Abstract
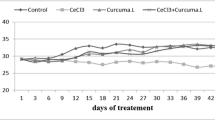
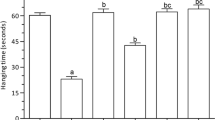
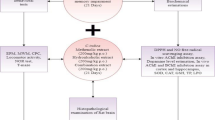
Aluminum (Al) is linked to the development of many neurological disorders such as Alzheimer’s disease (AD), Parkinson’s disease, and autism. Centella asiatica (CA) is a regenerating herb traditionally used to stimulate memory. This study was designed to assess the neuroprotective role of ethanolic extract of CA (CAE) in AlCl3-induced neurological conditions in rats. Adult rats were chronically treated with AlCl3 (100 mg/kg b.w./day) for 60 days to establish the dementia model, and co-administration of CAE was evaluated for its ability to attenuate the toxic effect of AlCl3. CAE was given orally at a dose of 150 and 300 mg/kg b.w./day, for 60 days. The behavioral performances of rats were tested through Y-maze and open field tests. Lipid peroxidation, superoxide dismutase, and catalase activity were evaluated to measure oxidative stress; and acetylcholinesterase (AChE) activity was assessed to evaluate cholinergic dysfunction in the rat brain. H&E staining was used to assess structural abnormalities in the cortex and hippocampus. The result showed that AlCl3 induces cognitive dysfunction (impaired learning and memory, anxiety, diminished locomotor activity), oxidative stress, cholinergic impairment, and histopathological alteration in the rat brain. Co-administration of CAE with AlCl3 markedly protects the brain from AlCl3-induced cognitive dysfunction, oxidative stress, AChE activity, and cytoarchitectural alterations. Furthermore, 15 days CAE treatment after 45 days AlCl3 administration markedly ameliorates the AlCl3-induced neurotoxicity indicating its potential for therapeutic use.
Graphical abstract








Similar content being viewed by others
Data Availability
Not applicable.
References
Exley C (2013) Human exposure to aluminium. Environ Sci Process Impacts 15(10):1807–1816
Grochowski C et al (2019) Increased aluminum content in certain brain structures is correlated with higher silicon concentration in alcoholic use disorder. Molecules 24(9):1721
Mold M et al (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol 46:76–82
Yuan C-Y, Lee Y-J, Hsu G-SW (2012) Aluminum overload increases oxidative stress in four functional brain areas of neonatal rats. J Biomed Sci 19(1):51
Gómez M et al (2008) Aluminum exposure through the diet: metal levels in AβPP transgenic mice, a model for Alzheimer’s disease. Toxicology 249(2–3):214–219
Becaria A, Campbell A, Bondy S (2002) Aluminum as a toxicant. Toxicol Ind Health 18(7):309–320
Wu Z et al (2012) Aluminum induces neurodegeneration and its toxicity arises from increased iron accumulation and reactive oxygen species (ROS) production. Neurobiol Aging 33(1): 199. e1–199. e12.
Al-Amin MM et al (2016) Astaxanthin ameliorates aluminum chloride-induced spatial memory impairment and neuronal oxidative stress in mice. Eur J Pharmacol 777:60–69
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol. https://doi.org/10.3389/fneur.2019.00399
Kosari S et al (2012) Effect of western and high fat diets on memory and cholinergic measures in the rat. Behav Brain Res 235(1):98–103
Batool Z et al (2016) Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res Bull 120:63–74
Wang B et al (2010) Effects of chronic aluminum exposure on memory through multiple signal transduction pathways. Environ Toxicol Pharmacol 29(3):308–313
Ribes D et al (2010) Impaired spatial learning and unaltered neurogenesis in a transgenic model of Alzheimer’s disease after oral aluminum exposure. Curr Alzheimer Res 7(5):401–408
Firdaus Z, Singh TD (2021) An insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev Med Chem 21(1):35–57
Gray NE et al (2018) Centella asiatica: phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev 17(1):161–194
Gohil KJ, Patel JA, Gajjar AK (2010) Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci 72(5):546
Krishnamurthy RG et al (2009) Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res 87(11):2541–2550
Rather MA et al (2018) Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front Biosci (Schol Ed) 10:262–275
Gupta R, Flora S (2006) Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J Appl Toxicol 26(3):213–222
Rao SB, Chetana M, Devi PU (2005) Centella asiatica treatment during postnatal period enhances learning and memory in mice. Physiol Behav 86(4):449–457
Mohandas Rao K, Muddanna Rao S, Gurumadhva Rao S (2006) Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evidence-Based Complementary and Alternative Medicine 3(3): 349–357
Chiroma SM et al (2019) Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their hippocampus. Int J Mol Sci 20(8):1871
Chiroma SM et al (2019) Protective effects of centella asiatica on cognitive deficits induced by D-gal/AlCl3 via inhibition of oxidative stress and attenuation of acetylcholinesterase level. Toxics 7(2):19
Chiroma SM et al (2019) Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed Pharmacother 109:853–864
Amjad S, Umesalma S (2015) Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain-histopathological, and biochemical approach. J Molec Biomark Diag 6(1):1
Firdaus Z et al (2020) Centella asiatica prevents D-galactose-induced cognitive deficits, oxidative stress and neurodegeneration in the adult rat brain. Drug Chem Toxicol. https://doi.org/10.1080/01480545.2020.1833907
Singla N, Dhawan D (2014) Zinc modulates aluminium-induced oxidative stress and cellular injury in rat brain. Metallomics 6(10):1941–1950
Kumar A, Prakash A, Dogra S (2011) Centella asiatica attenuates D-galactose-induced cognitive impairment, oxidative and mitochondrial dysfunction in mice. Int J Alzheimer’s Dis. https://doi.org/10.4061/2011/347569
Foyet HS et al (2015) Emilia coccinae (SIMS) G extract improves memory impairment, cholinergic dysfunction, and oxidative stress damage in scopolamine-treated rats. BMC Complement Altern Med 15(1):333
Poimenova A et al (2010) Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience 167(3):741–749
Lee M-S et al (2013) Novel antidepressant-like activity of propolis extract mediated by enhanced glucocorticoid receptor function in the hippocampus. Evidence-Based Complement Alternat Med. https://doi.org/10.1155/2013/217853
Wills E (1965) Mechanisms of lipid peroxide formation in tissues role of metals and haematin proteins in the catalysis of the oxidation of unsaturated fatty acids. Biochimica et Biophysica Acta (BBA)-Lipids Lipid Metabol 98(2): 238–251
Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmacological effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology 214(4):819–828
Aebi H (1974) Catalase. In: Bergmeyer, HU (ed) Methods of Enzymatic Analysis, 2nd edn. Verlag Chemie/Academic Press Inc., Weinheim/NewYork, 673-684. https://doi.org/10.1016/B978-0-12-091302-2.50032-3
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Ellman GL et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2): 88IN191–9095
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Yokel RA (2002) Brain uptake, retention, and efflux of aluminum and manganese. Environ Health Perspect 110(suppl 5):699–704
Wei L et al (2018) Asiatic acid attenuates CCl4-induced liver fibrosis in rats by regulating the PI3K/AKT/mTOR and Bcl-2/Bax signaling pathways. Int Immunopharmacol 60:1–8
Rao MK, Rao MS, Rao GS (2007) Treatment with Centalla asiatica (Linn) fresh leaf extract enhances learning ability and memory retention power in rats. Neurosciences 12(3):236–241
Bazzari FH, Abdallah DM, El-Abhar HS (2019) Chenodeoxycholic acid ameliorates AlCl3-Induced Alzheimer’s disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules 24(10):1992
Carola V et al (2002) Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav Brain Res 134(1–2):49–57
Li M et al (2020) Differentially expressed genes in the brain of aging mice with cognitive alteration and depression-and anxiety-like behaviors. Front Cell Develop Biol 8:814
Rather MA et al (2019) Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in wistar rats. Neurotox Res 35(4):955–968
Manoharan S et al (2016) The role of reactive oxygen species in the pathogenesis of Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease: a mini review. Oxidat Med Cell Longevity. https://doi.org/10.1155/2016/8590578
Arimon M et al (2015) Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis 84:109–119
Nayak P, Sharma SB, Chowdary NVS (2010) Augmentation of aluminum-induced oxidative stress in rat cerebrum by presence of pro-oxidant (graded doses of ethanol) exposure. Neurochem Res 35(11):1681–1690
Exley C (2004) The pro-oxidant activity of aluminum. Free Radical Biol Med 36(3):380–387
Zatta P et al (2002) Aluminium (III) as a promoter of cellular oxidation. Coord Chem Rev 228(2):271–284
Mathiyazahan DB, Thenmozhi AJ, Manivasagam T (2015) Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: a behavioural, biochemical and molecular approach. J Funct Foods 16:423–435
Benzi G et al (1989) Age-related effect induced by oxidative stress on the cerebral glutathione system. Neurochem Res 14(5):473–481
Jyoti A, Sethi P, Sharma D (2007) Bacopa monniera prevents from aluminium neurotoxicity in the cerebral cortex of rat brain. J Ethnopharmacol 111(1):56–62
Reddy PH (2006) Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem 96(1):1–13
Bhalla P, Dhawan D (2009) Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell Mol Neurobiol 29(4):513–521
Esparza JL et al (2005) Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J Pineal Res 39(2):129–136
Prakash A, Kumar A (2013) Mitoprotective effect of Centella asiatica against aluminum-induced neurotoxicity in rats: possible relevance to its anti-oxidant and anti-apoptosis mechanism. Neurol Sci 34(8):1403–1409
Mohd Salim R et al (2013) Statistical analysis of metal chelating activity of Centella asiatica and Erythroxylum cuneatum using response surface methodology. Biotechnol Res Int. https://doi.org/10.1155/2013/137851
Fadl N et al (2013) Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum Exp Toxicol 32(7):721–735
Jayant S, Sharma B, Sharma B (2016) Protective effect of transient receptor potential vanilloid subtype 1 (TRPV1) modulator, against behavioral, biochemical and structural damage in experimental models of Alzheimer’s disease. Brain Res 1642:397–408
Maurer SV, Williams CL (2017) The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol 8:1489
Kaizer RR et al (2005) Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J Inorg Biochem 99(9):1865–1870
H Ferreira-Vieira T et al (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14(1): 101–115
Moneim AEA (2012) Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol Trace Elem Res 150(1–3):328–336
Acknowledgements
We are thankful to Prof. Kathy W. Nordeen (Brain and Cognitive Sciences, University of Rochester, NY, USA) for her valuable suggestions and for editing the manuscript.
Funding
This work was supported by a fellowship grant to ZF from the Council of Scientific and Industrial Research (CSIR), India.
Author information
Authors and Affiliations
Contributions
ZF and TDS conceptualized and designed the study and prepared the manuscript. ZF carried the protocol and DY and SKS helped in behavioral and histological studies. All authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Central Animal Ethical Committee of the Banaras Hindu University (letter number—Dean/2017/CAEC/719, 30.03.2017).
Consent for publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Firdaus, Z., Kumar, D., Singh, S.K. et al. Centella asiatica Alleviates AlCl3-induced Cognitive Impairment, Oxidative Stress, and Neurodegeneration by Modulating Cholinergic Activity and Oxidative Burden in Rat Brain. Biol Trace Elem Res 200, 5115–5126 (2022). https://doi.org/10.1007/s12011-021-03083-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03083-5