Abstract
A series of novel 4β-triazole-podophyllotoxin glycosides were synthesized by utilizing the Click reaction. Evaluation of cytotoxicity against a panel of five human cancer cell lines (HL-60, SMMC-7721, A-549, MCF-7, SW480) using MTT assay shows that most of these compounds show weak cytotoxicity. It was observed that compound 16 shows the highest activity with IC50 values ranging from 2.85 to 7.28 μM, which is more potent than the control drugs etoposide and cisplatin against four of five cancer cell lines tested. Compound 16 is characterized with an α-d-galactosyl residue directly linked to the triazole ring and a 4′-OH group on the E ring of the podophyllotoxin scaffold. HPLC investigation of representative compound indicates that incorporation of a sugar moiety seems to improve the chemical stability of the podophyllotoxin scaffold.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
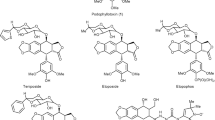
Podophyllotoxin (1, Fig. 1), a naturally occurring cyclolignan, which is mainly isolated from the roots of Podophyllotoxin peltatum, shows strong cytotoxic activity against various cancer cell lines by inhibiting tubulin through binding with part of its colchicine domain [1, 2]. Due to its severe side effects, podophyllotoxin has limited applications as a drug in cancer chemotherapy, but its semisynthetic derivatives etoposide 2 and teniposide 3 (Fig. 1) are in clinical use for the treatment of a variety of malignancies, including small cell lung cancer, testicular carcinoma, lymphoma, and Kaposi’s sarcoma [3, 4]. However, their therapeutic uses are often hindered by problems such as acquired drug-resistance and poor water solubility. Numerous studies [5–7] have shown that 4β-N-substituted derivatives of podophyllotoxin maintain the anticancer activity and function as topoisomerase II inhibitors. Since 1,2,3-triazole ring is a widespread functional group in drug [8], it is intriguing to attach 1,2,3-triazoles to podophyllotoxin derivatives.
Previously Reddy et al. [9] reported several glycosylated 4β-triazole-podophyllotoxin derivatives and their anticancer activity. In our earlier study [10], we reported a group of 4β-triazole-linked glucose podophyllotoxin conjugates as a new class of antitumor compounds. Reported here are the chemical synthesis of a series of 4β-triazole-podophyllotoxin α-glycosides (4, Fig. 1) of d-galactose, d-mannose, or d-xylose, and their in vitro anticancer activity against five human cancer cell lines, including HL-60 (leukemia), SMMC-7721 (hepatoma), A-549 (lung cancer), MCF-7 (breast cancer), and SW480 (colon cancer).
2 Results and Discussion
2.1 Chemical Synthesis
The Click reaction is a powerful means for linking two units, one with an azide functionality and the other an alkyne functional group. Typically, the cycloaddition reaction mediated by Cu(I) as the catalyst leads to the generation of a 1,4-disubstituted 1,2,3-triazole ring [11, 12]. The preparation of terminal alkynes is shown in Scheme 1. Using sulfuric acid on silica (H2SO4–silica) catalyzed Fischer type glycosylation with various alcohols and free sugars [13–15], α-glycosides with a propargyl group (7–12) were all obtained. The preparation of compounds 7 [16], 8 [17], 9 [18], 10 [19], and 11 [20] has been reported in the literature.
To introduce the azido functionality for the Click reaction, podophyllotoxin was readily converted to 4β-azido-4-deoxypodophyllotoxin 13 and 4β-azido-4-deoxy-4′-demethylpodophyllotoxin 14 according to previous reports [21, 22]. The azides 13 and 14 were to react with the terminal-alkynes 7–12 in the presence of copper (II) acetate and sodium ascorbate in t-butyl alcohol and water (1:2) at room temperature for 4 h to provide 4β-triazole-podophyllotoxin glycosides 15–26 in good yield (Scheme 2).
All the products were characterized by 1H-NMR, 13C-NMR, ESI-MS, and HRESI-MS. ESI-MS and HRESI-MS of all compounds showed the [M+Na]+ or [M+H]+ adduct as the molecular ion. In the 1H-NMR spectra, the formation of the triazole ring was confirmed by the resonance of its C5″–H signal (δ 7.81–8.31 ppm) in the aromatic region. The structure was further confirmed by the 13C-NMR spectra, which showed the two characteristic carbon signals at around 145 ppm (δ C-4″ ) and 126 ppm (δ C-5″ ) corresponding to the triazole residue. The configuration at C-4 position for target compounds 15–26 was confirmed based on the J 3,4 coupling constant, which is typically < 5.0 Hz for 4β-substituted compounds due to a cis relationship between H-3 and H-4 [23]. In some cases, 4β-substitution was further confirmed by 2D-NMR spectral data. For example, the ROESY of compound 15 shows strong correlation between H-4 ↔ H-3 (Fig. 2), indicating that the N-linked triazole ring moiety is attached to C-4 of podophyllotoxin via a β-linkage. The coupling constant of the anomeric proton of d-galactose and d-xylose residues (J 1′′′,2′′′ ) is typically < 5.0 Hz for the α-glycoside linkage. However, the coupling constant of the anomeric proton for d-mannose is usually small for both α- and β-mannosides. The α-linkage in d-mannosides was confirmed by the carbon-proton coupling constant (2 J C–H) of the anomeric carbon by acquiring the non-decoupled 13C NMR spectra. For example, the 2 J C–H is 167.9 Hz for the anomeric carbon of the d-mannose residue in compound 23, which confirms that 23 is an α-mannoside since the 2 J C–H of the anomeric carbon for a β-mannoside is typically below 160 Hz [24].
2.2 Evaluation of Biological Activity
All 4β-triazole-podophyllotoxin glycosides 15–26 were tested for their anticancer activity against five human cancer cell lines, including HL-60 (leukemia), SMMC-7721 (hepatoma), A-549 (lung cancer), MCF-7 (breast cancer), and SW480 (colon cancer). Etoposide (2) and cisplatin were taken as reference compounds. The screening procedure was based on the standard MTT method [25], and the results are reported in the terms of IC50 values (Table 1).
As it can be seen in Table 1, most of these compounds show weak cytotoxicity (IC50 > 40 μM). However, compound 16 shows strong anticancer activity against all cancer cell lines tested, with IC50 values ranging from 2.85 to 7.28 μM, which is significantly more potent than the control drug etoposide against four of the five cancer cells. It is interesting to note that the 4′-O-methylated analog 15 (IC50 > 40 μM) is much less potent than 16, indicating that this 4′-O-hydroxy group is perhaps important for the anticancer activity of glycosylated podophyllotoxin derivatives.
2.3 Chemical Stability Investigation
Compound 15 was selected for the investigation of chemical stability in aqueous phase with comparison to podophyllotoxin (1) and 4β-azido-4-deoxypodophyllotoxin (13). The results indicated that compound 15 exhibits higher chemical stability under the physiological condition (37 °C, pH 7.0, Fig. 3) than both podophyllotoxin (1) and 4β-azido-4-deoxypodophyllotoxin (13). Hydrolysis of the δ-lactone is anticipated to be the main degradation pathway under this condition [26]. It appears that the incorporation of the d-galactose moiety slows down the hydrolysis and improves the chemical stability of the podophyllotoxin scaffold.
3 Conclusions
In conclusion, we have used Fisher glycosylation strategy to prepare glycosylated terminal-alkynes. Then, all the glycosylated terminal-alkynes were reacted with podophyllotoxin-derived azides by the Click reaction to yield a series of 4β-triazole-podophyllotoxin glycosides 15–26 in good yields. All compounds were tested for anticancer activity against five human cancer cell lines. Most of these compounds show weak cytotoxicity while compound 16, having a galactose residue directly linked to the triazole ring and a 4′-OH group on the E ring, is more potent than the anticancer drug etoposide against four of the five cancer cell lines tested. In addition, chemical stability investigation indicates that the conjugated sugar residue seems to improve the stability of the podophyllotoxin scaffold under the physiological condition.
4 Experimental
4.1 General
d-Galactose, and d-xylose were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). d-Mannose was purchased from Acros Organics (New Jersey, USA). Podophyllotoxin was obtained from Shanghai yuanye Bio-Technology Co., Ltd (Shanghai, China). Melting points were uncorrected. MS data were obtained in the ESI mode on API Qstar Pulsar instrument. HRMS data were obtained in the ESI mode on LCMS-IT-TOF (Shimadzu, Kyoto, Japan). NMR spectra were acquired on Bruker AV-400 or DRX-500 or Bruker AVANCE Ш-600 (Bruker BioSpin GmbH, Rheinstetten, Germany) instruments, using tetramethylsilane (TMS) as an internal standard. Column chromatography (CC) was performed on flash silica gel (200–300 mesh; Qingdao Makall Group Co., Ltd; Qingdao; China). All reactions were monitored using thin-layer chromatography (TLC) on silica gel plates.
4.2 2-[2-[2-(2-Propyn-1-yloxy)ethoxy]ethoxy-α- d-xylopyranoside (12)
d-Xylose (150 mg, 1 mmol) and 2-[2-(2-propargyloxyethoxy)ethoxy]ethanol 7 (940 mg, 5 mmol) were stirred at 65 °C. H2SO4–silica (5 mg) was added and stirring was continued until all solids had dissolved (~2.5 h). After cooling to room temperature, the reaction mixture was purified by CC on silica gel (CHCl3:CH3OH = 9:1) to afford 12 (96 mg, 30 %). 1H-NMR (CD3OD, 400 MHz) δ 4.77 (d, 1H, J = 3.7 Hz, C1–H), 4.19 (d, 2H, J = 2.3 Hz, CH2–C≡C), 3.83–3.78 (m, 1H), 3.72–3.69 (m, 2H), 3.67–3.66 (m, 6H), 3.55–3.44 (m, 4H), 3.36–3.33 (m, 2H), 2.87 (t, 1H, J = 2.4 Hz, C≡CH); 13C-NMR (CD3OD, 100 MHz) δ 100.6 (C-1), 80.7 (C≡CH), 76.1 (C≡CH), 75.3, 73.7, 71.6, 71.5, 71.4, 70.1, 68.2, 63.1 (C-6), 59.1 (CH2–C≡C); ESIMS: m/z 343 [M+Na]+, HRESIMS: calcd for C14H24O18Na [M+Na]+ 343.1363, found 343.1364.
4.3 Click Chemistry: General Procedure for the Synthesis of Compounds 15–26
To a solution of a terminal-alkyne 7–12 (0.2 mmol) and 4β-azido-podophyllotoxin analogues 13 or 14 (0.2 mmol) in t– BuOH–H2O (1:2, 1.0 mL) at room temperature were added copper (II) sulfate pentahydrate (0.02 mmol) and sodium ascorbate (1.0 M in H2O, 3 drops). The reaction mixture was stirred at room temperature for 4 h until the starting material disappeared as indicated by TLC. Then, the mixture was diluted with water (10 mL) and extracted with ethyl acetate (3 × 10 mL), and the combined organic layer was dried over sodium sulfate. The solvent was evaporated and the residue was purified by CC (CHCl3/CH3OH, 9:1) to afford the cycloaddition product 15–26 (75–87 %).
4.3.1 4β-[4-(α-d-Galactopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxypodophyllotoxin (15)
White amorphous powder, yield 86 %; mp 153–155 °C; [α] 25.7D +18.6 (c 0.28, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.84 (s, 1H, C5′′–H), 6.69 (s, 1H, C5–H), 6.62 (s, 1H, C8–H), 6.41 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.98–5.96 (m, 2H, OCH2O), 4.93 (d, 1H, J = 4.0 Hz, C1′′′–H), 4.83 (s, 1H, C2′′′–H), 4.80–4.78 (m, 1H), 4.64–4.61 (m, 1H), 4.69 (t, 1H, J = 8.0 Hz), 3.87–3.86 (m, 2H), 3.83–3.78 (m, 5H), 3.74 (s, 6H, C3′, C5′-OCH3), 3.72 (s, 3H, C4′-OCH3), 3.43 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2′′′–H), 3.20–3.15 (m, 1H, C3–H,); 13C-NMR (CD3OD, 100 MHz) δ 175.9 (C-12), 153.9 (C-3′, C-5′), 150.6 (C-7), 149.3 (C-6), 145.7 (C-4′′), 138.3 (C-1′), 136.8 (C-9), 134.8 (C-10), 126.9 (C-4′), 126.0 (C-5′′), 111.2 (C-5), 109.9 (C-8), 109.4 (C-2′, C-6′), 103.3 (OCH2O), 100.0 (C-1′′′), 72.8, 71.4, 71.1, 70.1, 68.9 (C-11), 62.9 (C-6′′), 61.5 (C-6′′′), 61.1 (4′-OCH3), 59.8 (C-4), 56.6 (3′,5′-OCH3), 44.9 (C-1), 42.5 (C-2), 38.6 (C-3); ESIMS: m/z 680 [M+Na]+, HRESIMS: calcd for C31H35N3O13Na [M+Na]+ 680.2062, found 680.2056.
4.3.2 4β-[4-(α-d-Galactopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxy-4′-demethylpodophyllotoxin (16)
White amorphous powder, yield 88 %; mp 162 °C; [α] 25.6D –9.8 (c 0.10, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 8.23 (s, 1H, C5′′–H), 6.62 (s, 1H, C5–H), 6.59 (s, 2H, C2′, C6′–H), 6.27 (s, 1H, C8–H), 6.04–5.93 (m, 3H, C4–H, OCH2O), 4.98 (d, 1H, J = 4.0 Hz, C1′′′–H), 4.72–4.68 (m, 2H), 4.23–4.19 (m, 2H), 3.90–3.87 (m, 3H), 3.80 (s, 6H, C3′, C5′–OCH3), 3.77–3.70 (m, 5H), 3.54–3.48 (m, 1H, C3–H); 13C-NMR (CD3OD, 125 MHz): δ 176.1 (C-12), 149.8 (C-7), 149.2 (C-6), 148.8 (C-3′, C-5′), 145.9 (C-4′′), 134.3 (C-9), 131.7 (C-10), 129.9 (C-1′), 129.2 (C-4′), 125.7 (C-5′′), 111.0 (C-5), 109.6 (C-2′, C-6′), 107.3 (C-8), 103.1 (OCH2O), 100.2 (C-1′′′), 72.8, 71.5, 71.3 (C-11), 71.2, 70.2, 64.1 (C-4), 62.9 (C-6′′), 61.8 (C-6′′′), 57.0 (3′, 5′-OCH3), 46.8 (C-1), 45.2 (C-2), 40.0 (C-3); ESIMS: m/z 666 [M+Na]+, HRESIMS: calcd for C30H33N3O13Na [M+Na]+ 666.1906, found 666.1900.
4.3.3 4β-[4-(α-d-Mannopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxypodophyllotoxin (17)
White amorphous powder, yield 83 %; mp 117–119 °C; [α] 25.7D –8.8 (c 0.21, CH3OH); 1H-NMR (C5D5N, 500 MHz) δ 8.31 (s, 1H, C5′′–H), 6.85 (s, 1H, C5–H), 6.82 (s, 1H, C8–H), 6.76 (s, 2H, C2′, C6′–H), 6.58 (d, 1H, J = 4.8 Hz, C4–H), 5.99–5.98 (m, 2H, OCH2O), 5.63 (s, 1H, C1′′′–H), 5.27 (d, 1H, J = 5.0 Hz, C1–H), 5.05–5.02 (m, 3H), 4.62–4.58 (m, 2H), 4.54–4.52 (m, 2H), 4.41–4.35 (m, 3H), 3.98–3.93 (m, 1H, C2–H), 3.85 (s, 6H, C3′, C5′–OCH3), 3.82 (s, 3H, C4′–OCH3), 3.46–3.40 (m, 1H, C3–H); 13C-NMR (C5D5N, 125 MHz) δ 174.1 (C-12), 153.5 (C-3′, C-5′), 149.4 (C-7), 147.8 (C-6), 145.1 (C-4′′), 140.1 (C-1′), 138.3 (C-9), 134.0 (C-10), 126.6 (C-5′′), 125.2 (C-4′), 110.7 (C-5), 109.5 (C-8), 109.2 (C-2′, C-6′), 102.4 (OCH2O), 101.7 (C-1′′′), 75.8, 72.9, 71.9, 69.1, 68.0 (C-11), 63.2 (C-6′′), 61.1 (C-6′′′), 60.6 (4′-OCH3), 58.8 (C-4), 56.3 (3′, 5′-OCH3), 44.3 (C-1), 41.9 (C-2), 38.0 (C-3); ESIMS: m/z 680 [M+Na]+, HRESIMS: calcd for C31H35N3O13H [M+H]+ 658.2243, found 658.2236.
4.3.4 4β-[4-(α-d-Mannopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxy-4′-demethylpodophyllotoxin (18)
White amorphous powder, yield 80 %; mp 208 °C; [α] 26.7D –72.8 (c 0.27, Pyridine); 1H-NMR (C5D5N, 400 MHz) δ 8.30 (s, 1H, C5′′–H), 6.84 (s, 2H, C5–H, C8–H), 6.80 (s, 2H, C2′, C6′–H), 6.52 (d, 1H, J = 4.8 Hz, C4–H), 5.93 (s, 2H, OCH2O), 5.62 (s, 1H, C1′′′–H), 5.24 (d, 1H, J = 5.0 Hz, C1–H), 5.03–5.00 (m, 3H), 4.64–4.57 (m, 2H), 4.53–4.51 (m, 2H), 4.42–4.36 (m, 3H), 3.89 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2–H), 3.72 (s, 6H, C3′, C5′′–OCH3), 3.43–3.38 (m, 1H, C3–H); 13C-NMR (C5D5N, 100 MHz) δ 174.2 (C-12), 149.4 (C-7), 148.7 (C-3′, C-5′), 148.1 (C-6), 145.1 (C-4′′), 137.4 (C-1′), 134.5 (C-9), 130.1 (C-10), 126.5 (C-5′′), 125.1 (C-4′), 110.7 (C-5), 109.7 (C-2′, C-6′), 109.3 (C-8), 102.3 (OCH2O), 101.9 (C-1′′′), 75.8, 72.9, 72.0, 69.2, 67.9 (C-11), 63.2 (C-6′′), 61.1 (C-6′′′), 58.8 (C-4), 56.5 (3′, 5′-OCH3), 44.1 (C-1), 42.0 (C-2), 37.9 (C-3); ESIMS: m/z 666 [M+Na]+, HRESIMS: calcd for C30H33N3O13H [M+H]+ 644.2086, found 644.2079.
4.3.5 4β-[4-(α-d-Xylopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxypodophyllotoxin (19)
White amorphous powder, yield 75 %; mp 125–128 °C; [α] 25.7D –35.9 (c 0.27, Pyridine); 1H-NMR (C5D5N, 500 MHz) δ 8.26 (s, 1H, C5′′–H), 6.81 (s, 1H, C5–H), 6.79 (s, 1H, C8–H), 6.75 (s, 2H, C2′, C6′–H), 6.53 (d, 1H, J = 5.0 Hz, C4–H), 5.96 (s, 2H, OCH2O), 5.42 (d, 1H, J = 2.9 Hz, C1′′′–H), 5.18 (d, 1H, J = 4.5 Hz, C1–H), 5.02–4.97 (m, 2H), 4.47–4.43 (m, 1H), 4.40–4.36 (m, 1H), 4.20–4.15 (m, 3H), 4.10–4.08 (m, 1H), 4.05–4.03 (m, 1H), 3.82 (s, 6H, C3′, C5′–OCH3), 3.80 (s, 3H, C4′–OCH3), 3.66–3.60 (m, 1H, C2–H), 3.40–3.38 (m, 1H, C3–H); 13C-NMR (C5D5N, 125 MHz) δ 174.1 (C-12), 153.5 (C-3′, C-5′), 148.3 (C-7), 147.7 (C-6), 145.4 (C-4′′), 140.0 (C-1′), 138.3 (C-9), 134.0 (C-10), 126.6 (C-5′′), 125.1 (C-4′), 110.7 (C-5), 109.4 (C-8), 109.2 (C-2′, C-6′), 102.4 (OCH2O), 100.5 (C-1′′′), 75.4, 73.7, 71.7, 68.0 (C-11), 63.8 (C-5′′′), 61.7 (C-6′′), 60.6 (4′-OCH3), 58.7 (C-4), 56.2 (3′, 5′-OCH3), 44.2 (C-1), 41.9 (C-2), 38.1 (C-3); ESIMS: m/z 650 [M+Na]+, HRESIMS: calcd for C30H33N3O12H [M+H]+ 628.2137, found 628.2132.
4.3.6 4β-[4-(α-d-Xylopyranosyloxymethyl)-1,2,3-triazol-1-yl]-4-deoxy-4′-demethylpodophyllotoxin (20)
White amorphous powder, yield 84 %; mp 205–206 °C; [α] 26.6D –50.1 (c 0.14, Pyridine); 1H-NMR (C5D5N, 500 MHz) δ 8.24 (s, 1H, C5′′–H), 6.82 (s, 1H, C5–H), 6.78 (s, 2H, C2′, C6′–H), 6.76 (s, 1H, C8–H), 6.50 (d, 1H, J = 4.0 Hz, C4–H), 5.94–5.93 (m, 2H, OCH2O), 5.43 (d, 1H, J = 5.0 Hz, C1′′′–H), 5.20–5.18 (m, 1H), 5.02–5.00 (m, 1H), 4.96 (d, 1H, J = 5.0 Hz, C1–H), 4.47 (t, 1H, J = 8.0 Hz), 4.39 (t, 1H, J = 8.0 Hz), 4.20–4.17 (m, 3H), 4.10–4.08 (m, 1H), 4.06–4.04 (m, 1H), 3.73 (s, 6H, C3′, C5′–OCH3), 3.68–3.62 (m, 1H, C2–H), 3.42–3.39 (m, 1H, C3–H); 13C-NMR (C5D5N, 125 MHz) δ 174.2 (C-12), 149.4 (C-7), 148.8 (C-3′, C-5′), 148.2 (C-6), 145.4 (C-4′′), 137.4 (C-1′), 134.4 (C-9), 130.1 (C-10), 126.4 (C-5′′), 125.1 (C-4′), 110.8 (C-5), 109.7 (C-2′, C-6′), 109.3 (C-8), 102.4 (OCH2O), 100.5 (C-1′′′), 75.4, 73.8, 71.7, 68.0 (C-11), 63.8 (C-5′′′), 61.7 (C-6′′), 58.8 (C-4), 56.5 (3′, 5′-OCH3), 44.1 (C-1), 42.1 (C-2), 38.0 (C-3); ESIMS: m/z 636[M+Na]+, HRESIMS: calcd for C29H31N3O12H [M+H]+ 614.1980, found 614.1973.
4.3.7 4β-{4-[1-(α-d-Galactopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (21)
White amorphous powder, yield 82 %; mp 90 °C; [α] 25.8D +1.4 (c 0.17, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.82 (s, 1H, C5′′–H), 6.68 (s, 1H, C5–H), 6.62 (s, 1H, C8–H), 6.40 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.97–5.95 (m, 2H, OCH2O), 4.85 (s, 1H, C1′′′–H), 4.79 (d, 1H, J = 5.0 Hz, C1–H), 4.63–4.61 (m, 2H), 4.39 (t, 1H, J = 8.0 Hz), 3.87–3.80 (m, 7H), 3.73 (s, 6H, C3′, C5′–OCH3), 3.71 (s, 3H, C4′–OCH3), 3.69–3.60 (m, 12H, 3 × OCH2CH2O), 3.44 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2–H), 3.17–3.12 (m, 1H, C3–H); 13C-NMR (CD3OD, 125 MHz) δ 175.9 (C-12), 153.9 (C-3′, C-5′), 150.6 (C-7), 149.3 (C-6), 146.0 (C-4′′), 138.3 (C-1′), 136.8 (C-9), 134.8 (C-10), 127.0 (C-4′), 126.0 (C-5′′), 111.2 (C-5), 109.8 (C-8), 109.4 (C-2′, C-6′), 103.3 (OCH2O), 100.6 (C-1′′′), 72.4, 71.6, 71.4, 71.3, 71.1, 70.9, 70.4, 68.9 (C-11), 68.1, 65.0 (C-6′′), 62.8 (C-6′′′), 61.1 (4′-OCH3), 59.8 (C-4), 56.6 (3′, 5′-OCH3), 44.9 (C-1), 42.5 (C-2), 38.6 (C-3); ESIMS: m/z 812 [M+Na]+, HRESIMS: calcd for C37H47N3O16H [M+H]+ 790.3029, found 790.3013.
4.3.8 4β-{4-[1-(α-d-Galactopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4′-demethylpodophyllotoxin (22)
White amorphous powder, yield 87 %; mp 128 °C; [α] 25.6D –0.3 (c 0.16, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.82 (s, 1H, C5′′–H), 6.68 (s, 1H, C5–H), 6.64 (s, 1H, C8–H), 6.37 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.98–5.96 (m, 2H, OCH2O), 4.85 (s, 1H, C1′′′–H), 4.77 (d, 1H, J = 5.0 Hz, C1–H), 4.68–4.67 (m, 2H), 4.39 (t, 1H, J = 8.0 Hz), 3.87–3.83 (m, 7H), 3.74 (s, 6H, C3′′, C5′′–OCH3), 3.70–3.61 (m, 12H, 3 × OCH2CH2O), 3.41 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2–H), 3.17–3.12 (m, 1H, C3–H); 13C-NMR (CD3OD, 100 MHz) δ 176.0 (C-12), 150.5 (C-7), 149.2 (C-6), 148.7 (C-3′, C-5′), 145.9 (C-4′′), 136.0 (C-1′), 135.1 (C-9), 131.3 (C-10), 126.9 (C-4′), 126.0 (C-5′′), 111.2 (C-5), 109.7 (C-8), 109.3 (C-2′, C-6′), 103.2 (OCH2O), 100.6 (C-1′′′), 72.4, 71.6, 71.4, 71.3, 71.1, 70.9, 70.4, 68.9 (C-11), 68.1, 65.0 (C-6′′), 62.8 (C-6′′′), 59.9 (C-4), 56.7 (3′, 5′-OCH3), 44.7 (C-1), 42.7 (C-2), 38.5 (C-3); ESIMS: m/z 798 [M+Na]+, HRESIMS: calcd for C36H45N3O16H [M+H]+ 776.2873, found 776.2861.
4.3.9 4β-{4-[1-(α-d-Mannopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (23)
White amorphous powder, yield 81 %; mp 94 °C; [α] 26.8D –13.1 (c 0.20, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.82 (s, 1H, C5′′–H), 6.67 (s, 1H, C5–H), 6.63 (s, 1H, C8–H), 6.40 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.97–5.95 (m, 2H, OCH2O), 4.78 (s, 2H, C1′′′–H, C1–H), 4.64–4.61 (m, 2H), 4.40–4.36 (m, 1H), 3.84-3.79 (m, 7H), 3.73 (s, 6H, C3′, C5′–OCH3), 3.71 (s, 3H, C4′–OCH3), 3.65–3.59 (m, 12H, 3 × OCH2CH2O), 3.43 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2–H), 3.17–3.12 (m, 1H, C3–H); 13C-NMR (CD3OD, 100 MHz) δ 175.9 (C-12), 154.0 (C-3′, C-5′), 150.6 (C-7), 149.3 (C-6), 146.0 (C-4′′), 138.3 (C-1′), 136.8 (C-9), 134.8 (C-10), 127.0 (C-4′), 126.0 (C-5′′), 111.2 (C-5), 109.9 (C-8), 109.4 (C-2′, C-6′), 103.3 (OCH2O), 101.7 (C-1′′′), 74.6, 72.5, 72.1, 71.6, 71.5, 71.3, 70.9, 68.9 (C-11), 68.6, 67.7, 65.0 (C-6′′), 63.0 (C-6′′′), 61.1 (4′-OCH3), 59.8 (C-4), 56.6 (3′, 5′-OCH3), 44.9 (C-1), 42.5 (C-2), 38.6 (C-3); ESIMS: m/z 812 [M+Na]+, HRESIMS: calcd for C37H47N3O16H [M+H]+ 790.3029, found 790.3012.
4.3.10 4β-{4-[1-(α-d-Mannopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4′-demethylpodophyllotoxin (24)
White amorphous powder, yield 78 %; mp 108 °C; [α] 26.9D –26.2 (c 0.14, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.81 (s, 1H, C5′′–H), 6.68 (s, 1H, C5–H), 6.64 (s, 1H, C8–H), 6.38 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.98–5.97 (m, 2H, OCH2O), 4.78 (s, 2H, C1′′′–H), 4.77 (d, 1H, J = 5.0 Hz, C1–H), 4.64–4.62 (m, 2H), 4.41–4.37 (m, 1H), 3.84–3.79 (m, 7H), 3.75 (s, 6H, C3′, C5′–OCH3), 3.68–3.58 (m, 12H, 3 × OCH2CH2O), 3.41 (dd, 1H, J = 5.0 Hz, 10.0 Hz, C2–H), 3.18–3.13 (m, 1H, C3–H); 13C-NMR (CD3OD, 100 MHz) δ 176.0 (C-12), 150.5 (C-7), 149.2 (C-6), 148.7 (C-3′, C-5′), 146.0 (C-4′′), 136.0 (C-1′), 135.1 (C-9), 131.3 (C-10), 126.9 (C-4′), 125.9 (C-5′′), 111.2 (C-5), 109.7 (C-8), 109.3 (C-2′, C-6′), 103.2 (OCH2O), 101.7 (C-1′′′), 74.6, 72.5, 72.1, 71.6, 71.5, 71.3, 70.9, 68.9 (C-11), 68.6, 67.7, 65.0 (C-6′′), 63.0 (C-6′′′), 59.9 (C-4), 56.8 (3′, 5′-OCH3), 44.7 (C-1), 42.7 (C-2), 38.5 (C-3); ESIMS: m/z 798 [M+Na]+, HRESIMS: calcd for C36H45N3O16H [M+H]+ 776.2873, found 776.2863.
4.3.11 4β-{4-[1-(α-d-Xylopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxypodophyllotoxin (25)
White amorphous powder, yield 80 %; mp 113–115 °C; [α] 26.5D –8.3 (c 0.18, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.82 (s, 1H, C5′′–H), 6.68 (s, 1H, C5–H), 6.60 (s, 1H, C8–H), 6.41 (s, 2H, C2′, C6′–H), 6.25 (d, 1H, J = 4.8 Hz, C4–H), 5.97–5.95 (m, 2H, OCH2O), 4.78 (d, 1H, J = 5.2 Hz, C1–H), 4.75 (d, 1H, J = 4.0 Hz, C1′′′–H), 4.64–4.61 (m, 2H), 4.39–4.36 (m, 1H), 3.85–3.75 (m, 2H), 3.73 (s, 6H, C3′, C5′–OCH3), 3.71 (s, 3H, C4′–OCH3), 3.67–3.58 (m, 12H, 3 × OCH2CH2O), 3.52–3.40 (m, 4H), 3.36–3.32 (m, 1H, C2–H), 3.17–3.15 (m, 1H, C3–H); 13C-NMR (CD3OD, 100 MHz) δ 175.8 (C-12), 154.0 (C-3′, C-5′), 150.6 (C-7), 149.3 (C-6), 146.0 (C-4′′), 138.3 (C-1′), 136.8 (C-9), 134.8 (C-10), 127.0 (C-4′), 126.0 (C-5′′), 111.2 (C-5), 109.9 (C-8), 109.4 (C-2′, C-6′), 103.3 (OCH2O), 100.6 (C-1′′′), 75.3, 73.7, 71.5, 71.5, 71.3, 71.3, 70.9, 68.9 (C-11), 68.2, 65.0 (C-5′′′), 63.1 (C-6′′), 61.1 (4′-OCH3), 59.8 (C-4), 56.6 (3′, 5′-OCH3), 44.9 (C-1), 42.5 (C-2), 38.6 (C-3); ESIMS: m/z 782 [M+Na]+, HRESIMS: calcd for C36H45N3O15H [M+H]+ 760.2923, found 760.2914.
4.3.12 4β-{4-[1-(α-d-Xylopyranosyloxymethyl)-3,6,9-trioxadec-10-yl]-1,2,3-triazol-1-yl}-4-deoxy-4′-demethylpodophyllotoxin (26)
White amorphous powder, yield 84 %; mp 103 °C; [α] 26.5D –9.7 (c 0.27, CH3OH); 1H-NMR (CD3OD, 400 MHz) δ 7.83 (s, 1H, C5′′–H), 6.70 (s, 1H, C5–H), 6.66 (s, 1H, C8–H), 6.40 (s, 2H, C2′, C6′–H), 6.28 (d, 1H, J = 4.8 Hz, C4–H), 6.00–5.98 (m, 2H, OCH2O), 4.79 (d, 1H, J = 4.0 Hz, C1′′′–H), 4.77 (d, 1H, J = 3.6 Hz, C1–H), 4.66–4.64 (m, 2H), 4.41 (t, 1H, J = 12.0 Hz), 3.77 (s, 6H, C3′, C5′–OCH3), 3.70–3.62 (m, 12H, 3 × OCH2CH2O), 3.55–3.53 (m, 4H), 3.48–3.40 (m, 2H), 3.38–3.35 (m, 1H, C2–H), 3.17–3.15 (m, 1H, C3–H); 13C-NMR (CD3OD, 100 MHz) δ 176.4 (C-12), 150.9 (C-7, C-6), 149.7 (C-4′′), 149.1 (C-3′, C-5′), 136.4 (C-1′), 135.5 (C-9), 131.8 (C-10), 127.3 (C-4′), 126.4 (C-5′′), 111.7 (C-5), 110.2 (C-8), 109.8 (C-2′, C-6′), 103.7 (OCH2O), 110.0 (C-1′′′), 75.3, 73.7, 71.5, 71.4, 71.4, 71.3, 70.9, 68.9 (C-11), 68.2, 65.0 (C-5′′′), 63.1 (C-6′′), 60.3 (C-4), 57.2 (3′, 5′-OCH3), 45.2 (C-1), 43.1 (C-2), 39.0 (C-3); ESIMS: m/z 768 [M+Na]+, HRESIMS: calcd for C35H43N3O15H [M+H]+ 746.2767, found 746.2755.
4.4 Cell Culture and Cytotoxicity Assay
The following human tumor cell lines were used: HL-60, SMMC-7721, A-549, MCF-7, and SW480. All the cells were cultured in RMPI-1640 or DMEM medium (Hyclone, Logan, UT, USA), supplemented with 10 % fetal bovine serum (Hyclone) at 37 °C in a humidified atmosphere with 5 % CO2. Cell viability was assessed by conducting colorimetric measurements of the amount of insoluble formazan formed in living cells based on the reduction of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma, St. Louis, MO, USA). Briefly, adherent cells (100 μL) were seeded into each well of a 96-well cell culture plate and allowed to adhere for 12 h before drug addition, while suspended cells were seeded just before drug addition, both with an initial density of 1 × 105 cells/mL in 100 μL of medium. Each tumor cell line was exposed to the test compound at various concentrations in triplicate for 48 h. After the incubation, MTT (100 μg) was added to each well, and the incubation continued for 4 h at 37 °C. The cells lysed with SDS (200 μL) after removal of 100 μL of medium. The optical density of lysate was measured at 595 nm in a 96-well microtiter plate reader (Bio-Rad 680). The IC50 value of each compound was calculated by Reed and Muench’s method [25].
References
M.Á. Castro, J.M. Miguel del Corral, P.A. García, M.V. Rojo, J. de la Iglesia-Vicente, F. Mollinedo, C. Cuevas, A.S. Feliciano, J. Med. Chem. 53, 983–993 (2010)
J.L. Hartwell, A.W. Schrecker, J. Am. Chem. Soc. 73, 2909–2916 (1951)
I. Jardine, J.M. Cassady, J. Douros, In the anticancer agents based on natural product models (Academic Press, New York, 1980), p. 319
B.F. Issell, Cancer Chemother. Pharmacol. 7, 73–80 (1982)
T. Terada, K. Fujimoto, M. Nomura, J. Yamashita, K. Wierzba, R. Yamazaki, J. Shibata, Y. Sugimoto, Y. Yamada, T. Kobunai, S. Takeda, Y. Minami, K. Yoshida, H. Yamaguchi, J. Med. Chem. 36, 1689–1699 (1993)
Z.Y. Xiao, Y.D. Xiao, J. Feng, A. Golbraikh, A. Tropsha, K.H. Lee, J. Med. Chem. 45, 2294–2309 (2002)
R. Tawa, M. Takami, Y. Imakura, K.H. Lee, H. Sakuri, Bioorg. Med. Chem. Lett. 7, 489–494 (1997)
P. Thirumurugan, D. Matosiuk, K. Jozwiak, Chem. Rev. 113, 4905–4979 (2013)
P.B. Reddy, D.V. Paul, S.K. Agrawal, A.K. Saxena, H.M.S. Kumar, G.N. Qazi, Arch. Pharm. Chem. Life Sci. 341, 126–131 (2008)
C.T. Zi, F.Q. Xu, G.T. Li, Y. Li, Z.T. Ding, J. Zhou, Z.H. Jiang, J.M. Hu, Molecules 18, 1420–1430 (2013)
V.V. Rostovtsev, L.G. Green, V.V. Fokin, K.B. Sharpless, Angew. Chem. Int. Ed. 41, 2596–2599 (2002)
C.W. Tørnoe, C. Christensen, M. Meldal, J. Org. Chem. 67, 3057–3064 (2002)
B. Mukhopadhyay, Tetrahedron Lett. 47, 4337–4341 (2006)
B. Roy, B. Mukhopadhyay, Tetrahedron Lett. 48, 3783–3787 (2007)
V.K. Rajput, B. Mukhopadhyay, Tetrahedron Lett. 47, 5939–5941 (2006)
P. Simerská, M. Kuzma, D. Monti, S. Riva, M. Macková, V. Kǐren, J. Mol. Catal. B 39, 128–134 (2006)
O. Michel, B.J. Ravoo, Langmuir 24, 12116–12118 (2008)
T. Hasegawa, M. Numata, S. Okumura, T. Kimura, K. Sakurai, S. Shinkai, Org. Biomol. Chem. 5, 2404–2412 (2007)
L. Cui, J.A. Cohen, K.E. Broaders, T.T. Beaudette, J.M.J. Fréchet, Bioconjug Chem. 22, 949–957 (2011)
A.S. Rowan, N.I. Nicely, N. Cochrane, W.A. Wlassoff, A. Claiborneb, C.J. Hamilton, Org. Biomol. Chem. 7, 4029–4036 (2009)
A. Kamal, N. Laxman, G. Ramesh, Bioorg. Med. Chem. Lett. 10, 2059–2062 (2000)
X.M. Zhou, Z.Q. Wang, J.Y. Chang, H.X. Chen, K.H. Yung, J. Med. Chem. 34, 3346–3350 (1991)
S.W. Chen, Y.H. Wang, Y. Jin, X. Tian, Y.T. Zheng, D.Q. Luo, Y.Q. Tu, Bioorg. Med. Chem. Lett. 17, 2091–2095 (2007)
K.J. Doores, B.G. Davis, Org. Biomol. Chem. 6, 2692–2696 (2008)
L.J. Reed, H. Muench, Am. J. Hyg. 27, 493–497 (1938)
W.J. Gensler, C.D. Gatsonis, J. Org. Chem. 31, 3224–3227 (1966)
Acknowledgments
This work was financially supported by the Fund of State Key Laboratory of Phytochemistry and Plant Resource in West China (P2010-KF07). The authors thank the staff of analytical group of the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences, for measurements of all spectra.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zi, CT., Li, GT., Li, Y. et al. Synthesis and Anticancer Activity of 4β-Triazole-podophyllotoxin Glycosides. Nat. Prod. Bioprospect. 5, 83–90 (2015). https://doi.org/10.1007/s13659-015-0057-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-015-0057-3