Abstract
Memory disorders are the progressive neurological disorder, mainly causing dementia, memory loss and cognitive dysfunctions. The current study is aimed to experimentally validate the crude extract of Canna indica aerial parts (CIA) and root (CIR) against aluminium chloride induced altered memory in rats. Initially, methanolic extract of CIA, hydroalcoholic extract of CIR, and their combination of CIA + CIR were screened for Invitro antioxidant activity via 2,2-diphenyl-1-picrylhydrazyl (DPPH) and nitric oxide (NO) assays, acetylcholinesterase (AChE) inhibitory assay and were also screened for their memory enhancing activity by in-vivo models such as elevated plus maze (EPM), morris water maze (MWM), cooks pole climb (CPC), Actophotometer, novel object recognition (NOR), and T-maze. Aluminium chloride (AlCl3) (17 mg/kg/day p.o.) for 21 days, was used as an Alzheimer’s disease inducing agent and Donepezil (AChE inhibitor) as a standard treatment agent. The AChE, butyrylcholinesterase (BChE) activity and malondialdehyde (MDA) level were significantly increased, and glutathione (GSH), total protein (TP), catalase (CAT), and Dopamine were decreased only in AlCl3 treated rats and treatment with CIA 200 mg/kg and CIA + CIR 200 mg/kg significantly reversed these mechanisms.Histopathology of cortex and hippocampus was examined at 40× magnification, indicating maintain of integrity and architecture of CA1 and CA3 neuronal cells compared to control and standard groups. The in vivo studies of interospective and exteroceptive behavior models (EPM), MWM, CPC, Actophotometer, NOR, T-maze revealed that AlCl3 administration enhanced transfer latency (TL), escape latency time (ELT) and decreases locomotion, discriminatory index, and percentage alternation respectively. However, treatment with CIA and CIA + CIR 200 mg/kg highly significantly reversed the pathological changes of disease, extracts of Canna indica of both root and aerial parts phyto constituents are rich in flavonoids, phlobatannins, anthocyanin pigments, saponins, alkaloids, steroids, terpenoids etc. Which will decipher the acetylcholinestrase inhibitory, antioxidant and anti-inflammatory activity, will ameliorate the pathological state of Alzheimer disease.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer disease (AD) is a devastative neurodegenerative syndrome (progressive memory decline) reflecting the dysfunction of cerebral nerve cells and abnormal neurotransmitter activities. Currently more than 55 million people live with dementia worldwide, and there are nearly 10 million new cases every year (WHO report 2021). India is going through a demographic shift, with the elderly population significantly increasing. In India, life expectancy has nearly doubled, from 36.98 years in 1950–1960 to 69.27 years in 2015–2020. In the twenty first century, understanding the complete etiology, pathogenesis, and ascertaining disease modifying agents/therapy against AD is one of the most important challenges. AD is biochemically characterized by the Aβ elevation, insoluble protein aggregates (neuritic senile plaques and neurofibrillary tangles) accumulation, central cholinergic and dopaminergic systems impairment, increased cerebral oxidative stress etc., while it is clinically characterized by performance abnormality, behavior worsening, thought leisureliness etc. AD is associated with a heterogeneous cause due to the multiple gene mutations and accounted for complex multi- proteins and multi-pathways mechanisms. (Blennow et al. 2006; Korczyn and Vakhapova, 2007; Bachurin et al. 2017). The inducing compound Aluminium chloride causes the highly reactive oxygen species (ROS) leading the Neuroinflammation, Aβ and Tau protein degradation characterizing similar to AD pathogenesis (Prema et al. 2017).
To date, AD symptoms can be managed with two classes of drugs that are approved by the US-FDA, which includes (I) Cholinesterase (ChE) inhibitors (II) NMD Aantagonists (Colović et al., 2013). However, these agents provide a partial improvement of memory and cognitive function and are associated with several side effects viz. bronchospasm, bradycardia, increased respiratory secretion, hypotension, decreased intraocular pressure, nausea and vomiting during long term therapy. Also, these agents lose effectiveness as the disease progresses (Ali et al. 2015).
Traditional medicinal plants have received valuable attention in the management of various ailments, India is considered as a Goldmine for medicinal plants and their traditional uses with less side effects and multiple target mechanism for the neurodegenerative diseases. WHO has recommended and encouraged the use of traditional herbs remedies during the COVID-19 pandemic, because of their therapeutic efficacy, safety and availability (Gautam et al. 2020).
Canna indica, a member of the “Cannaceae” family and “Canna” genus. Traditionally, rhizomes are used for gonorrhoea, dropsy, febrifuge, dyspepsia, and as an antipyretic through decoction, and roots were utilized against amenorrhoea (Kanase and Vishwakarma 2018). The leaves were utilized for malaria, rheumatic pain, and arthritis and possess Haemostatic effect (Lin et al 2011). The flowers were used for eye diseases and their decoction for external wound healing. Numerous pharmacological experiments demonstrated its therapeutics against inflammation, diabetes, viral diseases, immune related disorders, brain disorders including its effect on Acetylcholinesterase enzyme. (Chigurupati et al. 2021; Talluri et al. 2018) Canna indica whole plant extracts exhibited potential radical scavenging, anti-inflammatory and were reported to possess neuroprotective activity (Chigurupati et al. 2021). Canna indicia enriched with flavonoids, phlobatannins, anthocyanin pigments, tannins, saponins, cardiac glycosides, alkaloids, steroids, terpenoids, etc. bioactive molecules (Oladotun et al 2019).
However, there is a paucity of scientific reports on Canna indica L., constituent’s molecular mechanisms in the treatment of AD. Herein, we designed this study to explore the possible mechanisms of Canna indica aerial part and root extract in AlCl3 induced memory impairment by utilizing Invivo studies by interospective and exteroceptive behavior models as well Invitro biochemical estimations.
Materials used in the current study
Chemicals
Aluminium chloride (AlCl3) (Sigma, USA), Donepezil (DPZ) (Cipla, Goa), Acetylthiocholine iodide (Sigma, USA), Butyrylthiocholine iodide (Sigma, USA). 2,2-diphenyl-1 picrylhydrazyl (DPPH) Dopamine, DTNB (Sigma, USA) and other analytical graded chemicals.
Plant material
The aerial parts and root of Canna indica L. gathered from Sujoy and Aditya Nursery, Chicalim, Goa, India and authenticated by the plant taxonomist from ICMR-NITM, Belagavi. Accession number: RMRC -1584. The collected plant specimen was stored as an herbarium for future reference. The fresh plant material was washed properly using running water and to remove dust particles and shade dried at room temperature. The dried plant material was further crushed into coarse powder using a grinder and exposed to the extraction method.
Extraction
-
1.
The aerial part dried coarse powder 1 kg were subjected to the maceration technique using Solvent: methanol (95%) as a solvent
-
2.
The dried root parts coarse powder 1 kg was subjected to the maceration technique using Hydroalcohol (Water:Ethanol 3:7).
Both the mixtures were kept for 7 days with random shaking. After 7 days, the solvent is filtered out and concentrated under reduced pressure at 45–55 °C using a rotary evaporator. The obtained extract was sticky, which were further air-dried, collected in an amber color glass container, and stored in the refrigerator.
The marc was used for Soxhlet extraction with the respective solvents i.e. aerial marc (methanol-75%), Root part (ethanol-70%). Phytoconstituents screening tests were carried out using standard protocols.
Flavonoid and phenolic content
Total flavonoid content of methanolic extract of aerial part and hydroalcoholic extract of root part of Canna indica was performed as explained by Zhishen et al. (1999) with equivalent to Rutin Similarly, Total phenolic content of both the extracts were performed as mentioned by Baba et al (2014) with equivalent to gallic acid and IC50 value is expressed
In-vitro antioxidant activity
The DPPH free radical scavenging assay of the methanolic extract of the aerial part and hydroalcoholic extract of root part of Canna indica was performed as explained by Tharun and Kumar (2013) and Noreen et al. (2017). Similarly, the nitrous oxide free radical scavenging assay for both the extracts was carried out as illustrated by Boora et al. (2014). Ascorbic acid was used as a standard. Appropriate controls were taken for standard and test compounds and IC50 value was calculated.
In-vitro acetylcholinesterase activity
Methanolic extract of the aerial part and hydroalcoholic extract of the root part of Canna indica was evaluated for acetylcholinesterase inhibitory activity by using Ellman’s method (Dhanasekaran et al. 2015). The concentration range from 10 to 320 μg/mL for extract and 1 to 32 μg/mL for Donepezil was tested. 1.7 mL (pH 8.0) and 250 µL different concentrations of extract and Donepezil was prepared. To the above mixture, 10 µL of 6.67 U mL−1 AChE enzyme and 20 µL of DTNB was added. The mixture was incubated for 15 min, after incubation added 10µL of Acetylthiocholine iodide, and the absorbance was read at 412 nm every 45 s for 3 min. The percentage inhibition was calculated from the change in Abs w.r.t. change in time.
IC50 was calculated between the inhibition percentage v/s extraction concentration.
Experimental animals
To evaluate the memory enhancing capacity of the Canna indica, male Wistar rats having 250–300 g weight of 8 weeks old rats were used. All the animals were housed in a hygienic and translucent polypropylene cage. Further animals were randomized and made into six groups (N = 6 and n = 6) N = Number of groups and n = Number of animals in each group. All the animals were kept under 12/12 h natural light–dark cycle [27 °C temp. and 45–55%RH]. The overall research procedure was revised and approved by the IAEC, KLE College of Pharmacy, Belagavi.Resolution No- KLECOP/CPCSEA- Reg.No.221/Po/Re/S/2000/CPCSEA, Res. No 29–03/09/2020.
Experimental study design
Male Wistar rats were divided into 6 groups (Study Design) LD50 of Canna indica was reported to be 2000 mg/kg for 28 days with no mortality. Thus, an intermediate dose of 200 mg/kg was chosen from 1/10th of the LD50 for the experiment. Control group animals received normal food and water throughout the experiment. Negative Control (NC) group received AlCl3 (17 mg/kg/day p.o.) for 21 days. Positive Control (PC) group received AlCl3 (17 mg/kg/day p.o.) along with Donepezil (DPZ) (3 mg/kg p.o.) for 21 days. CIA200, CIR200, and CIA + CIR 200 group received AlCl3 (17 mg/kg/day p.o.) along with extracts for 21 days. Before initiating the study, acquisition trials were carried out for all the animals using Elevated plus maze (EPM), Morris water maze (MWM), Cooks pole climb (CPC), T-maze, novel object recognition (NOR), and Actophotometer. After subjecting to the test agent, changes in the Latency were examined on 0th (first dose) 7th, 14th and 21st day. After completion of the in-vivo studies, rats were euthanatized, brains were isolated and cerebral cortex, hippocampus AChE and BChE enzyme level and whole brain Dopamine, lipid peroxidation (LPO), reduced glutathione GSH, malondialdehyde (MDA), total protein (TP), superoxide dismutase (SOD) and catalase (CAT) level were measured.
In-vivo screening model for memory: Morris water maze (MWM) test
MWM is a big round water tank “diameter: 110 cm and height: 60 cm” consisting of a white surface and that is filled using opaque water (Temp. 26 ± 2 °C) to a distance (depth) of 30 cm. The MWM tank circle is divided into 4 equal quadrants [North Quadrant 1, East Quadrant 2, West Quadrant 3, and South Quadrant 4]. Further, it consists of a 10 cm diameter platform in a constant position. The ELT of the individual rat was noted at the 60 s cut-off time (Ishola et al. 2018; Biradar et al. 2020).
Index of memory improvement- Escape Latency Time (ELT).
Elevated plus maze (EPM) test
EPM contains of 2 closed and 2 open arms (four arms; equal dimensions of 50 × 10 cm). These 4 arms connect at central with a 10 × 10 cm square gap as an initial point. The transfer latency (TL) was recorded via stopwatch by keeping rats independently toward one side of the open arm. The EPM TL cut-off was set to 60 s throughout the study. In EPM, when the rat does not enter the closed arm, such rat was directed back into one of the closed arms, and 60 s TL was given. (Bhuvanendran et al. 2018 and Biradar et al. 2020).
Index of memory improvement -A drop of transfer Latency(TL) by test drug.
Locomotor activity by actophotometer test
The individual rat locomotion was assessed via actophotometer at a cut-off time of 5 min. The system consists of a photocell connected to the circuit with a counter. The beam of light passing on the photocell is cut off by the moving animal, then a count is recorded. On the 0th, 7th, 14th, and 21st day, individual group readings were noted. Before initiating the experiment, 10% ethanol was used to wipe the chamber for avoiding the animal odour. (Goverdhan et al. 2012).
Index of memory improvement -Increase in locomator activity neuromuscular coordination and spatial learning by test drug.
Cook’s pole climbing (CPC) test
The escape latency/conditional avoidance of each rat during learning and its retention was screened by CPC. The ground part consists in grid rods that acted as a shock stimulus. First, individual rats were trained and readings were noted acquisition, the retention trail was noted 0th, 7th, 14th, and 21st day. The cut-off time of 120 s was considered for evaluation (Goverdhan et al. 2012).
Index of memory improvement - Exterceptive punishment based valence memory and strength of the neuromuscular activity of the treated drug.
Novel object recognition (NOR) test
This test is utilized to assess short- and long-term memory. The test includes both acquisition and retention trials. During the acquisition trial two identical objects (green balls) were placed equidistant from each other. Further, for 60 s animals were placed in the arm and then placed back in the cage. The process was repeated for all the animals for 7 days (training period). During this trial after a period of 30 min, to avoid the bias for a particular location of identical and novel objects, interchanged the objects from left to right. After training, the same methodology was performed for the retention trial after a period of 30 min. Here, one green ball (identical object) is replaced with the red ball (novel object), and the time is taken to explore an object was noted (Antunes et al 2012)
Object exploration recording includes:
-
Animal head to orient towards the object within a distance of 2 cm.
-
Animal to sniffing, touching, and licking the object with the paws.
Discrimination index (DI in %) was calculated by using DI (%) = (A1 − A2)/(A1 + A2) and DI (%).
= (A1 − B)/(A1 + B) formula.
-
1.
Time spent with familiar object 1 (A1)
-
2.
Time spent with the familiar object 2 (A2)
-
3.
Time spent with the novel object (B)
Index of memory improvement: assessment of working memory of the treated drug.
T-maze test
The test involves both acquisition and retention sessions. Initially, animals were trained for 7 consecutive days. After that, on the 0th day (Treatment period) food was placed in one side arm (left arm we selected) before beginning the experiment. The overnight fasted animal is placed in the start box for 60 s. Then, a slow sliding door was opened noted the number of entries in the left arm (right choices). Similarly, the tests were repeated on day 12th and 21st day.
% Alternation was calculated by dividing the number of entries into the left arm in the acquisition and during the treatment period (Wenk et al. 1998).
Index of memory improvement: Assessment of reward based memory of the treated drug.
Biochemical estimation
AChE and BChE level in the cerebral cortex and hippocampus
At the end of the study, the rats were decapitated, their brains removed quickly and kept in ice cold saline. Cortex and hippocampus were isolated and placed in an ice-cold water container to stop the enzymatic reactions. Ellman’s method 1961 was followed to estimate AChE and BChE level in the cortex and hippocampus regions of the brain. For this, approximately 100 mg of the isolated sections was weighed and homogenated in Phosphate buffer [0.1 M; pH 8.0]. To 0.4 mL above mixture (homogenate) was added, (1) 2.6 mL 0.1 M (pH 8) Phosphate buffer (2) 100µL of 10 mM 5,5-dithio-bis-(2-nitrobenzoic acid (DTNB) and mixed well. Further, 20µL of Acetylthiocholine iodide (AChI) (0.075 M) was added to the above mixture. The Abs was then noted at 412 nm for 5 min “The rate of moles of substrate hydrolyzed/min/g of tissue is calculated by”
where R Rate, in moles of substrate hydrolyzed per min per gram of tissue. ΔA change in Abs per min, Co Conc. of tissue (mg/mL).
Determination of MDA level in LPO
To the 0.2 mL of tissue homogenate was taken. 0.2 mL of SLS (8.1% w/v) was added followed by 1.5 mL of 20% CH3COOH and 1.5 mL TBA (0.8%). The above mixture was vortexed for 1 min and diluted up to 4 mL using DM water. Further, it was boiled at 900C for 1 h using the water bath. The whole mixture was cooled and 1 mL of DM water was added followed by 5 mL mixture of “pyridine:n-butanol” (1:15 v/v). Lastly, the mixture was centrifuged at 4000 rpm for 10 min. The Abs of the organic layer was read at 532 nm. (Ohkawa et al. 1979; Ramakrishnan et al. 2015).
Cerebrum malondialdehyde (MDA) mg−1 of LPO level was estimated and expressed in nanomoles using the formula,
MDA = nmoles of MDA/mg of LPO (P) in the cerebrum is
where A = Abs at 535 nm, V = Volume of mixture, E = Extinction coefficient.
Determination of reduced γ-L-Glutamyl-L-cysteinyl-glycine (GSH)
Ellman GL 1959 method was utilized to estimate whole-brain reduced GSH level. To 0.25 mL of tissue homogenate, 2.5mL sodium phosphate buffer was added followed by 50µL (5,5-dithio-bis-(2-nitrobenzoic acid) DTNB [pH 7.0]. The mixture was then vortexed the above mixture and incubated at 25-27°C for 2min. Within 15min, at 412nm, Abs of the mixture was read and expressed γ-L-Glutamyl-L-cysteinyl-glycine (GSH) level in µmoles/mg of tissue.
where Co is the original concentration A is Ab at 412 nm, N is molar extinction coefficient i.e. 13,600/M/cm. Dilution factor (D).
Determination of SOD
The capacity of SOD to antagonize the auto-oxidation of epinephrine to adrenochrome in the presence of alkaline ph. To the 25 µL of homogenate sample, added 0.1 mM of epinephrine in carbonate buffer (pH 10.2). At 295 nm, the adrenochrome formation in the above mixture was measured using the ELISA platereader. Further, the SOD level was determined from the total protein value and expressed in U/mg of protein (Misra et al. 1972).
Determination of total protein
Taken, 2.25 mL of 0.5MNaOH was added to 0.25 mL of tissue homogenate. Then, 0.5 mL was pipetted out from the above mixture and 0.5 mL of 10% TCA was added followed by centrifugation at 3000 rpm for 10 min at 4 °C. After, this the supernatant was discarded and the remaining precipitate was dissolved in 0.5 mL of 0.5 M NaOH and 2 mL of Alkaline mixture. Further, after waiting for 10 min 0.25 mL of Folin Ciocâlteu reagent was added followed by another span of 10 min after which absorbance was read at 540 nm (Sedlak et al. 1968).
Determination of catalase
50 µL of tissue homogenate was mixed with 1.95 mL of PBS7.0 pH and 1 mL of 0.7 mM H2O2 solution. The absorbance was read at 240 nm. (Claiborne et al. 1985).
Determination of dopamine level
The level of dopamine in the whole brain was estimated via UV–Visible spectroscopy. First, the standard linear curve of dopamine was derived via serial dilution of standard dopamine (50 to 500 ng/mL).The detect in range of dopamine was set to 240–280 nm and the linear curve was obtained for Conc. v/s Abs. The supernatant obtained from each test group was diluted10 times and the absorbance was read at 278 nm to detect the dopamine. Using the y = mx + c obtained equation from the linear curve and the sample absorbance, the whole brain dopamine level was estimated and expressed in ng/g of tissue (Khale et al. 2018).
Histopathology examination
Animals were euthanized at the end of the study, and the brain was isolated and placed in 10% formalin. Haematoxylin and eosin were used to stained the cortex and hippocampus slides. An electroscopic microscope with a magnification of 10× and 40× was used to examine the lesions.
Statistical analysis
The results were presented as Mean, Standard Error of the Mean (SEM) and SD. One-way and two-way ANOVA were used to determine the difference between the groups, followed by Bonferroni and Tukey's comparison tests, utilizing GraphPad Prism 6.0. The difference between the groups was considered significant and probability level at the degree of freedom at 0.05 *,**,***,**** indicates p < 0.05, 0.01, 0.001, 0.0001 compared. Treatment group with Control group respectively & #, ##, ###,#### indicates p < 0.05, 0.01, 0.001, 0.0001 compared to Negative control with Treatment group respectively.
Results
Plant extraction and phytochemical screening
The extract obtained from the aerial and root part is 64 g and 73 g respectively. The percentage yield (w/w) of Canna indica L. aerial part methanolic and root hydroalcoholic extract was found to be 6.4 and 7.4% respectively. The preliminary phytochemical screening of the aerial and root parts of Canna indica revealed the presence of carbohydrates, proteins, amino acids, alkaloids, flavonoids, terpenoids, steroids, polyphenols, tannins and cardiac glycosides.
Total flavonoid and phenolic content
The total flavonoid content of the methanolic extract of aerial part and hydroalcoholic extract of the root part of Canna indica was found to be 58.58 rutin equivalent/g of dry extract weight and 55.25 mg rutin equivalent/g of dry extract weight, rutin respectively. Similarly, the total phenolic content of the hydroalcoholic extract of aerial part and hydroalcoholic extract of the root part of Canna indica was found to be 15 mg gallic acidequivalent/g of dry extract weight and 8.83mg gallic acid equivalent/g of dry extract weight.
In-vitro antioxidant assay
DPPH free radical scavenging assay
The IC50 of Ascorbic acid was found to be 84.8153 µg/mL and the IC50 of Canna indica aerial part methanolic extract, root hydroalcoholic extract, and in combination was found to be 15.793, 130.589, 102.92 µg/mL respectively. The DPPH radical scavenging activity of Canna indica aerial part methanolic extract was found to be more potent compared to root alone and in a combination of aerial and root parts. The % inhibition and IC50 value are shown in Fig. 1a.
NO free radical scavenging assay
The IC50 of Ascorbic acid was found to be 133.20 µg/mL and the IC50 of Canna indica aerial part methanolic extract, root hydroalcoholic extract, and in combination was found to be 120.99, 397.77, 298.87 µg/mL respectively. The NO radical scavenging activity of Canna indica aerial part methanolic extract was found to be more potent compared to root alone and in a combination of aerial and root parts. The % inhibition and IC50 value are shown in Fig. 1b
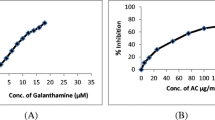
In-vitro AChE inhibitory activity
The IC50 of Donepezil was found to be 10.23 µg/mL and the IC50 of Canna indica aerial part methanolic extract and root hydroalcoholic extract was found to be 142.397 µg/mL and 214.666 µg/mL respectively. The AChE Inhibitory Activity of Canna indica aerial part methanolic extract was found to be more potent compared to root alone. The % inhibition and IC50 value are shown Fig. 2 and supplementary Table 1.
In-vivo studies
Elevated Plus maze (EPM): Effect of Canna indica on Transfer latency (TL)
The retrieval of memory “TL” from EPM was determined. A decline in the TL indicates the retrieval of memory. On the 0th day of the first treatment, the TL was found to be similar to that of Acquisition trial readings. However, on the 7th day a significant increase in transfer latency (TL) was observed in the Negative control group (NC) compared to the control group ***p < 0.001, whereas, treatment with Donepezil (PC) and CIA 200 showed a significant decline in the TL but no significant difference was observed in CIR 200 and CIA + CIR 200 group compared to NC group. Further, on the14th day and 21st day, a significant rise in TL was observed in the NC group compared to the control group ***p < 0.001, whereas the treatment groups saw a decline in the TL as compared to NC group Among the three treatment groups, CIA 200 and CIA + CIR 200 groups were found be most effective as compared to NC and Donepezil group ###p < 0.001. The effect of CIA, CIR, and CIA + CIR on TL is shown in Fig. 3a and supplementary Table 2.
Effect of Canna indica on a TL using EPM, b ELT using MWM, c) % change in locomotion using Actophotometer, d ELT using CPC.*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 is compared to Control group; #p < 0.05, ##p < 0.01 ###p < 0.001, and ****p < 0.0001 is compared to AlCl3 induced AD group
Morris water maze: effect of Canna indica on escape latency time (ELT)
Throughout the acquisition trial and on the 0th day no significant difference was observed, among the groups. However, on the 7th day, the Negative control group (NC) exhibited a rise in the Escape latency time (ELT) *** p < 0.001, whereas, treatment with CIA 200 and CIA + CIR 200 showed a decline in the ELT ## p < 0.01, however, no significant difference in ELT was observed in the Donepezil and CIR200 group compared to NC group## p < 0.01. Further, on the 14th day and 21st days a remarkable rise in ELT was observed in the NC group ****p < 0.0001compared to the control group, whereas, all the treatment groups exhibited a decline in ELT as compared to NC group #### p < 0.0001.The effect of CIA, CIR, and CIA + CIR on ELT is shown in Fig. 3b and supplementary table 3.
Actophotometer: effect of Canna indica on locomotion activity
Percentage change in locomotion was assessed by the actophotometer. On the 0th day and 7th day, no significant change in locomotion was observed among all the groups. However, on 14th and 21st day, a significant change in locomotion was observed in the NC group compared to the control group (− 1.57 ± 0.309) ****p < 0.0001. The treatment of Donepezil, CIA200 (0.762 ± 0.767) #### p < 0.0001 CIR 200, and CIA + CIR 200 (0.721 ± 0.583) #### p < 0.0001 reversed the decreased locomotion caused by AlCl3. The percentage change in locomotion is illustrated in Fig. 3c and supplementary Table 4.
CPC: effect of Canna indica on escape latency time (ELT)
During acquisition and 0th day, no significant difference in Escape latency time (ELT) was observed between the groups NC (16.33 ± 1.75)***p < 0.001 compared to CIR200 (15.67 ± 1.63). However, on the 7th, 14th and 21st days a significant increase in the ELT was seen in the NC group (23.5 ± 3.020)**** p < 0.0001as compared to the control group (11 ± 1.79), while treatment with Donepezil, CIA200 (12.5 ± 1.64) #### p < 0.0001 and CIA + CIR 200 decreased the ELT (14.5 ± 2.88) #### p < 0.0001,When compared to the NC group (23.5 ± 3.02)**** p < 0.0001.The effect of Canna indica on ELT in CPC is illustrated in Fig. 3c and supplementary Table 5.
NOR: effect of Canna indica on discriminatory index
The effect of Canna indica on memory was evaluated via NOR by discrimination index for both A1–A2 and A1-B1 (Supplementary table 6 and Fig. 4). On the 0th and 21st day, no significant difference was observed among all the groups for time spent with A1–A2 i.e., there was no significant difference in the time spent by the rats with the two familiar objects. However, on the 0th day, All the groups showed remarkable increase in time spent with B object compared to A object i.e., the time spent by the rats with the novel object (red box) was considerably higher than the time spent with the familiar object (green ball) suggestive of good retention of memory and natural exploratory behavior of the rats towards the novel object.
Effect of Canna indica on novel object recognition a A1–A2 on 0th day b A1–A2 on 21st day, c A1-B on 0th day, d A1-B on 21st day, e DI of A1-B on 0th day, and f DI of A1-B on 21st day.*p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 is compared to Control group; #p < 0.05, ##p < 0.01 ###p < 0.001, and ****p < 0.0001 is compared to AlCl3induced AD group
Similarly, on the 21st day, a significant increase in novel object recognition (time spent with object B) was observed in control, CIA 200 and CIA + CIR compared to A1 object (##p < 0.01). Whereas, NC, PC, CIR 200 groups animals didn’t show a significant increase in novel object recognition behavior. As for the discriminatory index, there was no significant difference in the DI among all the groups on the 0th day. On the other hand, a remarkable decline in DI was seen in the NC compared to the Control whereas PC, CIA 200, and CIA + CIR 200 group saw a remarkable rise in the DI.
T-maze
The effect of Canna.indica on % Alternation was screened via T-maze. During 0th and 12th day, no significant difference in %. Alternation is observed between the groups. However, on 21st day, a remarkable decline in % Alternation in the NC group was seen compared to the control group while % Alternation in the CIA 200 and CIA + CIR 200 group was remarkably raised when compared to the NC group(##p < 0.01). The effect of Canna. indica on % Alternation in T-maze is illustrated in Fig. 5 and Table 7.
In-vivo biochemical estimations
Acetylcholinesterase level [Hippocampus and Cerebral Cortex]:
-
(A)
Canna indica effect on AChE level.
The treatment of AlCl3 in the NC group remarkably increased the level of AChE in the cortex and hippocampus compared to the control group (4.66 ± 0.65). However, treatment with Donepezil, CIA200, CIR200, and CIA + CIR 200 significantly decreased the level of AChE in the cortex (###p < 0.001) and hippocampus (p < 0.001***) compared to the NC group. All three treatment groups of Canna indica exhibited a potent inhibitory effect on AChE. The effect of Canna indica on AChE enzyme level in the cerebral cortex and hippocampus is shown in supplementary table 8 and Fig. 6a, b respectively.
-
(B)
Canna indica effect on BChElevel.
Effect of Canna indica on brain AChE and BChE level. a AChE in cortex, b AChE in Hippocamous, c BChE in cortex, and d BChE in Hippocampus. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 is compared to Normal group; #p < 0.05, ##p < 0.01 ###p < 0.001, and ****p < 0.0001 is compared to AlCl3 induced AD group
The treatment of AlCl3 significantly increased the level of BChE in the cortex and hippocampus in the NC group as compared to the control group. However, treatment with Donepezil didn’t show a significant effect on BChE level in both cortex and hippocampus compared to the NC group while treatment with CIA 200, CIR 200, and CIA + CIR 200 (0.284 ± 0.153) #### p < 0.0001 significantly decreased the level of BChE in the cortex when compared to the NC group (1.07 ± 0.11)**** p < 0.0001, On the other hand, only CIA 200 and CIA + CIR 200 (0.326 ± 0.095) #### p < 0.0001 significantly decreased the level of BChE in the hippocampus and CIR200 didn’t show any effect as compared to the NC group (0.936 ± 0.079**** p < 0.0001. Among three treatment groups of Canna indica, CIA 200 and CIA + CIR 200 exhibited a potent inhibitory effect on BChE. The effect of Canna indica on BChE enzyme level in the cerebral cortex and hippocampus is shown in supplementary table 9 and Fig. 6c, d respectively.
Canna indica effect on reduced GSH
Treatment with AlCl3 in the NC group remarkably reduced GSH level in the whole brain compared to the control group. However, treatment with Donepezil, CIA 200, and CIA + CIR 200 (### p < 0.001) significantly increased the level of GSH compared to the NC group (***p < 0.001). Further, no significant change in GSH level is observed in the CIR200group compared to the NC group. The effect of Canna indica on whole brain GSH level is shown in supplementary Table 10 and Fig. 7a.
Effect of C. indica on whole brain antioxidant markers and dopamine level. a GSH, b LPO, c TP, d SOD, e CAT, and f Dopamine level. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 is compared to Normal group; #p < 0.05, ##p < 0.01 ###p < 0.001, and ****p < 0.0001 is compared to AlCl3 induced AD group
Canna indica effect on malondialdehyde (MDA) level
Treatment with AlCl3 in the NC group remarkably raised MDA level in the whole brain compared to the control group. However, treatment with Donepezil, CIA200, and CIA + CIR 200 (###p < 0.001) remarkably decreased the level of MDA compared to the NC group (***p < 0.001). Further, no significant change in MDA level is observed in the CIR200 group compared to the NC group. The effect of Canna indica on whole-brain MDA level is shown in supplementary table 10 and Fig. 7b.
Canna indica effect on total protein (TP)
The treatment with AlCl3 in the NC group didn’t show a significant change in TP level but showed a slight increase in TP level in the whole brain compared to normal. However, treatment with Donepezil showed, not significant but decrease in the TP level compared to the NC. Further, CIA + CIR 200 and CIR200 didn’t show a change in TP level, however, CIA 200 showed not significant but a slight decrease in TP level compared to the NC group. The effect of Canna indica on whole brain TP level is shown in supplementary table 10 and Fig. 7c respectively.
Canna indica effect on superoxide dismutase (SOD)
The treatment with AlCl3 in the NC group decreased the SOD level in the whole cerebrum compared to normal but data was found statistically non-significant. Also, Donepezil, CIA 200, CIR 200, and CIA + CIR 200 treatment didn’t show any significant change in SOD level compared to the NC group. The effect of Canna indica on whole brain SOD level is shown in supplementary Table 10 and Fig. 7d.
Canna indica effect on catalase CAT
Treatment with AlCl3 in the NC group didn’t show a significant change in CAT level but showed a decrease in CAT level in the whole cerebrum. Also, Donepezil treatment showed slight increase in the CAT level but didn’t show any significant change compared to the negative control. Further, CIA 200 and CIR 200 didn’t show CAT activity, however, combination therapy CIA + CIR 200 showed not significant but a slight increase in CAT level compared to the NC group. The effect of Canna indica on whole brain CAT level is shown in supplementary Table 10 and Fig. 7e.
Canna indica effect on dopamine level
Treatment with AlCl3 in the NC group remarkably decreased Dopamine level in the whole cerebrum compared to the control group. However, treatment with Donepezil, CIA 200, CIR 200 and CIA + CIR 200 (0.123 ± 0.026) #### p < 0.0001 remarkably increased the level of Dopamine as compared to the NC group (0.04 ± 0.007****). The effect of Canna indica on whole brain Dopamine level is shown in supplementary Table 11 and Fig. 7f.
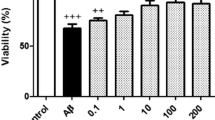
Effect of Canna indica on whole-brain histopathology
The histology of the control group cerebrum indicated mild cerebral oedema, cerebral congestion, neuronal eosinophilia, meningeal congestion, and no change was observed in RBC extravasation, macrophage influx, neuronal micro vacuolization, neuronal nuclear pyknosis, neutrophilic infiltration, neuronal karyorrhexis, reactive gliosis and vascular proliferation. However, the NC group histogram indicated severe damage in the above mentioned parameters (+ + + to +). The PC and CIA and CIA + CIR groups reversed the AlCl3 induced damage. The histopathology of the brain (cortex and hippocampus) at 10 × and 40 × magnification is shown in Figs. 8 and Fig. 9 respectively and Table 12 (Table 1).
Discussion
The neuroprotective and memory enhancing mechanism of action of Canna indica has been shown in this study via in vitro and in vivo. Firstly, using the interceptive and exterceptive models like MWM, EPM, Actophotometer, CPC, NOR, and T-Maze, analyzing the biochemical (AChE, BChE, GSH, MDA, TP, SOD, CAT, Dopamine) level from the cerebrum, the memory restoring function of Canna indica against AlCl3 mediated memory dysfunction in rats was investigated. The MWM was used to assess spatial memory. Administration of AlCl3 significantly raises the ELT and treatment with Donepezil and Canna indica [A+R] indicated a critical decline in ELT. EPM was used to determine the transfer latency (TL). The TL was significantly increased in the AlCl3 treated rats. However, prolonged Donepezil and Canna indica [A+R] treatment for 21 days lead to a remarkable reduction in TL. The change in body movement was observed by measuring locomotor activity through actophotometer. Administration of AlCl3 significantly decreased locomotory activity in rats and treatment with Donepezil and Canna indica reversed this mechanism. Further, CPC was used to investigate cognitive function, primarily a response to conditioned stimuli during learning and memory. AD induced using AlCl3 exhibited a noteworthy rise in ELT on day 21st and treatment with Canna indica [A+R] and Donepezil successfully reversed this change. Further, to analyze the recognition memory and the ability to discriminate between two discrete objects, the NOR apparatus was used. Treatment with AlCl3 exhibited a significant decline in the discriminatory index on day 21st while treatment with Canna indica [A andA+R] and Donepezil remarkably raised the discriminatory index. Finally, the exploratory behavior as well as the working memory of the rats was analyzed by using the T-maze apparatus. Treatment with AlCl3 exhibited a remarkable decline in % alternation on day 21st and treatment with Canna indica [A+R] significantly raised the % alternation. These findings suggest that Canna indica memory improving function protects against AlCl3 Induced memory loss.
Cholinesterase’s function, GSH, MDA, TP, SOD, CAT, and Dopamine level in the cerebrum were examined. The level of ACh and BCh chemicals associated with memory improvement, is diminished in AD because of fast hydrolysis by AChE and BChE action respectively. In the present study, the Invitro results suggest that CIA and CIR possess an AChE inhibitory potency. Similarly, chronic administration of CIA and CIR in rats exhibited a remarkable reduction in the AChE and BChE activity in both cortex and hippocampus, which suggests that Canna indica [A and R] both possess AChE and BChE inhibitory activity in a dose dependent manner. This may be due to the presence of various phytoconstituents in the plant such as flavonoids, terpenes, sesquiterpenes and sterols.
During physiological and pathological processes, free radicals and reactive oxygen species (ROS) are generated ROS are important in phagocytosis, enzyme activation, and cell cycle control, among other cell signaling pathways. (Manoharan et al. 2016) An imbalance in free radical and reactive oxygen species (ROS) formation weakest the antioxidant defense system, resulting in cell damage and inflammation. O3, H2O2, and NO2 are the primary free radicals involved in oxidative stress and the development of AD.Further, more the generation (production) of ROS causes a variety of undesirable effects, including DNA harm, lipid peroxidation, and protein mutilation. It is well-known that “plant extracts with higher flavonoid and phenolic content have the highest DPPH scavenging and nitric oxide scavenging activity” (Manoharan et al. 2016). In the present study, in vitro antioxidant assays showed that Canna indica to possess DPPH and NO radical scavenging activity. Further, administration of CIA and CIR showed remarkable change in the antioxidant level in the whole brain viz., GSH, MDA, TP, and CAT level. The antioxidant effect of Canna indica may be due to the abundant presence of flavonoids and triterpenoids in the plant. Further, Dopamine a neurotransmitter present in the VTA, is involved in reward motivated actions and aids in the regulation of expression and the formation of new memories. In the present study investigation, AlCl3 administration significantly decreased the level of Dopamine, and treatment with Donepezil and Canna indica reversed the above changes in dopamine level. Lastly, the histopathology examination of the brain of the normal animals revealed no significant changes, while the histopathology of the cerebrum in animals treated with AlCl3 revealed significant changes viz., cerebral oedema, cerebral congestion, neuronal eosinophilia, meningeal congestion etc. Also, it was found that CA1 neurons and CA3 neurons (subdivision of CA1 neurons) which are majorly implicated in the formation and retrieval of hippocampal dependent memories were significantly disrupted. (Bartsch et al. 2011) Treatment with Donepezil and Canna indica on the other hand, resulted in normal histopathology of the cerebrum, indicating that Donepezil and Canna indica reversed the harm caused by AlCl3.
Conclusion
In this study, In vivo and In vitro experiments were employed to decode the intramolecular interaction of Canna indica memory enhancement activity. Chronic Canna indica therapy improves memory damage caused by AlCl3 by restoring cholinergic system activity, regulating antioxidant pathways, and increasing dopamine levels. The study utilized MWM, EPM, CPC, Actophotometer, NOR, and T-Maze to demonstrate the memory functions modulated by both AlCl3 and Canna indica. It can be said that the study results are comparable with the clinically approved molecule Donepezil. However, the current study is only limited to preclinical and which further validation using other AD models and gene expression level studies are needed to be carryout to explore the Canna indica pharmacotherapy in Alzheimer disease.
Abbreviations
- AD:
-
Alzhemeir disease
- AlCl3 :
-
Aluminum chloride
- AChE:
-
Acetylcholinestrase enzyme
- BChE:
-
Butrylcholinestrase enzyme
- ELT:
-
Escape latency time
- CIA + CIR200 :
-
Canna indica aerial + root part (200 mg/kg) Group
- CIA200:
-
Canna indica aerial part (200 mg/kg) Group
- CIR200:
-
Canna indica root part (200 mg/kg) Group
- CPC:
-
Cook’s pole climbing
- CAT:
-
Catalase
- EPM:
-
Elevated plus maze
- DPZ:
-
Donepezil
- GSH:
-
Glutathione
- IC50 :
-
Inhibition concentration
- LPO:
-
Lipid peroxidation
- MDA:
-
Malondialdehyde
- LPO:
-
Lipid peroxidation
- MWM:
-
Morris water maze
- NOR:
-
Novel object recognition
- N:
-
Number of groups
- NC:
-
Negative control
- PC:
-
Positive control
- p.o.:
-
Per. oral
- TL:
-
Transfer latency
- SOD:
-
Super oxide dismutase
- STL:
-
Step through latency
- TP:
-
Total protein
- ROS:
-
Reactive oxygen species
References
Alan MP (2002) (2002) Pharmacotherapy for Alzheimer’s disease: progress and prospects. Trends Pharmacol Sci 23(9):426–433
Ali TB, Schleret TR, Reilly BM, Chen WY, Abagyan R (2015) Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance data bases of the United-States and Canada. PLoS ONE 10(12):1–10
Alistair B, Jane BE, Konrad M (2002) Alzheimer’s disease. Lancet 360:163–165
Ann EP, Vazdarjanova A (2003) McGaugh JL Muscarinic cholinergic influences in memory consolidation. Neurobio Learn Memory 80:178–193
Antunes M, Biala G (2012) The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13(2):93–110. https://doi.org/10.1007/s10339-011-0430-z
Baba SA, Malik SA (2014) Evaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurrooroyle. Saudi J Biol Sci 21:493–498. https://doi.org/10.1016/j.sjbs.2014.06.004
Bachurin SO, Bovina EV, Ustyugov AA (2017) Drugs in clinical trials for Alzheimer’s disease: the major trends. Med Res Rev 5:1186–1225
Bartsch T, Dohring J, Rohr A, Jansen O (2011) Deuschl G (2011) CA1 neurons in the human hippocampus are critical for autobiographical memory, mental time travel, and autonoetic consciousness. Proc Natl Acad Sci 108(42):17562–17567
Bhuvanendran S, Kumari Y, Othman I (2018) Amelioration of cognitive deficit by Embelin in a Scopolamine-induced Alzheimer’s disease-like condition in a rat model. Front Pharmacol 9(6):1–12
Biradar P, Patil V, Joshi H, Khanal P, Mallapur S (2020) Experimental validation and network pharmacology evaluation to decipher the mechanism of action of Erythrina variegata L. bark against scopolamine-induced memory impairment in rats. Adv Tradit Med 26:1–4
Blennow K, De LMJ, Zetterberg H (2006) Alzheimer’s Dis 368:387–403
Boora F, Chirisa E, Mukanganyama S (2014) Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J Food Process 2014:1–7
Cacabelos R, Alvarez A, Lombardi V (2000) Pharmacological treatment of Alzheimer disease: from psychotropic drugs and cholinesterase inhibitors to pharmacogenomics. Drug Today (barc) 36(7):415–499
Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, Dekosky ST (1998) Prevalence of Alzheimer’s disease and other demands in rural India. Neurology 51:1000–1008
Chigurupati S, Alharbi NA, Sharma AK, Alhowail A, Vardharajula VR, Vijayabalan S, Das S, Kauser F, Amin E (2021) Pharmacological and pharmacognostical valuation of Canna indica leaves extract by quantifying safety profile and neuroprotective potential. Saudi Journal of Biological Sciences. 2021 Jun 1
Claiborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC hand book of methods for oxygen radical research. CRC Press, Boca Raton, Florida, USA, pp 283–284
Colović MB, Krstić DZ, Lazarević-Pašti TD, Bondžić AM, Vasić VM (2013) Acetylcholinesterase inhibitors: pharmacology and toxicology. Currneuropharmacol 11(3):315–335
Cummings J, Lee G, Mortsdorf T, Ritter A, Zhong K (2017) Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s Dement Transl Res Clin Interv 3(3):367–384
Deutsch JA (1971) The cholinergic synapse and the site of memory. Science 174:788–794
Dhanasekaran S, Perumal P, Palayan M (2015) In-vitro Screening for acetylcholinesterase enzyme inhibition potential and antioxidant activity of extracts of Ipomoea aquatica Forsk: therapeutic lead for Alzheimer’s disease. J Appl Pharm Sci 5:12–126
Du X, Wang X, Geng M (2018) Alzheimer’s disease hypothesis and related therapies. Translational Neurodegeneration 7(2):1–7
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. BiochemPharmaco 7(2):88–95
Ezio G (2000) Cholinesterase inhibitors stabilize Alzheimer’s disease. Neurochem Res 25(9/10):1185–1190
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: Targeting the Cholinergic System. CurrNeuropharmacol 14(1):101–115
Gautam S, Gautam A, Chhetri S, Bhattarai U (2020) Immunity Against COVID-19: Potential Role of AyushKwath. J Ayurveda Integr Med
Gianni B, Antonio M (1998) Is there a rational effort the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease. Euro J Pharmacol 346:1–13
Goverdhan P, Sravanthi A, Mamatha T (2012) Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int J Alzheimer’s Dis
Hippius H, Neundörfer G (2003) The discovery of Alzheimer’s disease. Dialogues Clin Neurosci 5(1):101–108
Ishola IO, Ikuomola BO, Adeyemi OO (2018) Protective role of Spondiasmombin leaf and Cola acuminata seed extracts against scopolamine- induced cognitive dysfunction. Alexandria J Med 54(1):27–39
Kanase V, Vishwakarma S (2018) Treatment of various diseases by Canna indica L. promising herb. Asian J Pharm Clin Res 11(12):51–56
Kasture S (2003) Pharmacotherapy of Alzheimer’s disease. Proceedings of the AICTE sponsored national seminar; 2003 Aug 22–23; Guru Jambheshwar University (Haryana) 31–41
Khale AS, Kolhe SU, Tembhurne SV (2018) Neuropharmacological evaluation of Sesbania sesban using experimental animals. World J Pharm Res 7(12):698–717
Korczyn AD, Vakhapova V (2007) The prevention of the dementia epidemic. J Neurol Sci 257:2–4
Kosasa T, Kuriya Y, Matsui K, Yamanishi Y (2000) Inhibitory effect of orally administered donepezil hydrochloride (E2020), an over treatment for Alzheimer’s disease, on cholinesterase activity in rats 389:173–179
Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM (2011) Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology 25(6):763
Mahdi O, Baharuldin MT, Nor NH, Chiroma SM, Jagadeesan S, Moklas MA (2019) Chemicals used for the induction of Alzheimer’s disease-like cognitive dysfunctions in rodents. Biomed Res Ther 6(11):3460–3484
Manoharan S, Guillemin GJ, Abiramasundari RS, Essa MM, Akbar M, Akbar MD (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s Disease: a mini review. Oxid Med Cell Longev 2016:1–15
Margaret ME (2001) Is an effective immune intervention for Alzheimer’s disease in prospect? Trends Pharmacol Sci 22(1):2–3
Mayo clinic Alzheimer's: drugs help manage symptoms. https://www.mayoclinic.org/diseasesconditions/alzheimersdisease/indepth/alzheimers/art-20048103. Assessed on 05 Dec 2021
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a sample assay for superoxide dismutase. J Biol Chem 247:3170–3175
Nigel HG, Tada U (2001) Qian-Sheng Y A new therapeutic target in Alzheimer’s disease treatment: attention to butyrylcholinesterase. Curr Med Res Opin 17(3):159–165
Noreen H, Semmar N, Farman M, Mccullagh JSO (2017) Measurement of total phenolic content and antioxidant activity of aerial parts of medicinal plant Coronopusdidymus. Asian Pac J Trop Med
Ogura H, Kosasa T, Araki S (2000) Yamanishi Y Pharmacologicalpropertiesofdonepezil hydrochloride (Aricept) a drug for Alzheimer’s disease. Nihon YakurigakuZasshi 115(1):45–51
Ohkawa H, Ohishi N, Yagi K (1979) Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction 358:351–358
Oladotun PB, Akinsiku AA, Adeyemi AO et al (2019) Qualitative analysis, total phenolic content, FT-IR and GC-MS characterisation of Canna indica: bio reducing agent for nanoparticles synthesis. J Phys: Conf Ser 1299:012135
Palle S, Neerati P (2017) Quercetin nanoparticles attenuates scopolamine induced spatial memory deficits and pathological damages in rats. Bull Fac Pharmacy, Cairo Univ 55(1):101–106
Parihar MS, Hemnani T (2004) Alzheimer’s disease pathogenesis and therapeutic interventions. J Clin Neurosci 11(5):456–467
Paul EG (2003) Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Memory 80:194–210
Prema A, Justin Thenmozhi A, Manivasagam T, Mohamed Essa M, Guillemin GJ (2017) Fenugreek seed powder attenuated aluminum chloride-induced tau pathology, oxidative stress, and inflammation in a rat model of Alzheimer’s disease. J Alzheimers Dis 60(s1):S209–S220
Ramakrishnan P, Chandrasekhar T, Muralidharan P. (2015) Cognitive enhancing, antiacetylcholinesterase, and antioxidant properties of Tagetespatulaons copolamine induced amnesia in mice. Int J Green Pharm 167–174.
Raymond T, Bartus, Reginald L (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217:408–417
Sarje SK, Ingole K, Angad S, Priya B, Ghiware NB (2019) A pharmacognostic and pharmacological review on Canna indica Linn. Int J Res Pharm Chem 9(3):61–77
Scott A, Small MD (2001) Age-related memory decline. Arch Neurol 58:360–364
Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Shigeta M, Homma A (2001) Donepezil for Alzheimer’s disease: pharmacodynamic, pharmacokinetic, and clinical profiles. CNS Drug Rev 7(4):353–368
Sugimoto H (2008) The new approach in development of anti-Alzheimer’s disease drugs via the cholinergic hypothesis. Chemico Biol Interactions 175:204–208
Sweatt JD (2009) Aging related memory disorders-Alzheimer’s Disease. Elsevier Inc. 2009:292–319
Talluri MR, Killari KN, Viswanadha Murthy Manepalli N, Kanduri P, Kiran kumar B (2018) Protective effect of Canna indica on cerebral ischemia-reperfusion injury in rats, Agric Nat Resour
Terry Jr. AV, Buccafusco JJ (2003) The cholinergic hypothesis of age an Alzheimer’s disease related cognitived eficits: recent challenges and the implications for novel drug development. J Pharmacol Exp Ther 306(3):821–827
Tharun G, Kumar P (2013) Evaluation of antioxidant potential and antimicrobial activity of successive extracts of Pimpinella tirupatiensis. JOPR J Pharm Res 7(9):817–822
Wenk GL (1998) Assessment of spatial memory using the T maze. Curr Protoc Neurosci 4(1):8–5
Wikipedia. Canna indica.https://en.wikipedia.org/wiki/Canna_indica. Accessed on 24 Apr 2021
Zhishen J, Mengcheng T, Jianming W (1999) (1999) The determination of flavonoid content in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Acknowledgements
The authors thanks to Prof. Dr. S.S. Jalapure Principal, and Dr. N.A. Khatib, HOD of department of Pharmacology and Toxicology, KLE college of Pharmacy, Belagavi for providing necessary facilities to conduct the work. Authors thanks to Cipla, Verna, Goa for providing Donepezil HCl as a gift sample.
Funding
None.
Author information
Authors and Affiliations
Contributions
Prakash R. Biradar helped Conceptualization the study design, critical review and analysis of the manuscript and supervised the experiments. Prachi Ojha designed the study, conducted the experiments, and drafted the manuscript. Siddarth Tubachi, Vishal Patil assisted in data analysis and manuscript.
Corresponding author
Ethics declarations
Ethical statement
The study protocol was reviewed and approved by the Institutional Animal Ethics Committee, KLE College of Pharmacy, Belagavi, and Resolution No. KLECOP/CPCSEA- Reg.No.221/Po/Re/S/2000/CPCSEA, Res. No 29-03/09/2020. The animal experiments were carried out in accordance with the CPCSEA guidelines.
Conflict of interest
Prachi S. Ojha has no conflict of interest. Prakash R. Biradar has no conflict of interest. Siddarth Tubachi has no conflict of interest. Vishal S. Patil has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ojha, P.S., Biradar, P.R., Tubachi, S. et al. Evaluation of neuroprotective effects of Canna indica L against aluminium chloride induced memory impairment in rats. ADV TRADIT MED (ADTM) 23, 539–556 (2023). https://doi.org/10.1007/s13596-021-00627-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13596-021-00627-x