Abstract
Nectar is the resource that sustains honey bee colonies through periods of dearth and provides the surplus honey beekeepers harvest for human consumption. While extensive information is available for plants that honey bees visit for pollen and nectar, we lack knowledge on which nectars are stored long-term as honey for harvest and support of colonies through winter. Here, we used citizen science methods and pollen metabarcoding analysis to identify the plants contributing most to honey samples harvested by beekeepers from apiaries with variable intensities of surrounding agriculture. A total of 36 samples were collected from 36 apiaries in Ohio in 2019, with an average of 3 plant genera detected per sample. We found similarity in honey samples collected from all apiaries, regardless of the proportion of agricultural land within a 2-km foraging range, with substantial amounts of honey stored from spring trees, including Salix (willow) and Prunus (cherry). This result suggests the importance of early-season resources regardless of agricultural intensity in the surrounding landscape. This study contributes to a body of work aiming to identify the nectars making it to long-term honey storage and those that are being consumed within the hive shortly after collection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nectar is the source of carbohydrates for honey bee colonies; it fuels activities including foraging, wax production, and thermoregulation (Haydak 1970; Seeley 1992). Bees source nectar from flowers on the landscape, making decisions based on landscape factors including floral proximity and abundance and quality of the reward (e.g., nectar volume and sugar concentration) (Nicolson & Thornburg 2007; Corbet et al. 1984; Goulson 1999). Nectar that is brought back to a honey bee colony is either consumed immediately or placed in cells, dehydrated, capped, and stored as honey (Park 1925; Seeley 1989; Eyer et al. 2016). Honey bee foragers collect available nectar, often more than the immediate needs of the colony, leading to honey hoarding within the hive (Fewell & Winston 1996; Rinderer & Baxter 1978). Excess honey is stored for later consumption during periods when weather conditions are unsuitable for flying or when there is a nectar dearth. Beekeepers are also dependent on robust storage of honey by bees to harvest for human consumption.
During the foraging season, there is a limited window of time in which bees collect large quantities of nectar, when flowers that provide the bulk of seasonal nectar are blooming in abundance. However, the timing is variable depending on region and climate (Seeley & Visscher 1985; McLellan 1977; Bayir & Albayrak 2016). Many beekeepers extract this excess and provide supplemental carbohydrates to make up for honey harvested in order to augment honey stores for winter. In temperate climates, a honey bee colony can consume over 20 kg of honey over winter, and colonies depend on the energy in stored honey for thermoregulation in the winter cluster (Seeley & Visscher 1985). Starvation is frequently cited for winter colony failure by beekeepers (Steinhauer et al. 2021), and fall weight has been shown to strongly predict colony winter survival (Döke et al. 2019). Identifying the flowers that are contributing most to colony honey storage is important for beekeeper livelihood and colony winter survival.
Extensive surveys have been done to document pollen and nectar plants used by honey bees worldwide (Crane 1983; Bryant & Jones 2001); however, we lack knowledge about which nectar resources contribute most to honey stores which are most important for honey harvest and winter survival of colonies. Most existing studies have relied on observations of floral visitation, pollen collection, and targeted nectar sampling (Carreck & Williams 2002; Liolios et al. 2015; Park & Nieh 2017; Requier et al. 2015). Honey bees preferentially consume low-concentration sugar solutions immediately after collection while nectar with higher concentrations are stored as honey (Eyer et al. 2016), though honey storage also depends on nectar availability (McLellan 1977). Identifying the plants that are key for colony honey production is essential for supporting healthy honey bees. Additionally, assessing the role of landscape, including land in agricultural production, may contribute to the understanding of regional differences in floral availability or composition.
Here, we aim to determine the plants contributing most to Ohio honey production and identify differences in honey composition based on surrounding agricultural intensity. We used citizen science methods by collaborating with volunteer beekeepers in Ohio who collected honey from 36 apiaries across the state. Pollen metabarcoding methods were used to identify the taxonomic composition of honey samples and indicate the proportional contribution of plant taxa that were detected. A two-marker approach was used to increase our confidence in proportional values of detected plant taxa (Richardson et al. 2015). Landscape was analyzed around each apiary to determine the proportion of corn and soybean fields in a 2-km radius. This value was used to determine agricultural intensity around each apiary, classifying apiaries as high (> 50%), medium (20–50%), or low (< 20%). This metric was compared with honey sample composition to determine differences in honey related to agricultural intensity. We hypothesize that honey collected from apiaries located in areas with higher agricultural intensity will contain more pollen from soybeans (Glycine max). Previous research has identified soybeans as a major nectar resource for honey bees (Lin et al. 2022; McMinn-Sauder 2022; St. Clair et al. 2020), suggesting that it should be a large component of honey collected from highly agricultural sites. This work will help establish the plants that are contributing most to honey production in Ohio, with potential application to other regions with similar agricultural environments throughout the Midwestern United States.
2 Materials and methods
2.1 Honey identification
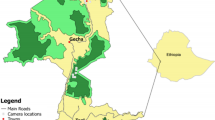
Honey samples (50 mL) and apiary location information were solicited from beekeepers at the annual Ohio State Beekeepers’ Association 2019 meeting in Plain City, Ohio, along with apiary location information (Fig. 1) (IRB study number: 2019E1019, Honey sources in an agricultural landscape and the impact of soybean fields on honey production, 2019). To be included in the study, honey must have been collected in 2019 from apiaries registered with the Ohio Department of Agriculture and with an identifiable apiary location included in the survey response. A total of 49 samples were submitted, and 36 samples fit the study criteria, each collected from a different apiary site. The dates for honey collection ranged from May through October, with most samples collected between July and September. Samples were stored in air-tight containers at room temperature until processing.
Map of study apiaries. Honey was collected in 2019 from 36 apiaries in Ohio, USA. Honey was brought to the Ohio State Beekeepers’ Association fall meeting and distributed to the Ohio State University bee lab. The Cropscape–cropland data layer overlays the study map with corn (yellow) and soybeans (green) representing the dominant crops in our study region
Honey samples were heated in a warm water bath at 65 °C to eliminate crystallization and reduce viscosity. Pollen was isolated from honey using dilution and centrifugation. First, 3.5 g of honey were added to a 50-mL plastic conical centrifuge tube and dissolved in 3.5 mL of warm, DI water. The solution was further diluted in 35 mL of 95% ethanol and centrifuged at 2849 RCF for 3 min. The supernatant was poured off and 0.5 mL of 95% ethanol was added to the tube to resuspend the pellet with agitation. Then, the mixture was transferred to a screw cap microcentrifuge tube (Fisherbrand Free-Standing Microcentrifuge Tubes; Fisher Scientific, Hampton, NH, USA), and centrifuged again at 1503 RCF. The supernatant was poured off, and residual ethanol was evaporated under a fume hood. To disrupt the pollen coat, 0.5 mL of 0.7-mm diameter zirconia beads (Fisher Scientific, Hampton, NH, USA) and 200 µL of DI water were then added to each microcentrifuge tube and agitated vigorously for three minutes with a Mini-BeadBeater-16 (BioSpec Products, Bartlesville, OK, USA). Samples were then prepared for a 3-step PCR protocol, the product of each step serving as the template for the subsequent step (PCR conditions detailed in Table S1) (Richardson et al. 2015, 2019). Universal primers for plant ITS2 and rbcL (Kress & Erickson 2007; Chen et al. 2010; Richardson et al. 2015) were used for PCR 1 with 1 µL of pollen homogenate serving as the template. In PCR 2 and 3, 1 µL of the previous reaction served as the template, with a linking sequence added to primers in PCR 2 and unique Illumina index sequences added in PCR 3. Gel electrophoresis was conducted on a subset of samples following PCR 3 for sample quality control. Samples were then combined and purified with a SequalPrep Normalization Plate kit (Thermo Fisher Scientific, Waltham, MA, USA). Libraries were sequenced at the Molecular and Cellular Imaging Center in Wooster, Ohio on a 15 million read, paired end 300 base-pair standard Illumina MiSeq Flow Cell.
The MetaClassifier protocol for taxonomic identification (Sponsler et al. 2020) was used to calculate proportional abundances of plant taxa detected in each honey sample using computing resources provided by the Ohio Supercomputer Center. Sequence paired-end reads were first merged and converted into FASTA format. Each sample sequence was then compared to library databases curated with the MetaCurator method (Richardson et al. 2020) and Taxonomizr (https://CRAN.R-project.org/package= taxonomizr) for each locus to identify sample taxonomy at the genus level. Sample alignment parameters were assigned as 92.5% sequence identity for ITS2 and 96% sequence identity for rbcL and sample coverage of 80% for both markers. Since each marker exhibits biases towards detection of certain taxa, a median value for each taxon was used. Proportional abundances were used for detected plant taxa by calculating the proportion of reads per marker for rbcL and ITS2 and calculating the median of those values. Sample taxonomy was visualized using the ggplot2 package (Wickham et al. 2016; R Development Core Team 2022) in R studio (version 4.0.3). Genera detected at 1% proportional abundance or greater were retained for further analysis. Sequences are available in GenBank (accession: PRJNA924028).
Honey sample richness and evenness and sample diversity, calculated with the Shannon–Wiener diversity index, were assessed. Diversity values were normally distributed and analyzed using a generalized linear model with agricultural intensity as the independent model effect and plant diversity as the dependent variable. Sample evenness and richness were nonnormally distributed and analyzed using a Kruskal–Wallis test and Wilcoxon rank sums test. Nonmetric multidimensional scaling (NMDS) was used to visualize dissimilarity between the floral composition of samples collected from apiaries with high, medium, and low surrounding agricultural intensity (Minchin 1987). We used the Bray–Curtis index distance metric, 2-dimensional scaling (k = 2), and defined the upper limit for stress at 0.2. The metaMDS (vegan, version 1.8–6) and ggplot2 R packages were used to perform the analysis and visualization. A permutational multivariate analysis of variance was performed using the adonis function in the vegan package (Oksanen et al. 2020) to assess differences in composition of honey samples collected from sites with variable surrounding agriculture.
2.2 Landscape analysis
The landscape surrounding each apiary was characterized at a 2-km radius, as colonies typically forage within 2 km during the summer (Couvillon et al. 2015). The amount of land in agriculture, development, forest, pasture, and roadside was quantified using the USDA CropScape 2019 Cropland Data Layer (USDA-NASS-RDD-2015). Apiaries were grouped by agricultural intensity for analysis, calculated by the proportion of row crop (primarily corn, soybean, and wheat) agriculture in the surrounding 2-km radius. Apiaries with greater than 50% agriculture were classified as high agriculture, those with 20–50% surrounding agriculture were classified as medium agriculture, and apiaries with less than 20% surrounding agriculture were classified as low agriculture.
3 Results
A total of 45 honey samples were collected, yielding an average of 258,715 raw reads per sample. Nine samples did not contain location information, excluding them from further analysis, leaving a total of 36 samples. Twelve samples were collected from apiaries with high (> 50%) surrounding agriculture, 10 samples were collected from apiaries with medium (20–50%) surrounding agriculture, and 14 samples were collected from apiaries with low (< 20%) surrounding agriculture.
Twenty-four genera were detected by each the rbcL and ITS2 marker, with 17 genera in rbcL and 16 genera in ITS2 above the 1% threshold (Tables S3 and S4). When median values were calculated for both markers, eighteen plant genera were detected at greater than 1% proportional abundance in samples, and 14 genera were detected at proportional abundances greater than 5% (Table S2). Salix (willow) was the genus detected at highest proportional abundance in averaged samples taken from all levels of agricultural intensity (Fig. 2). In addition, Malus (apple) and Prunus (cherry) were detected in high proportional abundances in samples collected from apiaries with high surrounding agriculture. In honey collected from apiaries with medium surrounding agriculture, Prunus, Pyrus (pear), and Trifolium (clover) were detected in proportional abundances greater than 10%. Samples collected from apiaries with low surrounding agriculture were largely (> 10%) composed of Prunus and Trifolium. Results of the NMDS ordination show high similarity in genus-level nectar composition of honey samples collected from apiaries with high, medium, and low surrounding agriculture (Fig. 3); however, the permutational multivariate analysis indicates differences in the taxonomic composition of honey samples collected from sites with low, moderate, and high surrounding agriculture (p < 0.001, F = 35.3, r2 = 0.68, df = 1). No significant effect of agricultural intensity was detected on the diversity, richness, or evenness of plants contributing to honey (p > 0.05) (Tables I and II).
Composition of honey samples collected from beekeepers in 2019. Pollen metabarcoding analysis was used to identify the proportional abundance of nectar from plant species contributing to samples. Average proportional abundance of combined reads from rbcL and ITS2 markers were used, as each marker displays individual biases for detection of different taxa. Study apiaries are classified as high (> 50%), medium (20–50%), or low (< 20%) surrounding agriculture
4 Discussion
Honey harvested by beekeepers over the summer of 2019 was predominantly composed of spring blooming trees, largely Salix (willow) and Prunus (cherry). These results imply that soybean and clover nectar either were not collected by bees in 2019 or that nectar collected from these plants was consumed by the colonies prior to honey harvest. This result differs from findings of previous studies conducted in Ohio, which detected large quantities of soybean in summer honey and nectar. However, this may be due to differences in methods between the studies. A previous study found that Glycine (soybeans) and Trifolium (clover) were major components of nectar collected by colonies in highly agricultural areas during summer months (McMinn-Sauder 2022). However, these samples were collected from uncapped nectar rather than end-of-season honey. Uncapped nectar is likely to represent recent foraging efforts by the colony. The composition of nectar collected from cells and extracted honey has shown to differ in plant composition (Layek et al. 2020). Honey collected in 2014 from colonies in agricultural landscapes consistently included soybean pollen (Lin et al. 2022). Differences in taxonomic abundance could be explained in part by method selection (Corby-Harris et al. 2018), with soybean pollen potentially being underrepresented in the metabarcoding analysis relative to microscopic analysis. The high abundance of Salix detected in honey samples is consistent with previous studies, identifying it as a key nectar resource for honey bees in spring, and a pollen resource for various specialist bees (McMinn-Sauder et al. 2022; Jones et al. 2022; de Vere et al. 2017; Richardson et al. 2015; Ostaff et al. 2015).
This region routinely experiences a nectar dearth period during August, resulting in colony weight loss (Couvillon et al. 2015; McMinn-Sauder et al., unpubl. data), following soybean bloom. It is possible that soybean nectar was collected by these colonies and consumed by bees in the hive either immediately, or during this dearth period and, therefore, absent in most of the stored honey. While soybean was detected in high abundance in one sample, it is possible that soybean pollen was present in other samples in trace amounts that fell below the 1% threshold for inclusion in analysis.
This study contributes to a body of research identifying which resources are consumed immediately and which are capped and stored for later consumption and for honey harvest by beekeepers (Park 1925; Seeley 1989; Eyer et al. 2016). The abundance of spring-collected nectar in summer honey demonstrates that much of the honey harvested throughout the summer and fall of 2019 was composed of spring-blooming plants. The absence of abundant pollen from plants flowering in summer suggests that bees did not visit them for nectar, or the nectar was consumed in the hive prior to honey extraction. This finding suggests that spring nectar resources may be important for colony survival in the subsequent winter, as the honey present at the end of the season sustains colonies through to the following spring.
An alternative explanation for this finding is related to colony management. Samples in this study were collected largely by hobbyist beekeepers. It is possible that inexperienced beekeepers did not provide sufficient space thereby limiting the quantity of nectar collected. If the available space was filled with abundant spring-collected nectar, there may not have been room for colonies to store resources from the summer soybean and clover nectar flow. Another potential explanation is the erratic weather conditions experienced in 2019 that resulted in late planting and a relatively poor soybean harvest that year, suggesting that soybean resource availability may have been different than in other years. It is also important to note the limitations of a single year of data collection. Honey floral composition collected from different years may vary due to annual differences in weather and floral availability. To better understand the resources most important for Ohio honey production, additional data are necessary.
A significant difference in genus-level nectar composition was found for colonies surrounded by high, medium, and low-intensity agriculture using a PERMANOVA test (p < 0.001). While the same plants were detected as major components of honey samples, differences related to agricultural intensity were present in minor nectar resources.
Using citizen science methods, we collected honey from a broad range of locations with different levels of agricultural intensity across Ohio. We worked with Ohio beekeepers to identify the plants contributing most to their honey. Though there was a wide range of sampling dates and levels of surrounding agriculture, the floral composition of honey was strikingly similar and was largely composed of nectar from spring trees, primarily willow, and cherry. In addition, we found that soybeans played a relatively small role in seasonal honey production in 2019. Access to these early-season resources may be crucial for maintaining colony strength during periods of resource scarcity. This highlights the importance of spring trees for seasonal colony performance, emphasizing the role of trees in the nectar diet of Ohio honey bees.
Data availability
The datasets generated during the current study are available in the GenBank repository (accession: PRJNA924028).
Code availability
The code used to analyze data in the current study is available in the GitHub repository (https://github.com/ewafula/MetaClassifier).
References
Bayir R, Albayrak A (2016) The monitoring of nectar flow period of honey bees using wireless sensor networks. Int J Distrib Sens Netw 12(11):1550147716678003
Bryant VM Jr, Jones GD (2001) The r-values of honey: pollen coefficients. Palynology 25(1):11–28
Carreck NL, Williams IH (2002) Food for insect pollinators on farmland: insect visits to flowers of annual seed mixtures. J Insect Conserv 6:13–23
Chen S, Yao H, Han J, Liu C, Song J, Shi L, Zhu Y, Ma X, Gao T, Pang X, Luo K (2010) Validation of the ITS2 region as a novel DNA barcode for identifying medicinal plant species. PLoS ONE 5:e8613
Corbet SA, Kerslake CJC, Brown D, Morland NE (1984) Can bees select nectar-rich flowers in a patch? J Apic Res 23(4):234–242
Corby-Harris V, Snyder L, Meador C, Ayotte T (2018) Honey bee (Apis mellifera) nurses do not consume pollens based on their nutritional quality. PLoS ONE 13(1):e0191050
Couvillon MJ, Fensome KA, Quah SK, Schürch R (2014) Summertime blues: August foraging leaves honey bees empty-handed. Commun Integr Biol 7(3):e93495
Couvillon MJ, Riddell Pearce FC, Accleton C, Fensome KA, Quah SK, Taylor EL, Ratnieks FL (2015) Honey bee foraging distance depends on month and forage type. Apidologie 46(1):61–70
Crane E (1983) A book of honey. Livraria Nobel SA
De Vere N, Jones LE, Gilmore T, Moscrop J, Lowe A, Smith D, Ford CR (2017) Using DNA metabarcoding to investigate honey bee foraging reveals limited flower use despite high floral availability. Sci Rep 7(1):1–10
Döke MA, McGrady CM, Otieno M, Grozinger CM, Frazier M (2019) Colony size, rather than geographic origin of stocks, predicts overwintering success in honey bees (Hymenoptera: Apidae) in the Northeastern United States. J Econ Entomol 112(2):525–533
Eyer M, Neumann P, Dietemann V (2016) A look into the cell: honey storage in honey bees. Apis Mellifera Plos One 11(8):e0161059
Fewell JH, Winston ML (1996) Regulation of nectar collection in relation to honey storage levels by honey bees. Apis Mellifera Behav Ecol 7(3):286–291
Goulson D (1999) Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect Plant Ecol Evol Syst 2(2):185–209
Haydak MH (1970) Honey bee nutrition. Annu Rev Entomol 15(1):143–156
Jones L, Lowe A, Ford CR, Christie L, Creer S, de Vere N (2022) Temporal Patterns of honeybee foraging in a diverse floral landscape revealed using pollen DNA metabarcoding of honey. Integr Comp Biol
Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE 2(6):e508
Layek U, Mondal R, Karmakar P (2020) Honey sample collection methods influence pollen composition in determining true nectar-foraging bee plants. Acta Botanica Brasilica 34:478–486
Lin CH, Suresh S, Matcham E, Monagan P, Curtis H, Richardson RT, Johnson RM (2022) Soybean is a common nectar source for honey bees (Hymenoptera: Apidae) in a Midwestern agricultural landscape. J Econ Entomol
Liolios V, Tananaki C, Dimou M, Kanelis D, Goras G, Karazafiris E, Thrasyvoulou A (2015) Ranking pollen from bee plants according to their protein contribution to honey bees. J Apic Res 54(5):582–592
McLellan AR (1977) Honeybee colony weight as an index of honey production and nectar flow: a critical evaluation. J Appl Ecol 401–408
McMinn-Sauder HB (2022) Complementary floral resources for honey bees in a midwestern agroecosystem (Doctoral dissertation, The Ohio State University)
McMinn-Sauder H, Lin CH, Eaton T, Johnson R (2022) A comparison of springtime pollen and nectar foraging in honey bees kept in urban and agricultural environments. Front Sustain Food Sys 66
Minchin PR (1987) An evaluation of the relative robustness of techniques for ecological ordination. In Theory and models in vegetation science (pp. 89–107). Springer, Dordrecht
Nicolson SW, Thornburg RW (2007) Nectar chemistry. In Nectaries and nectar (pp. 215–264). Springer, Dordrecht
Oksanen JF, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community Ecol Package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan
Ostaff DP, Mosseler A, Johns RC, Javorek S, Klymko J, Ascher JS (2015) Willows (Salix spp.) as pollen and nectar sources for sustaining fruit and berry pollinating insects. Can J Plant Sci 95(3):505–516
Park W (1925) The storing and ripening of honey by honeybees. J Econ Entomol 18(2):405–410
Park B, Nieh JC (2017) Seasonal trends in honey bee pollen foraging revealed through DNA barcoding of bee-collected pollen. Insectes Soc 64:425–437
R Development Core Team (2022) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Requier F, Odoux JF, Tamic T, Moreau N, Henry M, Decourtye A, Bretagnolle V (2015) Honey bee diet in intensive farmland habitats reveals an unexpectedly high flower richness and a major role of weeds. Ecol Appl 25(4):881–890
Richardson RT, Curtis HR, Matcham EG, Lin CH, Suresh S, Sponsler DB, Hearon L, Johnson RM (2019) Quantitative multi-locus metabarcoding and waggle dance interpretation reveal honey bee spring foraging patterns in Midwest agroecosystems. Mol Ecol 28(3):686–697
Richardson RT, Lin CH, Quijia JO, Riusech NS, Goodell K, Johnson RM (2015) Rank-based characterization of pollen assemblages collected by honey bees using a multi-locus metabarcoding approach. Appl Plant Sci 3(11):1500043
Richardson RT, Sponsler DB, McMinn-Sauder H, Johnson RM (2020) MetaCurator: a hidden Markov model-based toolkit for extracting and curating sequences from taxonomically-informative genetic markers. Methods Ecol Evol 11(1):181–186
Rinderer TE, Baxter JR (1978) Effect of empty comb on hoarding behavior and honey production of the honey bee. J Econ Entomol 71(5):757–759
Seeley TD, Visscher PK (1985) Survival of honeybees in cold climates: the critical timing of colony growth and reproduction. Ecol Entomol 10(1):81–88
Seeley TD (1989) Social foraging in honey bees: how nectar foragers assess their colony’s nutritional status. Behav Ecol Sociobiol 24(3):181–199
Seeley TD (1992) The tremble dance of the honey bee: message and meanings. Behav Ecol Sociobiol 31(6):375–383
Sponsler DB, Shump D, Richardson RT, Grozinger CM (2020) Characterizing the floral resources of a North American metropolis using a honey bee foraging assay. Ecosphere 11(4):e03102
St. Clair AL, Dolezal AG, O’Neal ME, Toth AL (2020) Pan traps for tracking honey bee activity-density: a case study in soybeans. InSects 11(6):366
Steinhauer N, Aurell D, Bruckner S, Wilson M, Rennich K, vanEngelsdorp D, Williams G (2021) United States honey bee colony losses 2020–2021: Preliminary results. https://beeinformed.org/2021/06/21/united-states-honey-bee-colony-losses-2020-2021-preliminary-results/
Wickham H, Chang W, Wickham MH (2016) Package ‘ggplot2’. Create elegant data visualisations using the grammar of graphics. Version 2(1):1–189
Acknowledgements
The authors would like to thank the Ohio beekeepers who donated honey samples for this project, as well as the Ohio State Beekeepers’ Association for allowing us to solicit samples at their annual meeting. Thank you to Isabel Nazarian for your work in processing these honey samples. We would like to thank the Ohio Supercomputer Center for their computational resources.
Funding
This work was supported by the US Department of Agriculture National Institute of Food and Agriculture (#2019–67013-29297 and #2022–67019-36437) and state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OHO01355-MRF and OHO01277).
Author information
Authors and Affiliations
Contributions
HMS, CHL, and RJ designed experiments. TE and HMS performed experiments and analysis. HMS, CHL, TE, and RJ wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is not applicable.
Consent to participate
This is not applicable.
Consent for publication
This is not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Cedric Alaux.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McMinn-Sauder, H., Lin, CH., Eaton, T. et al. The major role of spring trees in Ohio honey production for bees located in high and low agricultural intensity. Apidologie 54, 37 (2023). https://doi.org/10.1007/s13592-023-01016-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13592-023-01016-w