (1 mg/mL) played no significant role in intestinal or adipocyte glucose uptake. In HIT-T15 cells, the insulinotropic activity was inhibited by adrenaline (5 mM), somatostatin (1 mg/mL), verapamil (50 mM) and nifedipine (50 mM). Cyclic AMP concentration (cAMP) and 86Rb efflux were further measured and it was hypothesized that the insulinotropic effect of T. crispa was the result of calcium ion transport across the membrane of pancreatic β cells, and possibly closure of ATP-mediated potassium channels (Noor and Ashcroft 1998a). This was confirmed by a later study which revealed that the extract increased HIT-T15 cell sensitivity to extracellular calcium ions and resulted in increased intracellular accumulation of these ions caused by increased uptake and suppressed efflux. The physiological nature of the underlying mechanism suggested the presence of individual compounds in T. crispa which may serve as potential insulin secretagogues (Noor and Ashcroft 1998b). It was found in a later study that the administration of T. crispa powder in capsule form (1 g thrice daily) could not induce hypoglycemia in type-2 diabetic patients non-responsive to oral hypoglycemic drugs. It was postulated that these results reaffirm the insulinotropic nature of the antidiabetic activity of T. crispa (Sangsuwan et al. 2004).
An increase in glucose uptake and Glucose Transporter 1 (GLUT1) expression was reported when testing an aqueous extract of T. crispa on L6 myotubes. 2-Deoxy-[3H]-glucose (2-DG) uptake was measured following incubation up to 24 h with 100–1000 µg/mL of extract. At a dose of 400 µg/mL, 2-DG uptake increased by 151.5 ± 1.1, 166.7 ± 15.0, 179.6 ± 6.8 and 246.1 ± 0.1% following 4, 6, 8, and 24 h of incubation, respectively. The same dose also displayed a steady increase in mRNA levels of GLUT1 by 1.29 ± 0.06, 1.70 ± 0.22, and 2.04 ± 0.23 fold over a course of 4, 8 and 24 h, respectively. These were accompanied by boosted levels of extracellular signal-regulated kinases (ERK) 1/2, suggesting that this pathway is activated causing the increased GLUT1 expression. Increased AMPK levels were also observed in L6 myotubes (Noipha et al. 2011).
This ability to reverse the insulin resistance was also demonstrated in a study using Wistar rats fed a high fat diet. The aqueous extract of T. crispa at a dose of 1 g/mL resulted in a significant decrease in glucose (8.50 ± 0.30 mmol/L compared to 13.75 ± 0.25 mmol/L in the untreated group). Serum glucose, cholesterol and triglycerides levels decreased with the treatment, along with a fall in serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein, creatine and urea (Abu et al. 2015). A subsequent investigation established the capacity to abolish insulin resistance in insulin resistant IR-HEP-G2 cells using rosiglitazone maleate as a standard. It was observed that T. crispa methanol extract and the standard (both at doses of 100 µg/mL) led to a 2.5- and 1.5-fold increase in 2-DG uptake, respectively. It was found that the insulin receptor was upregulated, ultimately recruiting the PI3K/Akt pathways. Subsequent increase of GLUT4 expression was also observed resulting in a boosted 2-DG uptake. Additionally, T. crispa methanol extract triggered apoptosis in the IR-HEPG2 cells stimulated with insulin (Abu et al. 2017).
Another study revealed that an ethanol extract of T. crispa stems displayed α-glucosidase inhibitory activity, with a 78.34% inhibition at a concentration of 450 ppm compared to 81.01% when using the standard acarbose. The IC50 values for the extract and acarbose were 237 and 116 ppm, respectively (Tambunan et al. 2013). In a recent study, the ethanol and aqueous extracts of the stem have also been observed to inhibit the enzyme α-amylase in vitro with an IC50 of 10.348 ± 0.313 and 11.660 ± 0.310 mg/mL, respectively (Hartini et al. 2022). Interestingly, endophytic fungi isolated from T. crispa have been found to exhibit α amylase and α glucosidase inhibitory activity (Lestari et al. 2015; Pramitasari et al. 2017). The aqueous extract of the plant at a dose of 500 mg/kg has been reported to increase superoxide dismutase (SOD) and glutathione peroxidase (GPx) levels in streptozotocin-treated diabetic Sprague Dawley rats, thereby boosting antioxidant activity (Firdausa et al. 2018). The ethanol extract of T. crispa has showed an ability to increase lymphocytes, fibroblasts and enhanced healing activity in diabetic male Wistar rats with oral mucosal ulcers (Arundina et al. 2017; Roestamadji et al. 2017).
As there have been numerous studies on the antidiabetic potential of T. crispa extracts, the same can also be said for its phytoconstituents. Particularly, a number of clerodane type furanoditerpenoids and their glycosides have been reported to have significant hypoglycemic activity. Borapetosides A (32) and C (14) at a dose of 5 mg/kg significantly decreased blood glucose levels in normal and type-1 diabetic mice compared to the standard metformin (200 mg/kg). Borapetoside C (14) at a dose of 3 mg/kg also displayed activity against type-2 diabetes, evident from its insulin secretagogue activity. This was comparable to that of glibenclamide (5 mg/kg) and was exerted through an increased peripheral tissue glucose uptake and suppressed hepatic gluconeogenesis (Lam et al. 2012). Borapetoside C (14) (0.1 mg/kg) is also capable of increasing glycogen synthesis in skeletal muscles when given in combination with insulin in normal, type-1 and type-2 diabetic mice. It increased the serine phosphorylation of Akt, phosphorylation of the insulin receptor, and GLUT2 levels by 3.0, 1.4 and 1.3-fold when administered with insulin (Ruan et al. 2012). This demonstrated the versatility of this compound in terms of antidiabetic activity. Another compound with established insulin secretagogue activity is borapetol B (16), which was assessed on normoglycemic Wistar and spontaneously type-2 diabetic Goto-Kakizaki (GK) rats at a dose of 0.1 mg/kg. In the Oral Glucose Tolerance Test (OGTT), a significant decrease in glucose levels was observed in both animal models. This compound also enhanced insulin secretion in isolated pancreatic islets (Lokman et al. 2013). In a later study, borapetoside C (14) (IC50 value of 0.527 ± 0.008 mg/mL) and 4-hydroxybenzaldehyde (130) (IC50 value of 0.557 ± 0.004 mg/mL) were found to be the most potent α-glucosidase inhibitors. The alkaloids liriodenine (49), lysicamine (50) and N-formylanonaine (39) also strongly inhibited this enzyme, with IC50 values ranging from 0.5 to 0.8 mg/mL. Borapetoside C (14) (IC50 value of 0.775 ± 0.005 mg/mL) displayed the most prominent activity against α-amylase alongside notable activity observed for N-trans-feruloyltyramine (62), dihydrodiscretamine (53) and magnoflorine (51) (IC50 value of 0.8 to 0.9 mg/mL). It was suggested that the ring hybridization of these alkaloids allowed them to interact with the aforementioned enzymes, but that the presence of different functional groups weakened their activity (Hamid et al. 2015). Another clerodane furanoditerpenoid, borapetoside E (4) (40 and 80 mg/kg), caused stark improvements in hyperglycemia, insulin resistance, hyperlipidemia, hepatic steatosis and oxygen consumption in high fat diet-fed mice compared to the standard metformin (400 mg/kg). This compound also reduced the expression of sterol regulatory element binding proteins (SREBPs), which are important transcription factors in lipid synthesis and have emerged as novel targets for the treatment of type-2 diabetes (Xu et al. 2017). Tinosporol A (8) induced dose-dependent hypoglycemic activity in type-1 diabetic ICR (Institute of Cancer Research) mice and type-2 diabetic db/db mice, although it was found that the type-1 model was more sensitive to this compound than the type-2 one (Gao et al. 2016). In a study investigating the α-glucosidase inhibitory activity of acylated glucosylflavones (tested at a concentration of 10 μg/mL), isovitexin-2"-(E)-p-coumarate (89) displayed maximum inhibition (IC50 value of 4.3 ± 1.4 µM) compared to the standard acarbose (IC50 value of 0.033 ± 0.006 µM) (Chang et al. 2015).
Some clinical studies have been conducted to evaluate the effect of T. crispa on healthy volunteers, on patients with diabetes and patients with high risks of developing diabetes. For example, a clinical study conducted in Thailand, showed that pre-prandial administration of T. crispa (250 mg capsule twice daily for two months) in patients with metabolic syndrome resulted in a steady decrease in fasting blood sugar and triglyceride levels (Sriyapai et al. 2009). Another study reported a remarkable reduction in plasma glucose levels following oral administration of T. crispa powder (6 g) to healthy subjects (Rattanajarasroj et al. 2004). In both studies, however, T crispa caused a noticeable increase in ALT and AST serum levels, implying possible hepatotoxicity (Sriyapai et al. 2009; Rattanajarasroj et al. 2004). Other clinical studies also indicated the increased risk of hepatotoxicity associated with T. crispa and/or concluded that there was no evidence to support to use of this plant for the treatment of diabetes (Sangsuwan et al. 2004; Klangjareonchai and Roongpisuthipong 2012). In depth details and discussions on the clinical studies involving T. crispa can be found under the ‘Clinical Trials’ section.
In summary, the ethnomedicinal use of T. crispa in the treatment of diabetes has been underpinned by many scientific studies. The antihyperglycemic activity of this plant occurs mainly as a result of enhanced insulin secretion and inhibition of α- glucosidase and α-amylase. The pathways involved in the antidiabetic mode of action of T. crispa extracts and its phytoconstituents are similar (Fig. 9). Selected clerodane-type furanoditerpenoids present in T. crispa have been reported to possess insulin secretagogue properties. Further structure activity relationships (SAR) studies on this class of phytochemicals should be undertaken to determine the pharmacophore(s) responsible for the modulation of intracellular calcium ion levels. Other phytochemicals such as flavonoids, for example, have strong inhibitory activity against α-glucosidase and α-amylase and several SAR studies have been investigated these effects (Tadera et al. 2006; Proença et al. 2017, 2019; Zhu et al. 2020). Further research work on the antidiabetic potential of the various flavonoids present in T. crispa should be conducted.
Schematic diagram of the antidiabetic mode of action of Tinospora crispa. AE: Aqueous Extract, ME Methanol Extract, EE Ethanol Extract, ATP Adenosine triphosphate, GLUT Glucose transporter, ERK Extracellular signal-regulated kinase, AMPK AMP-activated protein kinase, IRS Insulin receptor substrate, P Phosphate, PI3K Phosphoinositide-3-kinase, PIP2 Phosphatidylinositol-4,5-bisphosphate, PIP3 Phosphatidylinositol-3,4,5-trisphosphate, PDK Phosphoinositide-dependent kinase, AKT Protein kinase B, SREBP Sterol regulatory element-binding protein
Cardiac activity and cardiovascular effects
Multiple extracts and fractions, at doses of 0.25–1 mg/mL, were evaluated for their cardioactive potential in isolated atria and aorta of male Sprague Dawley rats. Extraction was performed with petroleum ether, chloroform, methanol and water; and four fractions derived from the chloroform extract obtained following flash chromatography using chloroform/n-hexane and chloroform/methanol combinations. The fractions derived from the chloroform extract were found to be the most active, inhibiting the isoprenaline-induced positive chronotropic response in the left atrium by 80% at a dose of 1 mg/mL. From the dose–response curve obtained, it was concluded that all the extracts and fractions mentioned above functioned as non-competitive β-adrenoceptor antagonists. In the right atrium however, the extracts at high doses effectuated a complete inhibition of the isoprenaline-induced positive chronotropic response by suppressing the sinoatrial node. This could be rectified by high doses of isoprenaline. In the aorta, the fractions derived from the chloroform extract showed 85–99% inhibition of the noradrenaline-induced positive inotropic response, and the inhibition was commensurate with the increasing polarity of the fractions. The dose–response curve obtained suggested that these fractions acted as non-competitive α adrenoceptor antagonists (Bakhari and Isa 2010). The n-butanol fraction of the aqueous extract of T. crispa (1–100 mg/kg) was also tested in normal and reserpine-induced female Wistar rats. Whilst this fraction produced significant hypotensive and positive chronotropic activity in normal rats, dual effects were obtained following reserpine induction with a transient decrease followed by an increase in hypotensive activity. Similar dual effects were obtained for the positive chronotropic action. The mechanism of action was unravelled using post-treatment with propranolol (0.6 mg/kg), phentolamine (2 mg/kg), atenolol (2 mg/kg), the β2 antagonist ICI-118,551 (0.01 mg/kg), atropine (0.6 mg/kg) and hexamethonium chloride (10 mg/kg), either individually or in various combinations. This revealed that the action of the active constituents was mediated via β2-adrenergic receptors producing hypotension, as well as β1- and β2-adrenergic receptors effectuating a positive chronotropic response. Additionally, some constituents caused hypertension and increased heart rate via modulation of α-adrenergic receptors. The authors further concluded that compounds acting via non-adrenergic and non-cholinergic pathways were also present to cause a reduction in mean arterial pressure and heart rate (Praman et al. 2011).
Subsequent bioassay-guided fractionation resulted in the isolation of five cardio-active compounds from the n-butanol fraction, namely adenosine (140), uridine (142), salsolinol (65), higenamine (59) and tyramine (67). These compounds were assessed for their mechanism of action using the same model and chemicals including DMPX (an A2a adenosine receptor antagonist), suramin, phentolamine, ICI-118,551, atropine, prazosin and atenolol for post-treatment. Adenosine (140) (0.003–0.3 mg/kg) displayed hypotensive and negative chronotropic activity which was suppressed by DMPX. Uridine (142) (0.1–100 mg/kg) had a hypertensive and negative chronotropic effect in normal rats, which was inhibited by suramin. At high doses, it produced initial hypertension followed by hypotension. Salsolinol (65) (0.1–10 mg/kg) produced a hypotensive response with a decreased heart rate, which was suppressed significantly only by phentolamine. In reserpinized rats, however, hypertensive activity was observed for this compound, impeded by phentolamine, but not atenolol. Higenamine (59) (0.001–0.3 mg/kg) triggered hypotension in normal rats, which was obstructed by ICI-118,551 or atenolol. Similar results were observed in reserpinized rats, with prazosin increasing the hypotensive effect. Positive chronotropic effects were obtained in both animal models. Hypertension and increased heart rate were obtained in normal rats, but not in reserpinized ones, following treatment with tyramine (67) (0.003–1 mg/kg). The hypertensive effect dropped significantly by applying phentolamine, while the positive chronotropic effect was significantly boosted with atenolol. Salsolinol (65), higenamine (59) and tyramine (67) were reported to exert their effects through the adrenergic pathway, while adenosine (140) and uridine (142) exerted their action via the purinergic pathway. All constituents acted in a dose-dependent manner (Praman et al. 2012). The compounds were further assessed for their inotropic action on isolated left atria using the same animal model. Adenosine (140) (10−8—3 × 10−4 M) and uridine (142) (10−8—10−2 M) acting via the purinergic pathway produced a negative and slightly positive inotropic effect, respectively. On the other hand, higenamine (59) (10−8–10−5 M), salsolinol (65) (10−7—10−4 M) and tyramine (67) (10−8—3 × 10−5 M) increased the force of contractility in the left atria via the adrenergic pathway. Additionally, salsolinol (65) at higher concentrations (3 × 10−4—3 × 10−3 M) induced a greater release of acetylcholine, leading to the opposite outcome (Praman et al. 2013).
Other compounds from T. crispa have been investigated for their cardio-active potential. This includes cycloeucalenol (118) (5.6 × 10–5 M) and cycloeucalenone (119) (5.6 × 10–5 M). Both molecules had slightly positive inotropic activity in the isolated right atria of male Wistar rats. Conversely, these compounds initially demonstrated minimal negative inotropic activity, followed by significant negative inotropic activity in the left atria, thereby exhibiting mild cardiotonic activity compared to noradrenaline (1 × 10–8 M) (Kongkathip et al. 2002). A synthetic racemic mixture of N-formylnornuciferine (43) produced a negative inotropic and chronotropic response in isolated rat heart (Imphanban et al. 2009). The identified mechanisms through which the T. crispa modulates cardio-activity are presented in Fig. 10. However, it should be noted that the cardiac potential of this plant cannot be attributed to a particular class of compounds with much confidence, other than the purinergic action of its nucleosides. Moreover, when administered to diabetic rats, T. crispa powder produced a significant increase in hemoglobin concentration and red blood cells (RBC) alongside a notable decrease in White Blood Cells (WBC) compared to control (Suchantabud et al. 2008).
Schematic diagram of the cardioprotective and anticancer mode of action of Tinospora crispa. CE Chloroform Extract, BF n-Butanol Fraction, EE Ethanol Extract, AR Adrenergic Receptor, PR Purinergic Receptor, MAP Mean Arterial Pressure, HR Heart Rate, STAT3 Signal Transducer and Activator of Transcription 3, MMP13 Matrix Metalloproteinase 13, TIMP2 Tissue Inhibitor of Metalloproteinases 2
Anticancer activity
The cytotoxic potential of various extracts and fractions of T. crispa has been reported by multiple investigators using the brine shrimp lethality assay method. A petroleum ether fraction of the methanol extract was reported to have strong cytotoxic activity with IC50 of 173 ppm (Mackeen et al. 2000). Another study revealed that the methanol extract of the stem along with its chloroform and petroleum ether fractions at doses of 0.781–400 μg/mL showed comparable cytotoxicity (LC50 of 12.0, 11.5, and 12.6 μg/mL, respectively). Vincristine sulfate was used as a standard with an LC50 of 0.323 μg/mL (Haque et al. 2011). Stronger cytotoxicity (LC50 values of 6.43, 4.58, and 0.80 μg/mL, respectively) was later reported in another study on the same stem extract and fractions tested within the same concentration range. This study also evaluated the aqueous extract which showed a LC50 of 7.46 μg/mL (Islam et al. 2013). The ethanol extract of the leaves had a LC50 of 62.75 μg/mL, which is notably weaker compared to the previously mentioned extracts (Tarukbua et al. 2018). The methanol extract of the stems was found to suppress the proliferation of HL-60, HEP-G2 and Hep3B cancer cells in a dose- and time-dependent manner (Ahmad et al. 2016a). The aqueous extract showed moderate antiproliferative activity against MCF-7, Caov-3, HeLa and HEP-G2 cells (IC50 of 107, 100, 165 and 165 μg/mL, respectively) (Zulkhairi Jr et al. 2008). The aqueous, methanol and chloroform extracts of T. crispa stem revealed antiproliferative and cytotoxic activity against MCF-7, MDA-MB-231, 3T3 and HeLa cells. The extracts produced dose-dependent cytotoxicity, with the methanol extract being the most potent (Ibahim et al. 2011). The ethanol extract (12.5, 25, 50, and 100 μg/mL) showed inhibition of head and neck squamous cell carcinoma (HNSCC) metastasis on HN22 and HSC3 cells. In a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, this extract, at the maximum concentration used, significantly decreased cell viability in the two cell lines to 50% and 60%, respectively compared to the negative control dimethyl sulfoxide (DMSO). Administration of this extract at concentrations of 12.5, 25, and 50 μg/mL also downregulated MMP-13 gene expression in both cell lines. A stronger reduction in secreted MMP-13 levels was observed in HN22 compared to that of HSC3 cells. In the latter cell line, the ethanol extract at 25 and 50 μg/mL increased the expression of the tissue inhibitors of metalloproteinase-2 (TIMP-2). Moreover, pre-treatment with this extract (50 μg/mL) in a scratch wound healing assay using HN-22 cells caused cell migratory activity to drop to 65% compared to the control DMSO (Phienwej et al. 2015).
The chloroform extract of the stems was evaluated for its anti-angiogenic activity in the Chick embryo Chorioallantoic Membrane (CAM) induced by basic Fibroblast Growth Factor (bFGF) assay. Dose-dependent anti-angiogenic activity of 31.87 ± 9.01, 43.12 ± 8.01, 53.44 ± 2.70 and 62.81 ± 4.74% was obtained for concentrations of 15, 60, 240, and 960 μg/mL, respectively (Triastuti 2010). In contrast, no cytotoxic activity was reported for the methanol and aqueous extracts of the stems in a water-soluble tetrazolium (WST) or MTT assay employing HL-60, HEP-G2 and MCF-7 cancer cells (IC50 > 500 μg/mL) (Tungpradit et al. 2010). This apparent difference of activity on different cell lines may depend upon the nature of phytoconstituents present in the extracts. This, in turn, may be linked to differences in geographical areas of plant collection as has been reported previously when samples collected from different regions of the East Jawa province in Indonesia showed significant difference in cytotoxicity. The ethanol extract yielded LC50 values ranging from 30.64 ± 2.18 (strong activity) to 254.15 ± 30.77 μg /mL (weak activity) in an MTT assay carried out on MCF-7 breast cancer cells (Mutiah et al. 2019).
Tinocrisposide (14) (3.125–100 μg/mL) isolated from the dichloromethane fraction of the methanol stem extract was tested using an MTT assay on H1299 and MCF-7 cells. IC50 values of 70.9 and > 100 μg/mL were obtained in these cell lines, respectively. It was suggested that this compound, whilst not a viable cytotoxic agent, could still prove useful as a chemopreventive agent (Adnan et al. 2016). The cis-clerodane furanoditerpenoid crispene E (10) isolated from the n-hexane fraction of the methanol stem extract exerted notable inhibition of Signal Transducer and Activator of Transcription Protein 3 (STAT-3) both in a fluorescent polarization (FP)-based primary protein–protein binding assay and a MTT assay. In the FP assay, this compound exhibited an IC50 of 10.3 μM and 210% inhibition relative to the STAT-3 SH2 domain interacting molecule STA-21. The mentioned domain is pivotal for dimerization, which is in turn implicated in the development of different cancers. The IC50 values for the HeLa (cervical), MIA PaCa2 (pancreatic), NCI H1975 (non-small cell lung), MDA-MB-231 (breast) cancer cell lines in the MTT assay were 10.5, 8.3,11.8 and 5.4 μM, respectively (Mantaj et al. 2015). A subsequent study isolated two related compounds, crispene F (2) and crispene G (11), which yielded IC50 values of 42 and 17 μM, respectively, in the FP assay and 119% to 130% inhibition compared to STA-21, respectively. Both compounds had IC50 values of 10 and 7.8 μM on MDA-MB-231 cells using the MTT assay. Weak activity on A4 (STAT-3 independent) colon cancer cells indicated that the compounds possibly induced STAT-3-specific inhibition. Comparatively, crispene E (10) was identified as the most potent among the three derivatives (Noman et al. 2018).
The in vitro anticancer activity of T. crispa has been demonstrated against several cancer cell lines. Its effects on gene expression and the underlying mechanisms are illustrated in Fig. 10. There have been no studies reported on the anticancer activity of the plant in vivo, which warrants further investigations. Interestingly, pure compounds such as clerodane-type furanoditerpenoids have displayed promising activity, particularly on STAT-3 inhibition. Quantitative SAR (QSAR) studies are now required into the 38 compounds of this class that have been isolated from the plant. This may help to focus on specific chemical moieties that can interact with the binding sites of interest in the STAT-3 protein.
Antiparasitic activity
Although T. crispa has been reported as a traditional medicine against parasites, particularly Plasmodium (Vigneron et al. 2005; Malik 2015), investigations carried out to date have provided conflicting accounts on its antimalarial activity. The methanol stem extract (dose of 0.1–2.5 mg/mL) was evaluated for in vitro antiplasmodial activity against Plasmodium falciparum (FCR-3 strain). The highest dose of this extract showed 100% inhibition after 24 h of incubation. In vivo activity was further studied in adult female mice infected with Plasmodium berghei (chloroquine sensitive ANKA strain). At a dose of 5 mg/kg, the extract led to 0–32.7% parasitemia from days 1 to 5 post-infection, which was lower than the negative control. However, antiplasmodial activity was not considered to be significant (Rahman et al. 1999). Similarly, inconsequential results were obtained in another study testing the same extract against the same strain (Niljan et al. 2014). Tinospora crispa aqueous extract (1 mg/mL) yielded approximately 40% inhibition of P. falciparum and 80% inhibition of Babesia gibsoni in infected erythrocytes. In case of P. falciparum, the extract was considered to be inactive (Murnigsih et al. 2005). Similar inactivity against P. falciparum was also observed for the ethanol, ethyl acetate and n-hexane fractions of T. crispa stems (Ramadani et al. 2018). The methanol extract (0.5–3.0 mg/mL) showed IC50 values between 0.27–0.29 mg/mL against P. falciparum 3D7 strain. Artemisin was used as a standard and showed an IC50 of 10–8 mg/mL. The 2 mg/mL dose was found to significantly lower the parasitic load, with the percentage parasitemia and parasite DNA concentration reduced by 47.12% and 56.83%, respectively. At doses above 2.0 mg/mL, these effects did not correlate with the dose administered. It was postulated that antioxidant activity was responsible for the observed effects (Ihwan et al. 2014). In a different study using the same model, the ethanol extract was found to be more potent (IC50 of 0.344 ± 0.210 µg/mL). In the in vivo study using male Swiss mice infected with P. berghei NK65, the extract (doses of 50–400 mg/kg) had an ED50 of 271.89 ± 4.32, and consequently the plant was deemed to possess moderate activity (Abdillah et al. 2015). In another in vitro assay, the methanol extract displayed an EC50 value of 7.5 µg/mL, indicating strong antimalarial activity (Tran et al. 2003). The ethanol extract when administered at doses of 20, 40 and 80 mg/kg to ICR mice infected with P. yoelii 17XL demonstrated dose-dependent activity, with 53.68% parasitemia on day 18 at the highest dose (Rungruang and Boonmars 2009). In another assay using ICR mice infected with P. berghei (ANKA strain), 13-hydroperoxyoctadeca-9,11-dienoic acid (159) was identified as a probable antimalarial compound (Lee et al. 2020). The aqueous extract of the plant also exerted hepatoprotection in ICR mice infected with P. berghei. The liver damage, indicated by increased serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, was inhibited by this extract at a dose of 500 mg/kg (Somsak et al. 2015). In the same model, the aqueous extract at doses of 500, 1000 and 2000 mg/kg displayed renoprotective and antihemolytic effects. At higher doses, the blood urea nitrogen (BUN) and creatinine levels decreased significantly compared to the negative control. For the highest dose, the hematocrit percentage increased significantly compared to the untreated group (Nutham et al. 2015).
Three combinations of artesunate (32 mg/kg) were prepared using three doses of the aqueous extract (2.5, 3 and 3.5 mg/kg) and administrated to C57BL/6 J mice infected with P. berghei. This caused a substantial inhibition of Nuclear Factor Kappa B (NFκB) and Intracellular Adhesion Molecule-1 (ICAM1) compared to the artesunate or extract only groups (Izzati et al. 2016). The aqueous extract of T. cripsa stems was also assessed against Brugia malayi, amongst other parasites, to evaluate its antifilarial potential. Following an incubation period of 24 h, the extract produced relative mobility values of 25, 7 and 0 at doses of 1, 5 and 10 mg/mL, respectively (Zaridah et al. 2001). Another study reported that an ointment prepared from an oil extract of the stem displayed significant activity against Pediculus humanus capitis compared to a shampoo used as a positive control and containing 1% permethrin (Torre et al. 2017). The ethanol extract of the stem (1.56–200 μg/mL) also proved to be active against Toxoplasma gondii (RH strain) compared to standards of veratrine and clindamycin used at the same concentrations. This extract did not display any cytotoxicity in an MTT assay against Vero cells (IC50 value 179 μg/mL) compared to clindamycin (IC50 of 116.5 μg/mL) and veratrine (IC50 of 60.4 μg/mL). The antitoxoplasmic activity of the extract was established with an IC50 of 6.31 μg/mL compared to that of clindamycin (8.33 μg/mL) and veratrine (14.25 μg/mL). The good selectivity index calculated for this extract (28.4) suggests it may represent a promising source of new antitoxoplasmic agents (Sharif et al. 2019).
Overall, T. crispa has demonstrated in vitro and in vivo activity against various parasites, but there have been contradictory reports regarding the potency of its extracts against Plasmodium species. Further pharmacological investigation and bio-assay guided isolation of active compounds are required in the future.
Antimicrobial activity
An in vitro disk diffusion assay was carried out to evaluate the antimicrobial activity of the aqueous, ethanol and chloroform extracts of T. crispa (25, 50, 75, and 100%) against various Gram- positive (Staphylococcus aureus, Streptococcus pneumoniae, Corynebacterium diphtheriae, Bacillus cereus, Listeria monocytogenes) and Gram-negative (Escherichia coli, Salmonella typhi, Shigella flexneri, Klebsiella pneumoniae, Proteus vulgaris) bacteria using flemequine as a standard. All extracts dose-dependently inhibited S. pneumoniae, C. diphtheriae and S. flexneri compared to the standard. At concentrations above 50%, the aqueous and chloroform extracts inhibited S. aureus and E. coli. All extracts were ineffective against B. cereus and S. typhi (Zakaria et al. 2006). Additional testing of the aqueous extract on S. aureus and E. coli using an agar diffusion assay, led to a modest inhibitory effect with Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of 227.27 mg/mL each (Zakaria et al. 2011). Another study showed that the aqueous, ethanol, methanol and chloroform extracts of the plant were active against S. pneumoniae, E. coli and Candida albicans compared to the standards tetracycline and fluconazole (Asif Iqbal et al. 2012). The ethanol extract at a dose of 1 mg/disk was also active against Methicillin Resistant S. aureus (MRSA) compared to the standard vancomycin in a disk diffusion assay (Al-alusi et al. 2010). Furthermore, the ethanol extract, when administered as ointment (9% v/v) with zeolite, showed bactericidal activity against S. aureus and Pseudomonas aeruginosa compared to a preparation containing gentamicin (Susanti et al. 2016). Another disk diffusion assay study confirmed the efficacy of the ethanol extract against E. coli (zone of inhibition of 20–22 and 22–30 mm at concentrations of 8% and 32%, respectively) compared to the standard amoxicillin (19 mm) (Muslimin et al. 2018). The aforementioned extract also showed strong antifungal activity against Trichophyton rubrum at concentrations ≥ 40% (Erza et al. 2020). The n-hexane extract of the stem significantly inhibited the growth of S. aureus, Shigella boydii, S. dysenteriae, Vibrio mimicus, C. albicans and Aspergillus niger (Rahman et al. 2020). Two oxaporphine alkaloids isolated from the plant, namely lysicamine (50) and liriodenine (49), displayed activity on S. aureus and Enterococcus faecalis in a disk diffusion assay (Hamid et al. 2021). The plant ethanol extract, when employed as a 30% ointment, also revealed activity against Propionibacterium acnes (zone of inhibition of 9.13 mm), indicating its potential as an anti-acne treatment (Yusriani et al. 2018). One study tested the chloroform and petroleum ether fractions of the methanol extract of T. crispa using a disk diffusion assay against five Gram-positive bacteria (Bacillus subtilis, B. megaterium, B. cereus, S. aureus, Sarcina lutea), seven Gram-negative bacteria (E. coli, S. dysenteriae, S. typhi, S. paratyphi, S. boydii, V. mimicus, V. parahemolyticus) and three fungi (C. albicans, A. niger and Sacharomyces cerevisiae). The activity of the extract and fractions (400 μg/disc) was compared to that of the standard doxycycline (30 μg/disc). Zones of inhibition, albeit negligible, were only observed for the chloroform fraction (Haque et al. 2011). The weak activity of the chloroform fraction was confirmed by another study testing the same fractions against the aforementioned microorganisms and P. aeruginosa, and using kanamycin (30 μg/disc) as a standard. This study reported no activity for the petroleum ether fraction (Islam et al. 2014). The antibacterial activity of the protein extract of T. crispa was evaluated against B. cereus, S. aureus, K. pneumoniae and Salmonella typhimurium. Only B. cereus was found to be sensitive to the extract (zone of inhibition of 9.7 ± 0.5 mm) (Zin et al. 2016).
The antiviral activity of T. crispa was evaluated for the ethanol and aqueous extracts (3–100 µg/mL) against HIV-1 integrase. Weak activity was obtained (IC50 > 100 µg/mL) (Bunluepuech and Tewtrakul 2009). Another study reported the use of a molecular docking approach to investigate the interactions of a variety of T. crispa constituents (putatively detected by GC–MS) with the SARS-CoV2 main protease. Imidazolidin-4-one and 2-imino-1-(4-methoxy-6-dimethylamino-1,3,5-triazin-2-yl) (64) were found to bind with the active site of this enzyme in a similar manner to the standard nelfinavir (Rakib et al. 2020c).
Overall, T. crispa extracts have demonstrated in vitro activity against selected microorganisms, which should be further investigated particularly employing in vivo models of infection. Also noteworthy are bioassay-guided studies to identify the phytoconstituents responsible for such activity. Hamid et al. (2021) have reported that aporphine alkaloids had good activity against Gram-positive bacteria. A total of 13 alkaloids of this type have been isolated from T. crispa to date, warranting further testing and SAR studies. The molecular mechanisms underlying the antimicrobial activity of T. crispa extracts/constituents should also be elucidated. Considering the current global antimicrobial drug resistance issue, unravelling the specific microbial pathway(s) targeted and the chemical pharmacophores are particularly important as this may pave the way for future antibiotic design and development.
Immunomodulatory activity
The ability of T. crispa to modulate the innate and adaptive immune response has been demonstrated in several studies. The plant contains both anti-inflammatory and pro-inflammatory constituents. In the carrageenan-induced rat paw oedema model, the methanol extract of the stem at a dose of 10 mg/kg produced a 38% suppression of the oedema. The n-butanol fraction of the same extract was more effective than the diethyl ether and the aqueous fractions. When administered subcutaneously a dose of 3 mg/kg, the n-butanol fraction showed activity comparable to 250 mg/kg sulpyrine and 10 mg/kg diphenhydramine (Higashino et al. 1992). The anti-inflammatory activity of the plant was also assessed using an antigen-induced rat basophilic leukemia (RBL)-2H3 cell line where release of β hexoaminidase was measured. The ethanol extract and aqueous extract of the stem (concentration range of 0–100 μg/mL) revealed dose-dependent inhibition up to 44% and 65%, respectively. However, their IC50 values were higher (> 100 μg/mL and 83 μg/mL, respectively) compared to the standard ketotifen fumerate (20.2 μg/mL), suggesting weak activity. Interestingly, the ethanol extract of T. crispa combined with the ethanol extract of Piper nigrum (1:1, v/v) produced an IC50 of 26.7 μg/mL (Kraithep et al. 2008). The methanol extract was evaluated for its ability to inhibit reactive oxygen species (ROS) in whole blood, polymorphonuclear (PMN) leukocytes and macrophages during phagocytosis using a luminol/lucigenin-based chemiluminescence assay. The extract produced significant suppression of ROS in the metabolic phase of phagocytosis (IC50 of 0.6 ± 4.2 μg/mL compared to 3.0 ± 1.3 for the standard acetylsalicylic acid). It performed poorly in the other assays that were used in the study, including the PMN chemotaxis assay, compared to the standard ibuprofen (Jantan et al. 2011). Another study involving both the methanol and aqueous extracts of T. crispa stem was carried out on hydrogen peroxide-induced human umbilical vein endothelial (HUVEC) cells using a Tumor Necrosis Factor-α (TNF-α)-induced model of inflammation. The extracts inhibited Intracellular Adhesion Molecule- 1 (ICAM-1), Vascular Cell Adhesion Molecule-1 (VCAM-1) in a dose-dependent manner at concentrations ranging from 100–600 μg/mL. A significant and dose-dependent increase in Nitric Oxide (NO) production was observed in the presence of both extracts (Kamarazaman et al. 2012). In the carrageenan-induced paw oedema model, the aqueous extract of T. crispa (50, 100 and 150 mg/kg) showed inhibition comparable to ibuprofen (0.5%). In an in vitro membrane stabilization assay using hypotonic solution-induced lysis of human RBCs, the extract at a concentration of 2.5% was not active. At concentrations of 5 and 7.5%, however, it showed membrane stabilization comparable to ibuprofen (0.5%). The extract also dose-dependently inhibited the denaturation of protein in an albumin solution (Hipol et al. 2012). The ethanol extract (50, 100 and 200 mg/kg) was also tested on male Balb/C mice primed with sheep RBCs, using levimasole as a positive control. The results indicated that this extract increased peritoneal macrophage engulfment of E. coli, NO production, and lysozyme and myeloperoxidase serum levels. The extract at a dose of 200 mg/kg was equivalent to 2.5 mg/kg of levimasole. Upregulation of Immunoglobulin G (IgG) and Immunoglobulin M (IgM) also occurred, with the extract at the dose 100 mg/kg proving more potent than the standard. Dose-dependent delayed hypersensitivity was also observed in a footpad edema assay (Ahmad et al. 2016b).
A number of studies succeeded in elucidating the active constituents and their biological potential in immunomodulatory assays. Using a flow cytometry immunostaining assay on lipopolysaccharide (LPS)-induced RAW 264.7 cells, T. crispa ethanol extract and fractions were found to considerably boost the levels of the pro-inflammatory cytokines Interferon γ (IFN-γ), Interleukin 6 (IL-6) and IL-8. Cordioside (13), quercetin (82), eicosenoic acid (paullinic acid) (160) and boldine b were isolated from a fraction coded as Fraction 2 (Abood et al. 2014). In a chemotaxis assay carried out on RAW 264.7 cells with the chemoattractant formyl-methionylleucyl-phenylalanine, the ethanol extract (12.5–200 μg/mL) increased chemotaxis as compared to the standard. Compounds from the ethanol extract which displayed notable immunomodulatory activity were identified as N-formylanonaine (39), N-formylnornuciferine (43), lysicamine (50), magnoflorine (51), syringin (134) and 1-octacosanol (167). When tested in the chemotaxis assay at concentrations ranging from 1.56–25 μg/mL, the first four compounds—particularly magnoflorine (51)—showed a potentiating effect, while the last two—particularly syringin (134)—inhibited chemotaxis compared to the standards ibuprofen and levimasole. ROS production, phagocytosis, NO, prostaglandin E2 (PGE2), Monocyte chemoattractant protein-1 (MCP-1), IL-6, IL-1β and TNF-α levels were also boosted by the extract, magnoflorine (51), N-formylanonaine (39), N-formylnornuciferine (43) and lysicamine (50). Magnoflorine (51) proved to be most potent in this regard. Opposite effects were found for syringin (134) and 1-octacosanol (167). It was concluded that among the compounds tested, syringin (134) and 1-octacosanol (167) showed anti-inflammatory properties, while the rest activated the immune system (Ahmad et al. 2018). Magnoflorine (51) and syringin (134) were further confirmed to be important immunomodulatory constituents of the ethanol extract. In LPS-primed U937 human macrophages, both the ethanol extract and magnoflorine (51) enhanced Inhibitory κB Kinase (IKK) α/β and NFκB phosphorylation while simultaneously causing de-activation of IκBα. Subsequently, activation of NFκB occurred alongside release of IL-1β and TNF-α. In addition to this, the extract resulted in the upregulation of cyclooxygenase-2 (COX-2) and PGE2 along with phosphorylation of Akt, extracellular signal-regulated kinase (ERK) 1/2, p38 mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) 1/2 (Haque et al. 2020). Tinocrisposide (14) (100–1000 μg/mL) was another compound assayed for its hemolytic and anti-inflammatory potential. Its hemolytic value (< 10%) suggested it was non-hemolytic. Moreover, in an in vitro anti-inflammatory assay, this compound displayed membrane stabilizing activity comparable to the standard ibuprofen. Similar results were obtained for the aqueous extract of the plant (Adnan et al. 2019).
A recent in silico study postulated that tyramine (67) may act as a COX-2 inhibitor and exert anti-inflammatory activity (Widodo et al. 2021). While it is confounding that T. crispa phytoconstituents are able to both activate and suppress the immune system, it also opens up possibilities into designing new classes of immunomodulators. It is noticeable that the compounds of interest are not confined to a particular chemical class. This may also explain the marked diversity in the biochemical responses produced.
Antioxidant activity
The antioxidant activity of various extracts and fractions of the plant has been studied extensively. In this regard, the methanol extract was found to be more potent compared to the aqueous and chloroform extracts. In a 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay, the methanol extract had an IC50 of 12 μg/mL which was comparable to the standard ascorbic acid. The resultant inhibition also approached 100%. Its total phenolic and flavonoid contents were found to be 255.33 ± 10.79 mg Gallic Acid Equivalent (GAE)/g sample and 9.53 ± 0.50 mg Quercetin Equivalent (QE)/g sample, respectively (Ibahim et al. 2011). Another study used a DPPH free radical scavenging assay on the ethanol extract, aqueous fraction and ethyl acetate fraction. The ethyl acetate fraction displayed the strongest activity (53.77% inhibition at 200 μg/mL) (Irianti et al. 2011). Several in vitro and in vivo studies were performed on the aqueous extract. The latter at a concentration of 10% produced DPPH inhibition, Thiobarbituric Acid (TBA) inhibition and displayed a Ferric Reducing Antioxidant Power (FRAP) value of 86.51 ± 0.07%, 39.2 ± 5.14% and 0.89 ± 0.07 mmol/L, respectively compared to the standards ascorbic acid (96.36 ± 0.90%, 73.2 ± 5.14% and 1.05 ± 0.00 mmol/L, respectively) and butylated hydroxytoluene (96.51 ± 0.95%, 75.8 ± 6.08% and 1.03 ± 0.03 mmol/L, respectively). An in vivo study was carried out on hypercholesterolemic rabbits using the aqueous extract at doses of 150, 300 and 450 mg/kg. The extract reduced Total Cholesterol (TC), Triglyceride (TG) and Low-density Lipoprotein (LDL) while boosting High-density Lipoprotein (HDL) and restored malondialdehyde (MDA) levels to normal. Aortic atherosclerotic lesions were dose-dependently lessened up to 100%. This suggests that the antioxidant potential of T. crispa is linked to its inhibition of atherosclerosis and plasma lipid peroxidation (Amom et al. 2011). The aqueous extract of T. crispa stem showed anti-atherosclerotic and anti-hypercholesterolemic activity in adult male New Zealand albino rabbits. The animals were first conditioned with a 0.5% high cholesterol diet, which caused an increase of C-Reactive Protein (CRP) levels. A dose-dependent reduction of CRP levels was observed following administration of the extract. At 200 mg/kg, the extract did not change the CRP levels. At 450 mg/kg, it returned the CRP levels to normal levels while at 600 mg/kg it reduced the CRP levels to levels lower than normal. The extract also dose-dependently reduced atherosclerotic plaque coverage and foam cell formation to a considerable degree (Shah et al. 2021). Further investigations were carried out on the radical-scavenging activity of the methanol extract and its petroleum ether, chloroform, carbon tetrachloride and aqueous fractions, using a DPPH assay. The carbon tetrachloride fraction showed the strongest activity with an IC50 value of 30 μg/mL compared to the standard ascorbic acid (15 μg/mL) and BHT (25 μg/mL) (Haque et al. 2011). In another study using a DPPH assay, the ethanol extract, its water fraction and selected subfractions, showed IC50 values of 49.92 μg/mL, 38.25 μg/mL, 36.12 μg/mL, and 16.18 μg/mL, respectively. It was postulated that acid hydrolysis of the subfractions improved their antioxidant potential (Warsinah et al. 2020). Several other studies measuring the total phenolic content, total flavonoid content, DPPH free radical scavenging activity and Ferric Reducing Antioxidant Power of T. crispa all confirned the antioxidant potential of the plant (Zulkefli et al. 2013; Abood et al. 2014; Nguyen et al. 2020; Mahalle and Gupta 2021). In a metal chelating assay, the petroleum ether, chloroform, methanol and water extracts of the stem were first mixed and dried together. The mixed extract (1 mg/mL) produced 81.97% inhibition of Ferrozine-Fe2+ complex formation compared to ethylenediaminetetraacetic acid (EDTA) at the same concentration (98.51% inhibition) (Zulkefli et al. 2013). In an MTT cell viability assay, pre-treatment with the aqueous extract (50–1000 μg/mL) and the methanol extract (600 μg/mL) of T. crispa boosted viability to 69% and up to 76%, respectively. When assessed for antioxidant activity in hydrogen peroxide-induced HUVEC cells, antioxidant enzymes including Catalase (CAT), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx) were increased by the aqueous extract in a dose-dependent manner. The methanol extract on the other hand showed maximum CAT and SOD activity at 400 μg/mL and potentiated GPx activity dose-dependently. MDA levels were inhibited up to 58% and 60% for the aqueous and methanol extracts, respectively (Kamarazaman et al. 2012). A study using hyperlipidemic rabbits further confirmed the effect of the aqueous extract (administered at doses of 200, 450 and 600 mg/kg) on the cholesterol profile and the amelioration of atherosclerotic plaques compared to the standard simvastatin. Whilst SOD and GPx activity were also potentiated, the Total Antioxidant Status (TAS) did not improve substantially in the presence of T. crispa aqueous extract (Zamree et al. 2015). Three isolated constituents, N-trans-feruloyltyramine/moupinamide (62), N-cis-feruloyltyramine (63) and secoisolariciresinol (135) displayed stronger antioxidant activity than the standard BHT in a DPPH free radical scavenging assay (Cavin et al. 1998). Other compounds such as protoberberine alkaloids isolated from the plant, namely columbamine (54), dihydrodiscretamine (53) and 4,13-dihydroxy-2,8,9-trimethoxydibenzo[a,g]quinolizinium (55) showed IC50 > 500–800 μg/mL in a DPPH free radical scavenging assay (Hamid et al. 2021).
Whilst the antioxidant potential of T. crispa has been established in multiple in vitro studies, further in vivo studies are warranted, particularly focussing on how T. crispa extracts/constituents may interfere with antioxidant enzymes (Fig. 11). The numerous flavonoids present in the plant may contribute to the modulation of these enzymes, but this has yet to be assessed. Alkaloids, of the protoberberine class and others present in the plant should also be evaluated for their antioxidant potential so as to gain valuable insights into structure–activity relationships.
Hepatoprotective activity
The hepatoprotective potential of T. crispa has been demonstrated in many studies (Lee et al. 2017; Rakib et al. 2020a). The methanol extract of T. crispa was found to increase the activity of phase-1 metabolic enzymes in male Sprague Dawley rat hepatocytes. The extract produced a substantial increase in aminopyrine N-demethylase activity at a dose of 0.001–1.0 mg/mL. At lower (but not higher) doses, this effect was mediated by the cAMP pathway (Tin et al. 2005). In an in vitro study, the same extract (0.5 mg/mL) produced 61.3% inhibition of the CYP3A4 enzyme compared to the standard troleandomycin (62.1%) in a time-dependent manner (Usia et al. 2006) (Fig. 11). The activity of this extract on CYP3A4 and CYP2D6 yielded IC50 values of 428 and 488 μg/mL, respectively (Subehan et al. 2006). The ethanol extract also acted against tert-butyl hydroperoxide-induced hepatotoxicity in HEP-G2 cells (EC50 of 144.3 μg/mL). The underlying mechanism was established to be via the induction of Nrf2-mediated expression of HO-1 (Lee et al. 2017). Another study demonstrated that carbon tetrachloride-induced Swiss albino mice pre-treated with the methanol extract (doses of 100–400 mg/kg body weight) resulted in noteworthy hepatoprotection. Levels of ALT, AST, Alkaline Phosphatase (AP), Malondialdehyde (MDA) and total bilirubin were reduced comparably to the standard silymarin (Rakib et al. 2020a). The enzyme modulatory and hepatoprotective activity of T. crispa warrants further investigations. In particular, bio-assay guided isolation studies should be performed to assess the activity of phytochemicals.
Analgesic activity
Although used traditionally for pain management, the analgesic activity of the plant is not well studied. An extract of T. crispa stems was reported to demonstrate central analgesic activity in a tail flick response to radiant heat (Almeida et al. 2001). The ethanol extract (300 mg/kg) showed dose-dependent peripheral analgesia with 92% inhibition in the acetic acid-induced writhing test in mice, compared to the standard acetyl salicylic acid (81% inhibition at 100 mg/kg) (Sulaiman et al. 2008). In the same assay, the methanol extract, its petroleum ether and chloroform fractions (400 mg/kg) yielded 48.06, 51.94 and 43.41%, respectively, compared to 65.12% inhibition for the diclofenac sodium standard (100 mg/kg). The activity of the petroleum ether fraction was considered statistically significant (p < 0.05) compared to the standard (Islam et al. 2014). The methanol extract and the chloroform fraction (at doses 200 and 400 mg/kg) also displayed significant antinociceptive activity in the acetic acid-induced writhing and formalin-induced paw-licking tests, compared to the standard diclofenac (Rakib et al. 2020b). Having said that, the analgesic potential of the plant still requires further exploration. Future work should focus on investigations that aim to identify the phytoconstituents responsible for such activity. Studies on the molecular mode of action of the analgesic constituents must also be undertaken.
Antipyretic activity
The n-butanol fraction of T. crispa stems (3 mg/kg) suppressed LPS-induced fever in rats when administered intravenously. The activity was equivalent to that of 100 mg/kg sulpyrine and 1 mg/kg morphine hydrochloride administered intraperitoneally (Higashino et al. 1992). In DPT (Diphtheria-Pertussis-Tetanus) vaccine-induced male Wistar rats, a 40% ethanol extract of the plant produced significant antipyretic effect at 90- and 120-min post-treatment (Wulandari and Bestari 2016). Significant antipyretic activity was also observed for a methanol extract and its petroleum ether and n-hexane subfractions administered at a dose of 400 mg/kg to Swiss albino mice with Brewer’s Yeast-induced fever. The activity was found to be dose-dependent (Rakib et al. 2020a). These studies provide some evidence to support the ethnomedicinal use of T. crispa for the treatment of pyrexia. The specific molecular mode of action of such effects, however, remains to be elucidated.
Anticholinesterase activity
It is interesting to note that quaternary alkaloids are prevalent in T. crispa, indicating its probable acetylcholinesterase (AChE) inhibitory potential. One study assessed the potential of such alkaloids using a modified Ellman’s colorimetric method with physostigmine as the standard. Among the seven alkaloids studied, the least polar one—columbamine (54)—displayed significant inhibitory activity with an IC50 of 48.1 ± 1.3 μM compared to physostigmine (31.4 ± 0.5 μM). Dihydrodiscretamine (53) and N-formylanonaine (39) only showed moderate activity (Fig. 11). A preliminary SAR study was also performed on these alkaloids (Yusoff et al. 2014). QSAR studies employing the crystallized protein structure of AChE should be performed to gather information on the probable interactions of this target with bioactive ligands.
Central nervous system (CNS) activity
The activity of T. crispa on the CNS has not been studied extensively. A decoction of the plant was evaluated in a motor activity test, curiosity test, hanging test and rotary road test at various concentrations (6.5, 13 and 26%). It was found that the lowest concentration produced CNS-stimulant effects similar to the positive control caffeine (Merwanta et al. 2019). The methanol extract, its chloroform and n-hexane fractions at doses of 200 and 400 mg/kg were evaluated in the open field, hole board and elevated plus maze tests. A significant decrease in locomotion was observed in the open field test comparable to the standard diazepam (1 mg/kg). In the hole board test, the chloroform fraction at the highest dose yielded significant results, which indicated a reduced fearfulness. Additionally, the methanol extract (at the highest dose) and the chloroform extract (at the lowest dose) displayed anxiolytic activity in the elevated plus maze test comparable to the standard diazepam (1 mg/kg) (Rakib et al. 2020b). Additional investigations on the CNS activity of T. crispa are warranted, particularly focusing on the identification of the phytochemical(s) responsible for such activity.
Antihyperuricemic activity
The n-hexane insoluble fraction of the ethanol extract of T. crispa stem was evaluated in male BALB/C mice for its potential xanthine oxidase (XO) inhibitory activity. The extract reduced the levels of uric acid ranging from 49 to 78% at doses of 50–200 mg/kg. Peak activity was observed at the 100 mg/kg dose compared to the standard allopurinol (10 mg/kg) (Harwoko and Warsinah 2020) (Fig. 11). These results contradict a previous study carried out using the root of the plant, which showed an IC50 of 370.35 µg/mL compared to the standard allopurinol (0.022 µg/mL) (Vikneswaran and Chan 2005). This may suggest that the presence of phytoconstituents with prospective XO inhibitory activity is localized in certain parts of the plant. However, it is premature to drawing any conclusion on this aspect without supplementary evidence. Further identification of the phytoconstituents involved in the modulation of this enzyme are warranted.
Pesticidal activity
There is some evidence that T. crispa possesses pesticidal activity, although this has not been investigated exhaustively. Its chloroform, ethanol, petroleum ether and ethyl acetate extracts have been evaluated against the Small Mottled Millow Moth (Spodoptera exigua) which infests spinach. It was observed that the ethanol and petroleum ether extracts (five sprays over five days) reduced the moth population by 61.2% and 51.6%, respectively, compared to standard cyperin (91.5%). The other extracts did not produce significant inhibition (Isa et al. 2013). A similar study was carried out on Phyliotera sinuata ateph infesting mustard plants using the same extracts. Here, the ethanol and ethyl acetate extract (at a concentration of 1 g/L) reduced the insect population by 88.73% and 83.66%, respectively, compared to the standard cyperin (79.44%). Eight compounds namely, 1,2-benzenedicarboxylic acid (144), 2-propenoic acid, dodecyl ester (162), ethyl pentadecanoate (163), oxalic acid, decyl 2-ethylhexyl ester (164), 1-tetradecanol (165) 1-eicosanol (166), and 1-octacosanol (167) were isolated from the ethanol extract but their bioactivity was not evaluated (Nor Aziyah et al. 2014). The ethanol extract (0.312, 0.625, 1.25, 2.5 and 5%) was also found to have larvicidal activity against the diamondback moth (Plutella xylostella) with an IC50 of 0.894% (Suvannarat et al. 2015). Larvicidal activity was also demonstrated against Culex quinquefasciatus mosquito larvae. The petroleum ether extract (80–160 ppm) of T. crispa mature fruits showed LC50 values ranging from 79.58 to 127.19 mg/L during the 1st–4th instars of growth (Pal et al. 2016). Another study revealed that the aqueous extract (3.125, 6.25, 12.5, and 25 mg/L) of the stem produced LC50 values of 16.95 and 30.12 mg/L, respectively (Jiraungkoorskul 2019). Additionally, the chloroform, n-hexane, methanol, and aqueous extracts of the stem displayed time- and concentration-dependent molluscicidal activity on Pomacea canaliculata. The n-hexane, followed by the aqueous extract, were the least cytotoxic of all extracts tested. The chloroform and methanol extracts were more prominently molluscicidal than other extracts, with the methanol extract outperforming the rest (Aziz et al. 2021).
These studies suggest the usefulness of T. crispa as a biopesticide. Bio-assay guided isolation and analysis of active compounds should be carried out in the future in order to discover new natural chemical entities that could replace the harmful commercial pesticides currently used.
Clinical trials
The clinical trials conducted thus far with T. crispa have focused entirely on the assessment of its antidiabetic properties (Table 4). One placebo-controlled, double-blind, randomized trial was conducted on 20 type-2 diabetic patients who were non-responsive to oral antidiabetic drugs and did not receive insulin. Following administration of T. crispa (1 g dry powder thrice a day for 6 months), no significant differences were observed between the T. crispa-treated group and the control group in terms of fasting blood sugar, insulin, and glycosylated hemoglobin levels. Unexpectedly, the T. crispa-treated group displayed higher cholesterol and glycosylated hemoglobin concentrations. Interestingly, an average of 2 kg of body weight loss was observed among the treated patients (Sangsuwan et al. 2004). One placebo-controlled, double-blind, randomized, crossover study conducted on 36 patients with metabolic syndrome revealed that treatment with T. crispa (250 mg capsules daily for two months) significantly lowered fasting blood sugar and triglyceride levels, but induced hepatotoxicity with ALT and AST levels noticeably increased in about 16.7% of the patients (Sriyapai et al. 2009). Another trial, conducted in Thailand, showed that T. crispa administered as a single dose (6 g) to non-diabetic healthy volunteers neither induced acute changes in glucose metabolism nor significantly improve glucose tolerance in 9 subjects. A similar single 6 g dose administered to 6 different healthy volunteers led to a significant decrease in blood glucose levels, but no changes in insulin levels. To check the biochemical and hematological effects of the plant, 12 subjects were treated with T. crispa 1 g thrice daily for 8 weeks while 13 others received 1.05 g doses in a similar fashion. Serum glucose and other hematological parameters were unchanged, except for AST and ALT levels which were noticeably increased, indicating hepatotoxicity (Rattanajarasroj et al. 2004). A more recent study to observe the effects of T. crispa ingestion employed 10 healthy and 10 diabetic subjects. The subjects received 75 g of glucose with or without 250 mg of T. crispa supplements after overnight fasting, and serum samples were collected every 30–60 min for 3 h. No significant changes in glucose or insulin levels were observed between the control and test groups (Klangjareonchai and Roongpisuthipong 2012).
The clinical trials carried out so far are preliminary with small sample size and non-systematic. To evaluate the plant as a safe and effective antidiabetic agent for human use, a thorough, serious and more operationally randomized controlled trial have to be performed.
Safety and toxicological profile
Many studies have indicated that T. crispa extracts are relatively safe for oral ingestion. However, some studies have highlighted the hepatotoxicity potential of this plant. The ethanol extract of T. crispa, administered at a dose of 100–200 mg/kg, has displayed dose-dependent hepatotoxicity in thioacetamide-conditioned Sprague Dawley rats. The extract caused significant increases in the serum levels of ALT, AST, AP, bilirubin, and G-glutamyl transferase, and histological features of hepatocytic degeneration were also observed (Kadir et al. 2011). Similar elevation of AST and ALT were also reported in two Thai clinical studies involving T. crispa (Sriyapai et al. 2009; Rattanajarasroj et al. 2004). Two cases of toxic hepatitis following the use of T. crispa have been reported to date. The first one was a 49-year-old male who had been using a T. crispa-containing herbal medication (Langrand et al. 2014). The second was a 57-year-old man who ingested the aqueous extract of the plant (Cachet et al. 2018).
Clerodane-type furanoditerpenoids and borapetosides have been suggested as the constituents responsible for the observed in vivo toxicity of T. crispa. However, one study using a LPS-induced ND-4 mice model reported that borapetosides B (17), C (14), and F (25) did not produce hepatotoxicity, when administered both individually and in combination at a dose of 500 mg/kg for 21 days (Parveen et al. 2020). Tinospora crispa ethanol extract, and the n-hexane and chloroform fractions from its methanol extract, have been found to be quite safe in murine models. No harmful effects were observed neither following the administration of the ethanol extract (50–200 mg/kg) to male Balb/C mice, nor following the administration of fractions from the methanol extract (various doses with the maximal dose of 2000 mg/kg) on Swiss albino mice (Ahmad et al. 2016b; Rakib et al. 2020a). A dermal irritation test employing adult albino rabbits showed that a T. crispa-based ointment was non-irritant when administered topically (Torre et al. 2017).
Potential drug-drug interactions have been suggested between T. crispa and other co-administered drugs through its capacity of modulating the Pregnane X-receptor (PXR). In an in vitro luciferase reporter gene assay, the methanol extract of the plant and its chloroform and n-hexane fractions significantly activated PXR. Its ethyl acetate and the butanol fractions showed negligible activity. Several T. crispa constituents shared this activity, and the in vitro results were further reflected in silico (Parveen et al. 2022). Thus, any drug being metabolized or activated through PXR might experience altered pharmacokinetics when co-administered with T. crispa. Given the potential of T. crispa as a source for novel therapeutic lead compounds, further comprehensive studies should be conducted to establish its absolute safety.
Conclusion and future prospects
Multiple in vitro and in vivo studies on T. crispa have demonstrated its remarkable medicinal potential, particularly in the treatment of diabetes and hypertension, providing support to justify some of its ethnobotanical uses. Several clerodane-type furanoditerpenoids in T. crispa have been reported to possess significant antidiabetic activity, which is worthy of further exploration for the discovery of novel antidiabetic drugs. In addition, the adrenergic activity of its alkaloids may provide new avenues for the treatment of high blood pressure. While the cytotoxic prospects of this plant have been studied at length, the specific molecular mechanisms involved in this effect have yet to be elucidated. Clerodane-type furanoditerpenoids have revealed noticeable results as chemopreventive agents, which is also worthy of further investigations. The widespread use of T. crispa as an antimalarial agent has been supported by many studies, but with conflicting reports on its efficacy. It is interesting to note, however, that various extracts provide protection against malaria, which may help to offset the detrimental effects of this disease. These observations suggest the need for further bioassay-guided isolation in order to identify the antiplasmodial phytoconstituent(s) of T. crispa. Likewise, the immunomodulatory activity of various T. crispa extracts and phytoconstituents demands scrutiny. One of the most notable aspects in this regard is the ability of T. crispa phytoconstituents to both activate and suppress the immune system. Alkaloids from T. crispa have been found to be particularly potent and deserve closer inspection. Among the other pharmacological effects displayed by the plant, the inhibition of acetylcholinesterase by quaternary alkaloids looks promising and should be investigated further. Although the hepatotoxicity of T. crispa has been attributed to the presence of selected clerodane-type furanoditerpenoids, there are other compounds within that class that are not hepatotoxic. Therefore, in-depth investigations into this class of phytochemicals are essential in order to evaluate their relative safety and toxicity. The characteristic phytoconstituents of T. crispa may overall play an important part in the discovery of new drug leads. In that respect, additional in vivo pharmacological and toxicological studies on T. crispa are warranted to provide assurance of adequate efficacy and safety.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abdillah S, Tambunan RM, Farida Y et al (2015) Phytochemical screening and antimalarial activity of some plants traditionally used in Indonesia. Asian Pac J Trop Dis 5:454–457. https://doi.org/10.1016/S2222-1808(15)60814-3
Abood WN, Fahmi I, Abdulla MA, Ismail S (2014) Immunomodulatory effect of an isolated fraction from Tinospora crispa on intracellular expression of INF-γ, IL-6 and IL-8. BMC Complement Altern Med 14:1–12. https://doi.org/10.1186/1472-6882-14-205
Abu MN, Hassan HF, Yusoff R, Ismail WIW (2017) Tinospora crispa methanol crude extract activates apoptotic pathway of insulin resistant-HepG2 cell lines by improving the insulin sensitivity. Malays Appl Biol 46:145–152
Abu MN, Samat S, Kamarapani N et al (2015) Tinospora crispa ameliorates insulin resistance induced by high fat diet in wistar rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2015/985042
Adnan AZ, Armin F, Sudji IR et al (2019) In vitro anti-inflammatory activity test of tinocrisposide and freeze-dried aqueous extract of Tinospora crispa stems on human red blood cell by increasing membrane stability experiment. Asian J Pharm Clin Res. https://doi.org/10.22159/ajpcr.2019.v12i5.30690
Adnan AZ, Taher M, Afriani T et al (2016) Cytotoxic activity assay of tinocrisposide from Tinospora crispa on human cancer cells. Der Pharm Lett 8:102–106
Ahmad FB, Ismail G (2003) Medicinal plants used by Kadazandusun communities around Crocker Range. ASEAN Rev Biodivers Environ Conserv 1:1–10
Ahmad W, Jantan I, Bukhari SNA (2016) Tinospora crispa (L.) Hook. f. & Thomson: a review of its ethnobotanical, phytochemical, and pharmacological aspects. Front Pharmacol 7:59. https://doi.org/10.3389/fphar.2016a.00059
Ahmad W, Jantan I, Kumolosasi E et al (2018) Immunomodulatory effects of Tinospora crispa extract and its major compounds on the immune functions of RAW 264.7 macrophages. Int Immunopharmacol 60:141–151. https://doi.org/10.1016/j.intimp.2018.04.046
Ahmad W, Jantan I, Kumolosasi E, Bukhari SNA (2016b) Standardized extract of Tinospora crispa stimulates innate and adaptive immune responses in Balb/c mice. Food Funct 7:1380–1389. https://doi.org/10.1039/C5FO01531F
Ahmed SM, Manhas LR, Verma V, Khajuria RK (2006) Quantitative determination of four constituents of Tinospora sps. by a reversed-phase HPLC-UV-DAD method. Broad-based studies revealing variation in content of four secondary metabolites in the plant from different eco-geographical regions of India. J Chromatogr Sci 44:504–509. https://doi.org/10.1093/chromsci/44.8.504
Al-alusi NT, Kadir FA, Ismail S, Abdullah MA (2010) In vitro interaction of combined plants: Tinospora crispa and Swietenia mahagoni against Methicillin-resistant Staphylococcus aureus (MRSA). Afr J Microbiol Res 4:2309–2312. https://doi.org/10.5897/AJMR.9000399
Almeida RN, Navarro DS, Barbosa-Filho JM (2001) Plants with central analgesic activity. Phytomedicine 8:310–322. https://doi.org/10.1078/0944-7113-00050
Amom Z, Azman KF, Ismail NA et al (2011) An aqueous extract of Tinospora crispa possesses antioxidative properties and reduces atherosclerosis in hypercholesterolemic-induced rabbits. J Food Biochem 35:1083–1098. https://doi.org/10.1111/j.1745-4514.2010.00436.x
Amom Z, Bahari H, Isemaail S et al (2009) Nutritional composition, anti-oxidant ability and flavonoid content of Tinospora crispa stem. Adv Nat Appl Sci 3:88–95
Anulukanapakorn K, Pancharoen O, Bansiddhi J (1999) Hypoglycemic effect of Tinospora crispa (Linn.) Mier ex Hook f. & Thoms (Menispermaceae) in rats. Bull Dep Med Sci 41:231–243
Arcueno RO, Retumban JLB, Echano JE, Guerrero JJG (2015) Wound healing potential of Tinospora crispa (Willd.) Miers [Menispermaceae] stem on diabetic mice. J Med Plants Stud 3:106–109
Arundina I, Diyatri I, Budhy TI, Jit FY (2017) The effect of brotowali stem extract (Tinospora crispa) towards increasing number of lymphocytes in the healing process of traumatic ulcer on diabetic wistar rat. J Int Dent Med Res 10:975–980
Asif Iqbal CM, Gunjan M, Chellappan DK (2012) Antimicrobial activity of Tinospora crispa root extracts. Int J Res Ayurveda Pharm 3:417–419
Aziz NA, Abdullah NS, Harun A et al (2021) The molluscicidal effect of the stem extracts of Tinospora crispa in controlling the golden apple snail Pomacea canaliculata. J Teknologi 83(6):35–40. https://doi.org/10.11113/jurnalteknologi.v83.16779
Bakhari NA, Fadzillah SNAD, Isa N (2013) Chemical constituents of the insecticidal active extract of Tinospora crispa. Sci Res J 10:23–35
Bakhari NA, Isa N (2010) The effect of Tinospora crispa extracts on the contraction of isolated atrium and aorta of rats. Esteem Acad J 6:141–150
Bakhari NA, Sadikun A, Choon TS et al (2005) Aporphine alkaloids isolated from the cardiovascular active fraction of Tinospora crispa. Malays J Sci 24:161–165
Bao H, Zhang Q, Ye Y, Lin L (2017) Naturally occurring furanoditerpenoids: distribution, chemistry and their pharmacological activities. Phytochem Rev 16:235–270. https://doi.org/10.1007/s11101-016-9472-2
Bertani S, Bourdy G, Landau I et al (2005) Evaluation of French Guiana traditional antimalarial remedies. J Ethnopharmacol 98:45–54. https://doi.org/10.1016/j.jep.2004.12.020
Bisset NG, Nwaiwu J (1983) Quaternary alkaloids of Tinospora species. Planta Med 48:275–279. https://doi.org/10.1055/s-2007-969933
Bunluepuech K, Tewtrakul S (2009) Anti-HIV-1 integrase activity of Thai Medicinal Plants. Songklanakarin J Sci Technol 31:289–292
Cachet X, Langrand J, Riffault-Valois L et al (2018) Clerodane furanoditerpenoids as the probable cause of toxic hepatitis induced by Tinospora crispa. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-31815-6
Cavin A, Hostettmann K, Dyatmyko W, Potterat O (1998) Anti-oxidant and lipophilic constituents of Tinospora crispa. Planta Med 64:393–396. https://doi.org/10.1055/s-2006-957466
Chang CC, Ho SL, Lee SS (2015) Acylated glucosylflavones as α-glucosidase inhibitors from Tinospora crispa leaf. Bioorg Med Chem 23:3388–3396. https://doi.org/10.1016/j.bmc.2015.04.053
Chi S, She G, Han D et al (2016) Genus Tinospora: ethnopharmacology, phytochemistry, and pharmacology. Evid Based Complement Altern Med 2016:1–32. https://doi.org/10.1155/2016/9232593
Choudhary MI, Ismail M, Ali Z et al (2010a) Alkaloidal constituents of Tinospora crispa. Nat Prod Commun 5:1747–1750. https://doi.org/10.1177/1934578X1000501109
Choudhary MI, Ismail M, Shaari K et al (2010b) cis-Clerodane-type furanoditerpenoids from Tinospora crispa. J Nat Prod 73:541–547. https://doi.org/10.1021/np900551u
Chung SY (2011) Studies on the constituents of the dry stem of Tinospora crispa (Lour.) Merr. Masters dissertation, China Medical University
Da-Cunha EVL, Fechine IM, Guedes DN et al (2005) Protoberberine alkaloids. Alkaloids Chem Biol 62:1–75. https://doi.org/10.1016/S1099-4831(05)62001-9
Dapar MLG (2020) Tinospora crispa (L.) Hook. F. & Thomson Menispermaceae. Ethnobot Mt Reg Southeast Asia. https://doi.org/10.1007/978-3-030-14116-5_97-1
Dapar MLG, Alejandro GJD, Meve U, Liede-Schumann S (2020) Quantitative ethnopharmacological documentation and molecular confirmation of medicinal plants used by the Manobo tribe of Agusan del Sur, Philippines. J Ethnobiol Ethnomed 16:1–60. https://doi.org/10.1186/s13002-020-00363-7
Dweck AC, Cavin JP (2006) Andawali (Tinospora cripa): a review. Pers Care Mag 7:33–39
Elkington BG, Phiapalath P, Sydara K et al (2014) Assessment of the importance of medicinal plants among communities around Khiat Ngong of Southern Laos. J Environ Biol 35:607–615
Erza NN, Zulfa F, Setyaningsih Y (2020) Antifungal Test of the Ethanol Extract of Brotowali Stem (Tinospora crispa) on the Growth of Trichophyton Rubrum in vitro. In: The 7th international conference on public health 2020, Solo, Indonesia. https://doi.org/10.26911/the7thicph.05.02
Firdausa S, Cho MM, Maung KM et al (2020) The blood glucose lowering effect of Malaysian Tinospora crispa in rats. J Nat 20:20–23. https://doi.org/10.24815/jn.v20i1.15907
Firdausa S, Cho MM, Maung KM, et al (2018) The effect of Tinospora crispa on anti-oxidant status in streptozotocin induced diabetic rats. In: The 8th AIC: health and life sciences 2018, Syiah Kuala University
Forman LL (1981) A revision of Tinospora (Menispermaceae) in Asia to Australia and the Pacific: the Menispermaceae of Malesia and adjacent areas: X. Kew Bull. https://doi.org/10.2307/4113613
Gao Y, Niu YF, Wang F et al (2016) Clerodane diterpenoids with anti-hyperglycemic activity from Tinospora crispa. Nat Products Bioprospect 6:247–255. https://doi.org/10.1007/s13659-016-0109-3
Ge YC, Wang KW (2018) New analogues of aporphine alkaloids. Mini Rev Med Chem 18:1590–1602. https://doi.org/10.2174/1389557518666180423151426
Gimlette JD, Burkill IH (1930) The medical book of Malayan medicine. Botanic Gardens, Singapore
Global Biodiversity Information Facility (2021) Tinospora crispa (L.) Miers ex Hook. fil. & Thomson in GBIF Secretariat. https://doi.org/10.15468/39omei. Accessed 2 June 2021
Grenand P, Moretti C, Jacquemin H, Prévost MF (2004) Pharmacopées traditionnelles en Guyane: créoles, wayãpi, palikur. IRD (édn.)
Hamid HA (2013) Characterisation and Biological Activities of Tinospora crispa (Menispermaceae) Extract with Emphasis on Alkaloids. Doctorate Thesis. Universiti Malaysia Pahang
Hamid HA, Mutazah R, Yahya IH, Zeyohannes SS (2021) Anti-oxidant and antimicrobial screening of isolated alkaloids from Tinospora crispa. Mater Sci Forum 1025:163–168. https://doi.org/10.4028/www.scientific.net/MSF.1025.163
Hamid HA, Yusoff MM, Liu M, Karim MR (2015) α-Glucosidase and α-amylase inhibitory constituents of Tinospora crispa: Isolation and chemical profile confirmation by ultra-high performance liquid chromatography-quadrupole time-of-flight/mass spectrometry. J Funct Foods 16:74–80. https://doi.org/10.1016/j.jff.2015.04.011
Haque AM, Islam ASM, Shahriar M (2011) Antimicrobial, cytotoxicity and anti-oxidant activity of Tinospora crispa. J Pharm Biomed Sci 12:1–4
Haque MA, Jantan I, Bukhari SNA (2017) Tinospora species: an overview of their modulating effects on the immune system. J Ethnopharmacol 207:67–85. https://doi.org/10.1016/j.jep.2017.06.013
Haque MA, Jantan I, Harikrishnan H, Abdul Wahab SM (2018) Magnoflorine enhances LPS-activated pro-inflammatory responses via MyD88-dependent pathways in U937 macrophages. Planta Med 84:1255–1264. https://doi.org/10.1055/a-0637-9936
Haque MA, Jantan I, Harikrishnan H, Ahmad W (2020) Standardized ethanol extract of Tinospora crispa upregulates pro-inflammatory mediators release in LPS-primed U937 human macrophages through stimulation of MAPK, NF-κB and PI3K-Akt signaling networks. BMC Complement Med Ther 20:1–13. https://doi.org/10.1186/s12906-020-03039-7
Harwoko H, Warsinah W (2020) Phytochemical analysis and evaluation of purified extract of Tinospora crispa stem for in vivo antihyperuricemic effect. J Rep Pharm Sci 9:46–51. https://doi.org/10.4103/jrptps.JRPTPS_45_19
Hartini YS, Setyaningsih D, Chrismaurin F et al (2022) Brotowali (Tinospora crispa L.) stem extract activity as an α-Amylase enzyme inhibitor. Pharm Educ 22(2):275–277. https://doi.org/10.46542/pe.2022.222.275277
Hassani MMRS, Ahmad A, Asmawi MZ, Mahmud R (2016) Preliminary investigation of normoglycemic, anti-hyperglycemic and dyslipidemic activities of different extracts of Tinospora crispa on diabetic rat. Acta Pol Pharm 73:129–134
Higashino H, Suzuki A, Tanaka Y, Pootakham K (1992) Inhibitory effects of Siamese Tinospora crispa extracts on the carrageenin-induced foot pad edema in rats (the 1st report). Nihon Yakurigaku Zasshi 100:339–344. https://doi.org/10.1254/fpj.100.339
Hipol RLB, Cariaga MFNM, Hipol RM (2012) Anti-inflammatory activities of the aqueous extract of the stem of Tinospora crispa (Family Menispermaceae). J Nat Stud 11:88–95
Hossen F, Ahasan R, Haque MR et al (2016) Crispene A, B, C and D, four new clerodane type furanoid diterpenes from Tinospora crispa (L.). Pharmacogn Mag 12:S37. https://doi.org/10.4103/0973-1296.176116
Hout S, Chea A, Bun SS et al (2006) Screening of selected indigenous plants of Cambodia for antiplasmodial activity. J Ethnopharmacol 107:12–18. https://doi.org/10.1016/j.jep.2006.01.028
Ibahim MJ, Wan-Nor I’zzah WMZ, Narimah AHH et al (2011) Anti-proliperative and anti-oxidant effects of Tinospora crispa (Batawali). Biomed Res 22:57–62
Ihwan I, Rifa’i M, Fitri LE (2014) Antiplasmodial test of Tinospora crispa stem extract against Plasmodium falciparum 3D7 strain in vitro. J Kedokt Brawijaya 28:91–96
Imphanban K, Kongkathip N, Dhumma-upakorn P et al (2009) Synthesis of N-formylnornuciferine with cardiotonic activity. Agric Nat Resour 43:738–744
Irianti T, Puspitasari A, Suryani E (2011) The activity of radical scavenging of 2,2-diphenyl-1-pycrilhydrazil by ethanolic extracts of (Tinospora crispa (L.) Miers) stem and its fractions. Tradit Med J 20:180131
Isa N, Satar SA, Bakhari NA et al (2013) The effect of Tinospora crispa extract against Spodoptera exigua on Spinacia oleracea. Malays J Fundam Appl Sci. https://doi.org/10.11113/mjfas.v9n2.93
Islam F, Jahan FI, Seraj S et al (2011) Variations in diseases and medicinal plant selection among folk medicinal practitioners: a case study in Jessore district, Bangladesh. Am Eurasian J Sustain Agric 5:282–291
Islam MA, Amin MR, Mahmud ZA (2014) Evaluation of analgesic and antimicrobial activity of different fractions of crude methanol extract of Tinospora crispa stem. Int J Pharm Sci Rev 5:16–21
Islam MA, Mahmud ZA, Rahman SMA et al (2013) Evaluation of thrombolytic activity and brine shrimp lethality bioassay of methanol extract of stems of Tinospora crispa. Int J Pharm Sci Res 4:1148–1153
Ismail M, Choudhary MI (2016) Compounds isolated from Tinospora crispa. Chem Nat Compd 52:1151–1153. https://doi.org/10.1007/s10600-016-1892-0
Izzati N, Fitri LE, Dalhar M (2016) Artesunate-tinospora combination treatment decreases nuclear factor kappa-B and intercellular adhesion molecule-1 expression in mouse malarial models. Univ Med 35:222–228. https://doi.org/10.18051/UnivMed.2016.v35.222-228
Jantan I, Harun NH, Septama AW et al (2011) Inhibition of chemiluminescence and chemotactic activity of phagocytes in vitro by the extracts of selected medicinal plants. J Nat Med 65:400–405. https://doi.org/10.1007/s11418-010-0492-8
Jiraungkoorskul W (2019) Efficiency of Tinospora crispa against Culex quinquefasciatus larva. Environ Sci Pollut Res 26:14712–14716. https://doi.org/10.1007/s11356-018-2429-9
Kadir FA, Othman F, Abdulla MA et al (2011) Effect of Tinospora crispa on thioacetamide-induced liver cirrhosis in rats. Indian J Pharmacol 43:64. https://doi.org/10.4103/0253-7613.75673
Kadir MF, Sayeed MSB, Setu NI et al (2014) Ethnopharmacological survey of medicinal plants used by traditional health practitioners in Thanchi, Bandarban Hill Tracts, Bangladesh. J Ethnopharmacol 155:495–508. https://doi.org/10.1016/j.jep.2014.05.043
Kamarazaman IS, Amom ZH, Ali RM et al (2012) Protective effects of Tinospora crispa extracts on H2O2-induced oxidative stress and TNF-α-induced inflammation on human umbilical vein endothelial cells (HUVECs). J Med Plants Res 6:3013–3021. https://doi.org/10.5897/JMPR11.1510
Klangjareonchai T, Roongpisuthipong C (2012) The effect of Tinospora crispa on serum glucose and insulin levels in patients with type 2 diabetes mellitus. J Biomed Biotechnol 2012:808762. https://doi.org/10.1155/2012/808762
Koay YC, Koay F (2013) A review of the secondary metabolites and biological activities of Tinospora crispa (Menispermaceae). Trop J Pharm Res 12:641–649. https://doi.org/10.4314/tjpr.v12i4.30
Kongkathip N, Dhumma-upakorn P, Kongkathip B et al (2002) Study on cardiac contractility of cycloeucalenol and cycloeucalenone isolated from Tinospora crispa. J Ethnopharmacol 83:95–99. https://doi.org/10.1016/S0378-8741(02)00210-6
Kongsaktrakoon B, Temsiririrkkul R, Suvitayavat W et al (1984) The antipyretic effect of Tinospora crispa Mier ex Hook. f. & Thoms. Mahidol Univ J Pharm Sci 21:1–6
Kraithep S, Oungbho K, Tewtrakul S (2008) Anti-allergic activity of Thai medicinal plants used in longevity formulation. Songklanakarin J Sci Technol 30:621–625
Lam SH, Ruan CT, Hsieh PH et al (2012) Hypoglycemic diterpenoids from Tinospora crispa. J Nat Prod 75:153–159. https://doi.org/10.1021/np200692v
Langrand J, Regnault H, Cachet X et al (2014) Toxic hepatitis induced by a herbal medicine: Tinospora crispa. Phytomedicine 21:1120–1123. https://doi.org/10.1016/j.phymed.2014.04.031
Lee DS, Keo S, Cheng SK et al (2017) Protective effects of Cambodian medicinal plants on tert-butyl hydroperoxide-induced hepatotoxicity via Nrf2-mediated heme oxygenase-1. Mol Med Rep 15:451–459. https://doi.org/10.3892/mmr.2016.6011
Lee WC, Mahmud R, Perumal S et al (2020) In vivo antimalarial potential of Tinospora crispa miers in mice and identification of the bioactive compound. Pharmacogn Mag 16:76–82. https://doi.org/10.4103/pm.pm_10_19
Lestari Y, Velina Y, Rahminiwati M (2015) Metabolites activity of endophytic Streptomyces SP. IPBCC. B. 15.1539 from Tinospora crispa L. Miers: α-glucosidase inhibitor and anti-hyperglycemic in mice. Int J Pharm Pharm Sci 7:235–239
Li S, Long C, Liu F et al (2006) Herbs for medicinal baths among the traditional Yao communities of China. J Ethnopharmacol 108:59–67. https://doi.org/10.1016/j.jep.2006.04.014
Lin YH (2009) Studies on the Chemical Constituents of Tinospora crispa and Synthesis of the Analogous of Penta-O-Galloyl-d-Glucopyranose. Masters Dissertation. China Medical University
Lokman FE, Gu HF, Mohamud WNW et al (2013) Antidiabetic effect of oral borapetol B compound, isolated from the plant Tinospora crispa, by stimulating insulin release. Evid Based Complement Altern Med. https://doi.org/10.1155/2013/727602
Longuefosse JL, Nossin E (1996) Medical ethnobotany survey in Martinique. J Ethnopharmacol 53:117–142. https://doi.org/10.1016/0378-8741(96)01425-0
Mackeen MM, Khan MN, Samadi Z, Lajis NH (2000) Brine shrimp toxicity of fractionated extracts of Malaysian medicinal plants. Nat Prod Sci 6:131–134
Mahalle D, Gupta A (2021) Estimation of total phenol, flavonoids and alkaloid content and evaluation of anti-oxidant and antimicrobial activity of Tinospora crispa leaves and flower extracts. J Adv Sci Res 12:208–212
Malik MM (2015) The potential of brotowali stem extract (Tinospora crispa) as an alternative antimalarial drug. J Major 4:45–49
Mantaj J, Rahman SMA, Bokshi B et al (2015) Crispene E, a cis-clerodane diterpene inhibits STAT3 dimerization in breast cancer cells. Org Biomol Chem 13:3882–3886. https://doi.org/10.1039/C5OB00052A
Marlina M, Sudding S, Salempa P (2017) Isolasi Dan Identifikasi Senyawa Metabolit Sekunder Ekstrak n-Heksan Batang Brotowali (Tinospora crispa Linn). Chem J Ilm Kim Dan Pendidik Kim 16:77–84. https://doi.org/10.35580/chemica.v16i2.4561
Merwanta S, Pameswari P, Maria O (2019) Uji aktivitas sistem saraf pusat decocta batang brotowali (Tinospora crispa (L.) Hook. F. & Thomson) pada mencit putih jantan. J Acad Pharm Pray 4:43–56
Mohamad S, Zin NM, Wahab HA et al (2011) Antituberculosis potential of some ethnobotanically selected Malaysian plants. J Ethnopharmacol 133:1021–1026. https://doi.org/10.1016/j.jep.2010.11.037
Murnigsih T, Subeki MH et al (2005) Evaluation of the inhibitory activities of the extracts of Indonesian traditional medicinal plants against Plasmodium falciparum and Babesia gibsoni. J Vet Med Sci 67:829–831. https://doi.org/10.1292/jvms.67.829
Musa WAJ, Duengo S, Kilo AK (2019) Campesterol compound from methanol fraction of Brotowali (Tinospora crispa) Stem Bark. In: National seminar on chemistry 2019 (SNK-19). Atlantis Press, pp 243–245
Muslimin L, Hasrah NR, Jamaludin AW (2018) Sensitivity test of bacterium (Escherichia coli) against Brotowali’s extract (Tinospora crispa). Adv Heal Sci Res 5:105–108
Mutiah R, Azizah LN, Annisa R, Listyana A (2019) Profile of anticancer activities of brotowali (Tinospora crispa L.) plants of various regions in East Jawa. J Pharm Sci Community 16:68–77. https://doi.org/10.24071/jpsc.002020
Nguyen TP, Bang LH, Nguyen TTB, Nguyen TP (2020) Bioactive compounds analysis and anti-oxidant activities of Tinospora crispa miers stem extract. Sci J Tra Vinh Univ 1:58–69. https://doi.org/10.35382/18594816.1.40.2020.617
Niljan J, Jaihan U, Srichairatanakool S et al (2014) Antimalarial activity of stem extract of Tinospora crispa against Plasmodium berghei infection in mice. J Heal Res 28:199–204
Noipha K, Ratanachaiyavong S, Purintrapiban J et al (2011) Effect of Tinospora crispa on glucose uptake in skeletal muscle: role of glucose transporter 1 expression and extracellular signal-regulated kinase1/2 activation. Asian Biomed 5:361–369. https://doi.org/10.5372/1905-7415.0503.047
Noman MAA, Hossain T, Ahsan M et al (2018) Crispenes F and G, cis-clerodane furanoditerpenoids from Tinospora crispa, inhibit STAT3 dimerization. J Nat Prod 81:236–242. https://doi.org/10.1021/acs.jnatprod.7b00377
Noor H, Ashcroft SJH (1998a) Pharmacological characterisation of the antihyperglycaemic properties of Tinospora crispa extract. J Ethnopharmacol 62:7–13. https://doi.org/10.1016/S0378-8741(98)00008-7
Noor H, Ashcroft SJH (1998b) Insulinotropic activity of Tinospora crispa extract: effect on ß-cell Ca2+ handling. Phyther Res 12:98–102. https://doi.org/10.1002/(SICI)1099-1573(199803)12:2%3c98::AID-PTR195%3e3.0.CO;2-F
Noor H, Hammonds P, Sutton R, Ashcroft SJH (1989) The hypoglycaemic and insulinotropic activity of Tinospora crispa: studies with human and rat islets and HIT-T15 B cells. Diabetologia 32:354–359. https://doi.org/10.1007/BF00277258
Nor Aziyah B, Norain I, Nor Aimi AW et al (2014) Biopesticidal effect of Tinospora crispa extracts against flea beetles, Phyliotera sinuata ateph. Res J Biotechnol 9:1–5
Nutham N, Sakulmettatham S, Klongthalay S et al (2015) Protective effects of Tinospora crispa stem extract on renal damage and hemolysis during Plasmodium berghei infection in mice. J Pathog. https://doi.org/10.1155/2015/738608
Pachaly P, Adnan AZ, Will G (1992) NMR-Assignments of N-Acylaporphine Alkaloids from Tinospora crispa. Planta Med 58:184–187. https://doi.org/10.1055/s-2006-961425
Pal JK, Singh A, Rawani A, Chandra G (2016) Larvicidal activity of Tinospora crispa (Menispermaceae) fruit extract against filarial vector Culex quinquefasciatus. J Mosq Res. https://doi.org/10.5376/jmr.2016.06.0035
Parveen A, Alhusban M, Fantoukh OI et al (2022) Probing PXR activation and modulation of CYP3A4 by Tinospora crispa and Tinospora sinensis. J Ethnopharmacol 291:115159. https://doi.org/10.1016/j.jep.2022.115159
Parveen A, Huang Y, Fantoukh O et al (2019) Rearranged clerodane diterpenoid from Tinospora crispa. Nat Prod Res 35:369–376. https://doi.org/10.1080/14786419.2019.1633648
Parveen A, Maqbool MT, Wang YH et al (2020) Evaluation of the hepatotoxic potential of Tinospora crispa and its isolated borapetosides B, C and F in a murine model. Planta Med 86:489–495. https://doi.org/10.1055/a-1127-7503
Patel N, Patel S, Krishnamurthy R (2013) Indian Tinospora species: natural immunomodulators and therapeutic agents. Int J Pharm Biol Chem Sci 2:1–9
Pathak AK, Jain DC, Sharma RP (1995) Chemistry and biological activities of the genera Tinospora. Int J Pharmacogn 33:277–287. https://doi.org/10.3109/13880209509065379
Paudel HR, Bhattarai S, Kunwar RM (2020) Tinospora crispa (L.) Hook. f. & Thomson (Menispermaceae): a new record for Nepal. Feddes Repert 131:159–162. https://doi.org/10.1002/fedr.202000001
Phienwej H, Swasdichira I, Amnuoypol S et al (2015) Tinospora crispa extract inhibits MMP-13 and migration of head and neck squamous cell carcinoma cell lines. Asian Pac J Trop Biomed 5:738–743. https://doi.org/10.1016/j.apjtb.2015.07.001
Praman S, Mulvany MJ, Allenbach Y et al (2011) Effects of an n-butanol extract from the stem of Tinospora crispa on blood pressure and heart rate in anesthetized rats. J Ethnopharmacol 133:675–686. https://doi.org/10.1016/j.jep.2010.10.052
Praman S, Mulvany MJ, Williams DE et al (2013) Crude extract and purified components isolated from the stems of Tinospora crispa exhibit positive inotropic effects on the isolated left atrium of rats. J Ethnopharmacol 149:123–132. https://doi.org/10.1016/j.jep.2013.06.010
Praman S, Mulvany MJ, Williams DE et al (2012) Hypotensive and cardio-chronotropic constituents of Tinospora crispa and mechanisms of action on the cardiovascular system in anesthetized rats. J Ethnopharmacol 140:166–178. https://doi.org/10.1016/j.jep.2012.01.006
Pramitasari PD, Pujiyanto S, Suprihadi A (2017) Aktivitas inhibitor α-amilase isolat khamir endofit dari tumbuhan brotowali (Tinospora crispa L.). J Akad Biol 6:76–84
Proença C, Freitas M, Ribeiro D et al (2017) α-Glucosidase inhibition by flavonoids: an in vitro and in silico structure-activity relationship study. J Enzyme Inhib Med Chem 32:1216–1228. https://doi.org/10.1080/14756366.2017.1368503
Proença C, Freitas M, Ribeiro D et al (2019) Evaluation of a flavonoids library for inhibition of pancreatic α-amylase towards a structure-activity relationship. J Enzyme Inhib Med Chem 34:577–588. https://doi.org/10.1080/14756366.2018.1558221
Quisumbing E (1951) Medicinal plants of the Philippines. Dep Agric Commer Philipp Islands Tech Bull 16:1234
Rahman M, Rahman MH, Chowdhury TA (2020) Phytochemical and biological activity studies of Tinospora crispa stem. Dhaka Univ J Sci 68:167–170
Rahman NNNA, Furuta T, Kojima S et al (1999) Antimalarial activity of extracts of Malaysian medicinal plants. J Ethnopharmacol 64:249–254
Rahmatullah M, Azam MNK, Rahman MM et al (2011) A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am Eurasian J Sustain Agric 5:350–357
Rahmatullah M, Noman A, Hossan MS et al (2009) A survey of medicinal plants in two areas of Dinajpur district, Bangladesh including plants which can be used as functional foods. Am Eurasian J Sustain Agric 3:862–876
Rakib A, Ahmed S, Islam MA et al (2020a) Antipyretic and hepatoprotective potential of Tinospora crispa and investigation of possible lead compounds through in silico approaches. Food Sci Nutr 8:547–556. https://doi.org/10.1002/fsn3.1339
Rakib A, Ahmed S, Islam MA et al (2020b) Pharmacological studies on the antinociceptive, anxiolytic and antidepressant activity of Tinospora crispa. Phyther Res 34:2978–2984. https://doi.org/10.1002/ptr.6725
Rakib A, Paul A, Chy MNU et al (2020c) Biochemical and computational approach of selected phytocompounds from Tinospora crispa in the management of COVID-19. Molecules 25:3936. https://doi.org/10.3390/molecules25173936
Ramadani AP, Paloque L, Belda H et al (2018) Antiprotozoal properties of Indonesian medicinal plant extracts. J Herb Med 11:46–52. https://doi.org/10.1016/j.hermed.2017.06.004
Rattanajarasroj S, Pinthong T, Warachit P et al (2004) Effect on blood sugar level and safety of Tinospora crispa in healthy Thai volunteers. Bull Depart Med Sci 46:72–88
Roestamadji RI, Arundina I, Diyatri I et al (2017) Brotowali extract (Tinospora crispa) for oral traumatic ulcer in diabetes mellitus wistar rat. J Int Dent Med Res 10:991–996
Roosita K, Kusharto CM, Sekiyama M et al (2008) Medicinal plants used by the villagers of a Sundanese community in West Java, Indonesia. J Ethnopharmacol 115:72–81. https://doi.org/10.1016/j.jep.2007.09.010
Ruan CT, Lam SH, Chi TC et al (2012) Borapetoside C from Tinospora crispa improves insulin sensitivity in diabetic mice. Phytomedicine 19:719–724. https://doi.org/10.1016/j.phymed.2012.03.009
Rungruang T, Boonmars T (2009) In vivo antiparasitic activity of the Thai traditional medicine plant Tinospora crispa against Plasmodium yoelii. Southeast Asian J Trop Med Public Health 40:898–900
Sangsuwan C, Udompanthurak S, Vannasaeng S, Thamlikitkul V (2004) Randomized controlled trial of Tinospora crispa for additional therapy in patients with type 2 diabetes mellitus. J Med Assoc Thail 87:543–546
Shah ZB, Hasan MKBN, Kadir KKBA et al (2021) The effects of Tinospora crispa aqueous extract on C-reactive protein level and development of atherosclerotic plaques. Int J Progress Sci Technol 26:25–35. https://doi.org/10.52155/ijpsat.v26.1.2931
Sharif AA, Unyah NZ, Nordin N et al (2019) Susceptibility of Toxoplasma gondii to ethanolic extract of Tinospora crispa in vero cells. Evid Based Complement Altern Med. https://doi.org/10.1155/2019/2916547
Somsak V, Kittitorn J, Chachiyo S et al (2015) Effect of aqueous crude extract of Tinospora crispa on Plasmodium berghei induced liver damage in mice. Malar Chemother Control Elimin 4:127. https://doi.org/10.4172/MCE.1000127
Srithi K, Balslev H, Wangpakapattanawong P et al (2009) Medicinal plant knowledge and its erosion among the Mien (Yao) in northern Thailand. J Ethnopharmacol 123:335–342. https://doi.org/10.1016/j.jep.2009.02.035
Sriyapai C, Dhumma-upakorn R, Sangwatanaroj S et al (2009) Hypoglycemic effect of Tinospora crispa dry powder in outpatients with metabolic syndrome at King Chulalongkorn Memorial Hospital. J Health Res 23(3):125–133
Subehan UT, Iwata H et al (2006) Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J Ethnopharmacol 105:449–455. https://doi.org/10.1016/j.jep.2005.12.001
Suchantabud A, Talubmook C, Chomko S, Narkkong N (2008) Some hematological values and ultrastructure of blood cells in Piper sarmentosum Roxb. and Tinospora crispa Miers ex Hook. F & Thoms. treated diabetic rats. J Microsc Soc Thail 22:65–70
Sulaiman MR, Zakaria ZA, Lihan R (2008) Antinociceptive and anti-inflammatory activities of Tinospora crispa in various Animal models. Int J Trop Med 3:66–69
Sunthikawinsakul A (2005) Isolation of Active Constituents with Cardiotonic and Anti-HIV-1 Activities from Tinospora crispa Miers (Menispermaceae). Doctorate Thesis. Kasetsart University, Bangkok
Susanti L, Widodo S, Bahri S, Indriasari W (2016) Formulation test of brotowali stem extract (Tinospora Crispa L. Miers) combination zeolite against Staphylococcus Aureus bacteria and Pseudomonas Aeruginosa. Inov Pembang J Kelitbangan 4:234–243
Suvannarat S, Junmatong C, Thongchai W, et al (2015) Larvicidal activity of Tinospora crispa (Menispermaceae) extract against larvae of diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). In: 7th international science, social sciences, engineering and energy conference
Syarifah VB, Rafi M, Wahyuni WT (2017) High perfomance liquid chromatography fingerprint analysis for quality control of brotowali (Tinospora crispa). J Phys Conf Ser 835:12016
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of α-glucosidase and α-amylase by flavonoids. J Nutr Sci Vitaminol 52:149–153. https://doi.org/10.3177/jnsv.52.149
Tambunan RM, Kartiningsih YD, Pithaloka LD (2013) α-Glucosidase inhibitory activity of ethanolic extract of brotowali stem (Tinospora crispa Miers.) in vitro. In: First international conference on pharmaceutical nanotechnology/nanomedicine, Jakarta, Indonesia
Tarukbua YSF, Queljoe ED, Bodhi W (2018) Skrining fitokimia dan uji toksisitas ekstrak etanol daun Brotowali (Tinospora crispa (L.) Hook F. & T) dengan metode Brine Shrimp Lethality Test (BSLT). Pharmacon. https://doi.org/10.35799/pha.7.2018.20600
The Plant List (2013) A working list of all plant species. Version 1.1. http://www.theplantlist.org/tpl/record/tro-50053822. Accessed 02 May 2021
Thomas A, Rajesh EK, Kumar DS (2016) The significance of Tinospora crispa in treatment of diabetes mellitus. Phyther Res 30:357–366. https://doi.org/10.1002/ptr.5559
Tin TP, Lam CK, Hussin AH (2005) Molecular mechanism of Tinospora crispa on herb–drug interaction in rat hepatocytes. Malaysian J Sci 24:229–232
Torre GLTD, Ponsaran KMG, De Guzman ALDP et al (2017) Safety, efficacy, and physicochemical characterization of Tinospora crispa ointment: a community-based formulation against Pediculus humanus capitis. Korean J Parasitol 55:409. https://doi.org/10.3347/kjp.2017.55.4.409
Tran QL, Tezuka Y, Ueda J et al (2003) In vitro antiplasmodial activity of antimalarial medicinal plants used in Vietnamese traditional medicine. J Ethnopharmacol 86:249–252. https://doi.org/10.1016/S0378-8741(03)00045-X
Triastuti A (2010) Antiangiogenic effect of the chloroform extract of Tinospora crispa (L.) miers stem in the chick embryo chorioallantoic membrane (CAM) induced by bFGF. Eksakta J Sci Data Anal 11:1–1
Tungpradit R, Sinchaikul S, Phutrakul S et al (2010) Anti-cancer compound screening and isolation: Coscinium fenestratum, Tinospora crispa and Tinospora cordifolia. Chiang Mai J Sci 37:476–488
Umi Kalsom Y, Noor H (1995) Flavone O-Glycosides from Tinospora Crispa. Fitoter 66:280
Usia T, Iwata H, Hiratsuka A et al (2006) CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine 13:67–73. https://doi.org/10.1016/j.phymed.2004.06.022
Vigneron M, Deparis X, Deharo E, Bourdy G (2005) Antimalarial remedies in French Guiana: a knowledge attitudes and practices study. J Ethnopharmacol 98:351–360. https://doi.org/10.1016/j.jep.2005.01.049
Vikneswaran M, Chan KL (2005) Xanthine oxidase inhibitory activity of some Malaysian plants. Malays J Sci 24:263–266
Warsinah W, Baroroh HN, Harwoko H (2020) Phytochemical analysis and anti-oxidant activity of Brotowali (Tinospora crispa L. Mier) stem. Molekul 15:73–78. https://doi.org/10.20884/1.jm.2020.15.2.533
Widodo WT, Widyarti S, Sumitro SB, Santjojo DH (2021) In silico study of tyramine-Fe complex in Brotowali (Tinospora crispa) as anti-inflammatory. In: 11th annual international conference on industrial engineering and operations management, Singapore
World Flora Online (2021) Tinospora crispa (L.) Hook. f. & Thomson. http://www.worldfloraonline.org/taxon/wfo-0001228223. Accessed 02 May 2021
Wulandari Y, Bestari RS (2016) Uji efek antipiretik infusa batang Brotowali (Tinospora crispa (L.) Miers) pada tikus putih jantan galur wistar yang diinduksi vaksin dpt. Thesis. Universitas Muhammadiyah Surakarta
Xu Y, Niu Y, Gao Y et al (2017) Borapetoside E, a clerodane diterpenoid extracted from Tinospora crispa, improves hyperglycemia and hyperlipidemia in high-fat-diet-induced type 2 diabetes mice. J Nat Prod 80:2319–2327. https://doi.org/10.1021/acs.jnatprod.7b00365
Yusoff M, Hamid H, Houghton P (2014) Anticholinesterase inhibitory activity of quaternary alkaloids from Tinospora crispa. Molecules 19:1201–1211. https://doi.org/10.3390/molecules19011201
Yusriani Y, Ermawati E, Dewi R (2018) Uji daya hambat krim ekstrak batang brotowali (Tinospora crispa L.) terhadap Propionibacterium acnes. J Kesehat Yamasi 2:1–6
Zakaria ZA, Mat Jais AM, Somchit MN et al (2006) The in vitro antibacterial activity of Tinospora crispa extracts. J Biol Sci 6:398–401
Zakaria ZA, Zakaria ML, Amom Z, Desa MNM (2011) Antimicrobial activity of the aqueous extract of selected Malaysian herbs. Afr J Microbiol Res 5:5379–5383. https://doi.org/10.5897/AJMR11.874
Zamree MS, Ihsan SK, Khairul KAK et al (2015) Lipid lowering and anti-atherosclerotic properties of Tinospora crispa aqueous extract on high-cholesterol diet-induced hyperlipidemic rabbits. Afr J Biotechnol 14:2604–2610. https://doi.org/10.5897/AJB2015.14787
Zaridah MZ, Idid SZ, Omar AW, Khozirah S (2001) In vitro antifilarial effects of three plant species against adult worms of subperiodic Brugia malayi. J Ethnopharmacol 78:79–84. https://doi.org/10.1016/S0378-8741(01)00286-0
Zhu J, Chen C, Zhang B, Huang Q (2020) The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit Rev Food Sci Nutr 60:695–708. https://doi.org/10.1080/10408398.2018.1548428
Zin NH, Ahmad NF, Bunnori NM et al (2016) Antibacterial activities of Protein Extracts From Andrographis paniculata, Tinospora crispa and Centella asiatica. IIUM Med J Malays. https://doi.org/10.31436/imjm.v15i1.1370
Zulkefli HN, Mohamad J, Abidin NZ (2013) Anti-oxidant activity of methanol extract of Tinospora crispa and Tabernaemontana corymbosa. Sains Malays 42:697–706
Zulkhairi A Jr, Abdah MA, Kamal NHM et al (2008) Biological properties of Tinospora crispa (Akar Patawali) and its antiproliferative activities on selected human cancer cell lines. Malays J Nutr 14:173–187
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haque, E., Bari, M.S., Khandokar, L. et al. An updated and comprehensive review on the ethnomedicinal uses, phytochemistry, pharmacological activity and toxicological profile of Tinospora crispa (L.) Hook. f. & Thomson. Phytochem Rev 22, 211–273 (2023). https://doi.org/10.1007/s11101-022-09843-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-022-09843-y