Abstract
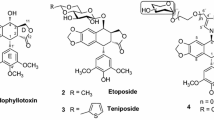
A series of triazolyl-modified podophyllotoxin analogs have been designed and synthesized by utilizing Huisgen 1,3-dipolar cycloaddition in order to develop potent antitumor agents. The synthesized analogs were assessed for in vitro anticancer activity against four human cancer cell lines, including MDA-MB-231 and MCF-7 (human breast cancer cells), A549 (human lung cancer), PC3 (human prostate cancer), and non-tumor human breast epithelial cell line FR2 by using MTT assay. Some of the tested derivatives exhibited potent biological profiles in comparison to podophyllotoxin. p-Nitrophenyltriazolyl and p-hydroxyphenyltriazolyl analogs were found to be the most potent with IC50 values of 19.0 (against PC3 cell line and 20.0 mM (against A549 cell line), respectively.


Similar content being viewed by others
REFERENCES
Kelly, M.G. and Hartwell, J.L., J. Natl. Cancer Inst., 1954, vol. 14, p. 967. https://doi.org/10.1093/jnci/14.4.967
Imbert, T.F., Biochimie, 1998, vol. 80, p. 207. https://doi.org/10.1016/S0300-9084(98)80004-7
Canel, C., Moraes, R.M., Dayan, F.E., and Ferreira, D., Phytochemistry, 2000, vol. 54, p. 115. https://doi.org/10.1016/S0031-9422(00)00094-7
Garcia, E S. and Azambuja, P., Toxicon, 2004, vol. 44, p. 431. https://doi.org/10.1016/j.toxicon.2004.05.007
Williams, A.H.W., Dai, X.D., Blot, W., Xu, Z.Y., Sun, X.W., Xiao, H.P., Stone, B.J., Yu, S.F., Feng, Y.P., and Ershow, A.G., Br. J. Cancer, 1990, vol. 62, p. 982. https://doi.org/10.1038/bjc.1990.421
Kamal, A., Tamboli, J.R., Nayak, V.L., Adil, S.F., Vishnuvardhan, M.V.P.S., and Ramakrishna, S., Bioorg. Med. Chem., 2014, vol. 22, p. 2714. https://doi.org/10.1016/j.bmc.2014.03.021
Zhou, J., Qu, Z., Yan, S., Sun, F., Whitsett, J.A., Shapiro, S.D., and Xiao, G., Oncogene, 2015, vol. 34, p. 3804. https://doi.org/10.1038/onc.2014.318
Utsugi, T., Shibata, J., Sugimoto, Y., Aoyagi, K., Wierzba, K., Kobunani, T., Terada, T., Ohhara, T., Tsuruo, T., and Yamada, Y., Cancer Res., 1996, vol. 56, p. 2809.
Subrahmanyam, D., Renuka, B., Rao, C.B., Sagar, P.S., Deevi, D.S., Babu, J.M., and Vyas, K., Bioorg. Med. Chem. Lett., 1998, vol. 8, p. 1391. https://doi.org/10.1016/S0960-894X(98)00232-7
Wang, J.Z., Tian, X., Tsumura, H., Shimura, K., and Ito, H., Anti-Cancer Drug Des., 1993, vol. 8, p. 193.
Huang, T.S., Lee, C.C., Chao, Y., Shu, C.H., Chen, L.T., Chen, L.L., Chen, M.H., Yuan, C.C., and Wang-Peng, J., Pharm. Res., 1999, vol. 16, p. 997. https://doi.org/10.1023/A:1018971313256
Gordaliza, M., Garcı́a, P.A., Miguel del Corral, J.M., Castro, M.A., and Gómez-Zurita, M.A., Toxicon, 2004, vol. 44, p. 441. https://doi.org/10.1016/j.toxicon.2004.05.008
Xu, H., Lv, M., and Tian, X., Curr. Med. Chem., 2009, vol. 23, p. 327. https://doi.org/10.2174/092986709787002682
You, Y.J., Curr. Pharm. Des., 2005, vol. 11, p. 1695. https://doi.org/10.2174/1381612053764724
Lv, M., Xu, H., Mini-Rev. Med. Chem., 2011, vol. 11, p. 901. https://doi.org/10.2174/138955711796575461
Zhang, X., Rakesh, K.P., Shantharam, C.S., Asiri, A.M., Marwani, H.M., and Qin, H.L., Bioorg. Med. Chem., 2018, vol. 26, p. 340. https://doi.org/10.1016/j.bmc.2017.11.026
Mukherjee, A.K., Basu, S., Sarkar, N., and Ghosh, A.C., Curr. Med. Chem., 2001, vol. 8, p. 1467. https://doi.org/10.2174/0929867013372094
Stähelin, H.F., Eur. J. Cancer, 1973, vol. 9, p. 215. https://doi.org/10.1016/S0014-2964(73)80021-0
Hande, K.R., Eur. J. Cancer, 1998, vol. 34, p. 1514. https://doi.org/10.1016/S0959-8049(98)00228-7
Sanghvi, Y.S., Bhattacharya, B.K., Kini, G.D., Matsumoto, S.S., Larson, S.B., Jolley, W.B., Robins, R.K., and Revankar, G.R., J. Med. Chem., 1990, vol. 33, p. 336. https://doi.org/10.1021/jm00163a054
Pawelec, G., Ehninger, G., Rehbein, A., Schaudt, K., and Jaschonek, K., Int. J. Immunopharmacol., 1991, vol. 13, p. 299. https://doi.org/10.1016/0192-0561(91)90111-J
Yan, S.J., Liu, Y.J., Jiang, Y., Chen, Y.L., Liu, L., and Lin, J., Bioorg. Med. Chem. Lett., 2010, vol. 20, p. 5225. https://doi.org/10.1016/j.bmcl.2010.06.141
Shaw, J., Chen, B., Bourgault, J.P., Jiang, H., Kumar, N., Mishra, J., Valeriote, F.A., Media, J., Bobbitt, K., Pietraszkiewicz, H., Edelstein, M., and Andreana, P.R., Am. J. Biomed. Sci., 2012, vol. 4, p. 14. https://doi.org/10.5099/aj120100014
Kumbhare, R.M., Kosurkar, U.B., Ramaiah, M.J., Dadmal, T.L., Pushpavalli, S.N.C.V.L., and PalBhadra, M., Bioorg. Med. Chem. Lett., 2012, vol. 22, p. 5424. https://doi.org/10.1016/j.bmcl.2012.07.041
Zhou, C.H. and Wang, Y., Curr. Med. Chem., 2012, vol. 19, p. 239. https://doi.org/10.2174/092986712803414213
Bhat, B.A., Reddy, P.B., Agrawal, S.K., Saxena, A.K., Kumar, H.M.S., and Qazi, G.N., Eur. J. Med. Chem., 2008, vol. 43, p. 2067. https://doi.org/10.1016/j.ejmech.2007.09.015
Reddy, P.B., Paul, D.V., Agrawal, S.K., Saxena, A.K., Kumar, H.M.S., and Qazi, G.N., Arch. Pharm., 2008, vol. 341, p. 126. https://doi.org/10.1002/ardp.200700116
Reddy, D.M., Srinivas, J., Chashoo, G., Saxena, A.K., and Sampath Kumar, H.M., Eur. J. Med. Chem., 2011, vol. 46, p. 1983. https://doi.org/10.1016/j.ejmech.2011.02.016
Zi, C.T., Xu, F.Q., Li, G.T., Li, Y., Ding, Z.T., Zhou, J., Jiang, Z.H., and Hu, J.M., Molecules, 2013, vol. 18, p. 13992. https://doi.org/10.3390/molecules181113992
Rashid, S., Bhat, B.A., and Mehta, G., Org. Lett., 2015, vol. 17, p. 3604. https://doi.org/10.1021/acs.orglett.5b01707
Rashid, S., Bhat, B.A., and Mehta, G., Tetrahedron Lett., 2017, vol. 58, p. 3800. https://doi.org/10.1016/j.tetlet.2017.08.026
Rashid, S., Bhat, B.A., and Mehta, G., Tetrahedron Lett., 2019, vol. 60, p. 1122. https://doi.org/10.1016/j.tetlet.2019.03.037
Rashid, S., Bhat, B.A., Sengupta, S., and Mehta, G., Asian J. Org. Chem., 2020, vol. 9, p. 449. https://doi.org/10.1002/ajoc.201900714
Rashid, S., Bhat, B.A., and Mehta, G., Asian J. Org. Chem., 2020, vol. 9, p. 1726. https://doi.org/10.1002/ajoc.202000401
Lone, A.M., Dar, N.A., Hamid, A., Shah, W.A., Ahmad, M., and Bhat, B.A., ACS Chem. Neurosci., 2016, vol. 7, p. 82. https://doi.org/10.1021/acschemneuro.5b00267
Masood-ur-Rahman, Mohammad, Y., Fazili, K.M., Bhat, K.A., and Ara, T., Steroids, 2017, vol. 118, p. 1. https://doi.org/10.1016/j.steroids.2016.11.003
Wang, Y., Shao, Y., Wang, Y., Fan, L., Yu, X., Zhi, X., Yang, C., Qu, H., Yao, X., and Xu, H., J. Agric. Food Chem., 2012, vol. 60, p. 8435. https://doi.org/10.1021/jf303069v
ACKNOWLEDGMENTS
B.A.G is highly thankful to Director NIT Srinagar for providing all types of facilities. B.A.G. also acknowledges Ministry of Human Resource Development (MHRD), New Delhi, for providing the Institute fellowship to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ganaie, B.A., Banday, J.A., Bhat, B.A. et al. Synthesis and In Vitro Anticancer Activity of Triazolyl Analogs of Podophyllotoxin, a Naturally Occurring Lignin. Russ J Org Chem 57, 2039–2047 (2021). https://doi.org/10.1134/S1070428021120216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428021120216