Abstract
The simultaneous wing movement by multiple worker bees in a colony produces a hissing sound, which is a novel acoustic and vibrational signal of the honey bees. Hissing of honey bees is thought to be a response to direct, threatening stimuli. However, we discovered Japanese honey bees (Apis cerana japonica) can hiss even without obvious disturbances in previous study. In this study, to understand the temporal characteristics of honey bee hissing, we conducted 24-h sound recordings over 7 months in 2015 and investigated when A. cerana japonica hissed every day. Additionally, we also investigated the relationship of hissing onset and offset times with sunrise and sunset times, and environmental factors. We found that honey bees hiss daily during daytime and most frequently at dawn, with hissing onset/offset occurring mostly within 30 min of sunrise/sunset time. Hissing onset and offset were significantly related to sunrise and sunset times, respectively, and also to solar radiation intensity. The findings reveal that A. cerana japonica hissing has unique temporal patterns, and also shed a new light on vibrational collective behavior in honey bees.
Similar content being viewed by others
1 Introduction
How and why some animals behave in a group and show coordinated behavior is one of the most interesting topics in animal ecology. Many researchers have tried to address these questions by investigating the circumstances of when and how animals behave. Honey bees are one of the most well-known eusocial insects. As such, many studies have investigated how they behave and make group decisions. One of the most famous signals in honey bees is the waggle-dance that recruits other bees in the colony to visit possible forage sites. Recently, because of the development of acoustic devices and techniques of analyses, it is becoming apparent that honey bees also use a variety of vibrational communication signals (Fuchs and Koeniger 1974; Hrncir et al. 2005; Fuchs and Tautz 2011; Schlegel et al. 2012). For example, Seeley et al. (Seeley et al. 2012) reported that honey bees can produce “stop signals” that play a role in stopping other honey bees from performing waggle dances. Worker piping is also known as a vibrational signal, and it is reported to be observed in cases like when Apis mellifera start swarming (Seeley and Tautz 2001) or when Apis florea face threatening stimuli (Sarma et al. 2002).
Hissing behavior is one of the acoustic/vibrational signals in honey bees, involving simultaneous wing movements of multiple bees (Koeniger and Fuchs 1972; Fuchs and Koeniger 1974; Seeley et al. 1982; Sarma et al. 2002). It has also been referred to as shimmering (Butler 1954; Seeley et al. 1982), which describes the visual impression of coordinated wing movements (Hrncir et al. 2005). Hissing behavior is observed in different honey bee species, such as A. cerana (Butler 1954; Sakagami 1960), A. florea (Sarma et al. 2002), and A. mellifera (Papachristoforou et al. 2008). Honey bees emit short buzz sounds or vibrations (0.5–1.0 s) during such behavior (Hrncir et al. 2005; Fuchs and Tautz 2011). The coordinated movement of hissing is contagious and spreads from one bee to another like the propagation of the audience wave.
Regarding the function of hissing, it is thought to serve as an aposematic signal in response to disturbances, such as physical disturbance of a colony or the approach of predator, such as hornets (Fuchs and Koeniger 1974; Sarma et al. 2002; Hrncir et al. 2005; Fuchs and Tautz 2011; Wehmann et al. 2015); a correlation formed on the basis of circumstantial and observational evidence over time. However, findings from the previous study (Kawakita et al. 2018) showed that A. cerana japonica could hiss even without an obvious disturbance. Although our previous study (Kawakita et al. 2018) suggested A. cerana japonica make hissing sound in diurnal patterns, the daily and seasonal patterns of hissing in A. cerana japonica, however, remain unknown, and if identified, could uncover the potential function of this behavior.
The purpose of this study is to investigate the patterns of hissing behavior in A. cerana japonica and to contribute to understanding the hissing behavior of honey bees. We conducted 24-h sound recordings inside A. cerana japonica colonies for 7 months and investigated the patterns of its occurrence. Based on the previous finding that (Kawakita et al. 2018) A. cerana japonica hiss at daytime, we defined the first/last occurrence time of hissing in a day as hissing onset/offset, respectively. We then developed statistical models and clarified the relationship between hissing onset/offset time and environmental factors. We expect that the findings of this research will contribute to our understanding of vibrational behavior of honey bees and its daily and seasonal occurrence patterns would be useful to understand the function of hissing for honey bees.
2 Materials and methods
We conducted sound recordings in two A. cerana japonica colonies between May and October and in December in 2015 in Kyoto, Japan, and these were partly used in our previous study (Kawakita et al. 2018). The colonies we used in this study were located outdoors and were formed in artificial nest boxes. The nest boxes had only one gap in the form of an entrance of approximately 1 cm × 15 cm, through which the bees could pass and were placed at the Kitashirakawa Experimental Station, Kyoto University, which was surrounded by the woods and shrubs.
We conducted 24-h sound recordings inside the honey bee colonies and collected 40 days of sound recordings, in total (960 h, 40 × 24 h), using two colonies (Colony 1 and 2). We investigated changes in hissing onset and offset time during a day by defining the time when hissing occurred for the first time as hissing onset, and the time when hissing occurred for the last time as hissing offset. In addition to this, we also investigated when all hissing happened in a day using 16 days from the 40 days of sound data (typical samples of 2–3 days from each month) to understand daily and seasonal hissing patterns.
We extracted hissing sounds recorded inside the colony based on the method described in the previous study (Kawakita et al. 2018). Only hissing sound that consisted of broadband noises of 0.5–3.6 kHz, with greater than 0.25-s duration were extracted as hissing sounds and counted each bout. The raw sound data was visualized using Adobe Audition CC (Adobe Systems Incorporated, CA, United States of America), and the hissing sounds were manually detected.
Pearson product-moment correlation coefficients were conducted to determine whether hissing onset or offset was associated with sunrise or sunset, respectively. To understand the temporal patterns, we separated a day into four time-categories. We defined 30 min before and after sunrise as dawn and defined 30 min before and after sunset as dusk. Time between dawn and dusk was defined as day, and the rest of the time was defined as night. By comparing the number of hissings recorded in the four time categories in a day, temporal patterns were investigated using the Wilcox multiple comparisons test with Bonferroni correction. Sunrise and sunset times for each experiment date were obtained from the National Astronomical Observatory of Japan home page (http://eco.mtk.nao.ac.jp/koyomi/). In addition to this, we also investigated the time lag between the timing of hissing onset or offset and sunrise or sunset, respectively. Mean absolute values of the differences between sunrise and hissing onset time, and sunset and hissing offset time were calculated. Hissing onset time and offset time were measured on the time scale of seconds and these were rounded down to the nearest minutes.
We developed linear mixed effect models to investigate the relationship between hissing onset and offset and environmental factors. We referred to an approach used previously (Narendra et al. 2010), in which the relationship between the outbound activity time of the ancient ant (Myrmecia pyriformis) and sunrise and other environmental factors, such as ambient temperature or intensity of solar radiation were investigated. Sunrise and sunset time, ambient temperature, the intensity of solar radiation, and humidity were set as fixed effects, and colony ID was set as a random effect. If light intensity had effects on the occurrence of hissing, hissing onset and offset would follow sunrise and sunset time, since the light intensity would dramatically change around sunrise and sunset. Terrain and weather conditions, such as cloud cover, might cause changes in ambient light levels at sunrise and sunset; so, we also used the intensity of solar radiation in the models. The environmental data were obtained at about 30 m from the nest box. Temperature (HMP45A, Vaisala, Helsinki, Finland), humidity (HMP45A, Vaisala, Helsinki, Finland), and intensity of solar radiation (CMP-3, Kipp&Zonen, Delft, Netherlands) were measured every minute, and their mean was calculated every 10 min. Hissing onset time, offset time, and sunrise and sunset time were transformed into variables ranging from 0 to 1 by dividing the time (h) by 24 (h) The intensity of solar radiation was log-transformed. All statistical analyses were carried out using the R Statistical Computing Software (v 3.2.2, R Foundation for Statistical Computing, Vienna, Austria). We used Pseudo r-squared (R2) values (Nakagawa and Schielzeth 2013; Johnson 2014) for assessing the models we made, using the MuMIn package (Barton 2018) in R.
3 Results
The 16-day investigations of hissing patterns over 24 h showed that A. cerana japonica not only hiss every day, but also opportunistically throughout the day (Figure 1). The mean number of hissing occurrences in 1 day was 295.75 ± 134.45 (mean ± SD), while the number of hissing occurrences between May and December fluctuated (Figure 2). The minimum and maximum number of hissing occurrences in 1 day was 133 (Jul 22) and 549 (May 24), respectively. There was no correlation between the total number of hissings in a day and mean temperature (Pearson correlation: r = − 0.17, p > 0.05). Temperature ranged from around 0 to 35 °C at the study site during the experiment dates. Regarding rainfall, it rained throughout the day and night especially on June 26 and July 22 (more than 30 mm in a day) during the experiments, and the number of hissing occurrences recorded on June 26 and July 22 were 319 and 133, respectively. In December, we observed that very few honey bees went outside to forage. Piping sounds were sometimes recorded in our experiments but did not always accompany hissing sounds.
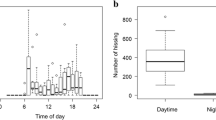
Comparisons of the times of occurrence in the four time-categories revealed that no hissing was recorded at night (Figure 3), and the bees made hissing sounds more frequently at dawn than during the day and dusk (Wilcoxon rank sum test with Bonferroni correction, dawn vs. day: p < 0.01; dawn vs. dusk: p < 0.01, Figure 3). There was no significant difference between the number of hissing occurrences at day and at dusk (Wilcoxon rank sum test with Bonferroni correction, p = 0.88, Figure 3).
Throughout the experiment, most A. cerana japonica hissing onsets (39 out of 40 instances) occurred during dawn, and the mean absolute value of the difference between sunrise and hissing onset was 9.58 ± 7.72 min (mean ± SD, Figure 4). Furthermore, there was a significant correlation between hissing onset and sunrise (Pearson correlation: r = 0.98, p < 0.01). Contrarily, throughout the experiment, most A. cerana japonica hissing offsets (36 out of 40 instances) were observed at dusk, and the mean absolute value of differences between sunset and hissing offset was 16.15 ± 12.72 min (mean ± SD, Figure 4). There was also a significant correlation between hissing offset time and sunset (Pearson correlation: r = 0.93, p < 0.01).
Hissing onset time was strongly predicted by sunrise and the solar radiation intensity (Figure 5a, Table I); the Pseudo R2 value of the model was 0.99. Hissing offset time was strongly predicted by sunset and solar radiation intensity (Figure 6a, Table II); the Pseudo R2 value of the model was 0.95. There were no significant effects of temperature and humidity on the timing of hissing onset (Table I) and offset (Table II).
4 Discussion
In this study, we investigated hissing occurrence patterns over 40 days, which consistently showed that a colony of A. cerana japonica hiss in the daytime (Figure 1). As we did not visually monitor the colonies over 24 h, we are not able to confirm the individual situations under which A. cerana japonica hissed; however, based on the findings of the previous study (Kawakita et al. 2018), we can assert that A. cerana japonica hiss without predatory disturbances. As such, we consider it suitable to discuss A. cerana japonica hissing occurrence patterns using the data we collected in this study.
Our experiments clearly show that A. cerana japonica hiss during the daytime, which corroborates the findings of the previous research (Kawakita et al. 2018). The number of hissing events during a day had a wide variance (Mean ± SD 295.75 ± 134.45), and there was no significant correlation between the number of occurrences in a day and the mean temperature (Pearson correlation − 0.17, p > 0.05). It is suggested that the change of the seasons influence the timing but does not influence the frequency of A. cerana japonica hissing. It is reported that European honey bees (Apis mellifera) maintained the thorax temperature at high level (37.0–38.5 °C) and collected water in the winter at air temperatures as low as 3 °C (Kovac et al. 2010). Considering this, it is likely that A. cerana japonica can also regulate their thorax temperature at high enough to move their wings and hiss more than 200 times even in winter. Regarding temperature and sound production, some cicada species exhibit endothermy, allowing them to generate sounds under relatively cold conditions (Wiley and Richards 1978; Henwood and Fabrick 1979). In the case of honey bees, it is known that the central brood nest of a colony maintains the temperature at 34 to 36 °C (Seeley 1989); so, this may also allow them to hiss under cold ambient temperatures. Regarding rainfalls, we only observed it in only 2 days (June 26 and July 22) in the 16-day experiments, but it is suggested that rainfall does not influence the number of occurrences of hissing in A. cerana japonica considering it is natural phenomenon and the result of the other dates (Figure 2).
Comparisons of the number of hissing occurrences that were recorded in each of the four time-categories indicated that the bees hissed most frequently at dawn (Figure 3). This result is surprising since A. cerana japonica is diurnal and do not be active at night so that it is thought to be still inactive at dawn. It is still unclear why the honey bee hissed frequently in dawn but most of the hissing onset times were around sunrise and the ambient light conditions changed dramatically around sunrise; so, we expected that the change in environmental factors would influence the timing of hissing.
A. cerana japonica exhibited hissing behavior between sunrise and sunset throughout the experiment. The occurrence of hissing onset and offset was around sunrise and sunset, respectively (Figure 4). These results suggested that A. cerana japonica hissing behavior is not an internal behavior, but a behavior adjusted based on exogenous environmental factors. If honey bee hissing was an internal periodic behavior, it would occur at specific intervals, but this was not the case. Since the light conditions varied depending upon weather conditions (e.g., cloud cover), honey bees adjusted the timing of hissing, which explains why the light intensity conditions, as well as sunrise and sunset, had significant effects in our statistical model. Our study could not separate the influence of sun movement or light conditions on hissing, but Narendra et al. (Narendra et al. 2010) showed that ambient light levels, rather than sun movement, influenced activity in primitive ants. To further understand the factors that influence the occurrence of hissing, several controlled experiments are needed. We expected that hissing onset and offset would occur at a certain range of temperature; however, this was not the case. This result was surprising, since it is widely known that ambient temperature affects insect activity (Mellanby 1939; Taylor 1963). Although there was clear relationship between the ambient temperature and hissing onset/offset time, it is assumed that there is spurious relationship between them considering the relationship between sunrise/sunset and hissing onset/offset (Figures 5 and 6) and the result of our statistical model (Tables I and II).
Our results show that A. cerana japonica clearly hiss during the daytime, and that the start and end of the hiss is adjusted to the timing of sunrise and sunset. This result was also interesting, since the hissing behavior of honey bees is typically regarded as an aposematic signal acting as a conditional reflex behavior against disturbance, as reported in many other bees (Wehmann et al. 2015). Considering our results, it is likely that there are other potential functions or, at least, unknown characteristics, of the hissing behavior of A. cerana japonica.
One of the possible functions of hissing in A. cerana japonica may be to inform other honey bees about the outside light conditions, considering that hissing onset and offset happened close to the times of sunrise and sunset. A. cerana japonica usually makes their colony in a closed space, like in the hollow of a tree, so that the colony is in shade even during the day. Since it is expected to be hard for the bees inside the closed space to know the outside light conditions, several early foragers perhaps make frequent hissing sounds and vibrations in the colony to inform the rest of the colony about the conditions outside. Foragers usually work during the day; so, it is important for them to know the outside light conditions in order to collect enough resources, such as nectar or pollen. More hissing may happen at dawn than at other times during the day because the start of foraging is important to maximize resources obtained by the foragers.
In addition to this, we also consider the possibility that the hissing sounds or vibrations function in arousing bees in the colony. It is known that Apis mellifera foragers sleep and remain inactive at night (Klein et al. 2008, 2014). It would be interesting if A. cerana japonica hiss to arouse the sleeping foragers. Furthermore, Klein et al. (Klein et al. 2014) reported that some foragers sleep and remain inactive even during the day. It is possible that A. cerana japonica may also hiss frequently in the daytime to keep the foragers active.
Another possibility is that the signal of hissing has a role in controlling the foraging behavior of honey bees. Sarma et al. (Sarma et al. 2002) reported that foraging activity decreased after A. florea made piping and hissing sounds, but A. cerana japonica did not make piping sounds with the hissing sounds in our experiments although it is likely that the piping sound of A. cerana japonica was so faint that we were not able to record it. The honey bees we observed showed hissing behavior every day and they kept hissing even when few of them foraged in December. If hissing in A. cerana japonica has a role in stopping foragers from going outside, hissing would be expected to happen more often at dusk, since foragers usually stop foraging when it becomes dark; however, our results show that hissing happened more at dawn than at dusk (Figure 3). Thus, we do not think that hissing is directly connected to the foraging behavior of A. cerana japonica, but that other environmental factors, such as ambient temperature possibly limit their foraging behavior. Since we did not investigate whether flight activity of foragers changed or not before and after hissing happened, further study is necessary for testing whether foraging behavior change before or after hissing in A. cerana japonica.
Although our study showed that A. cerana japonica produced hissing sounds frequently in the daytime, we do not deny the possibility that all the signals could have a role in aposematic signaling. Sarma et al. (2002) reported that A. florea can produce hissing sounds even there is a distant (20 m) stimuli. Although we were not able to detect such stimuli, but unknown daytime stimuli, we did not observe might threaten the colony of A. cerana japonica and made them produce hissing sound. Indeed, it is likely that A. cerana japonica produced hissing sounds steadily (during the day) to deter potential predators. Frequent occurrence of loud hissing sounds in a colony can have a function of threating potential (daytime) predators approaching the colony. Furthermore, among some group-living birds and insects, such as the pied babbler (Bell et al. 2009), greylag geese (Kahlert 2006), or treehopper (Hamel and Cocroft 2012), it is known that false alarms are common, often occurring more frequently than the correct detections (Cresswell et al. 2000; Beauchamp 2010). Since hissing is the simultaneous wing movement of multiple honey bees, if the first group of individuals detected a non-threatening stimulus and mistakenly hissed, neighboring bees may imitate their movement, resulting in a false alarm through the colony. The previous study (Kawakita et al. 2018) showed that A. cerana japonica hissed without obvious disturbance stimuli; it would be interesting to find out if these insects are highly sensitive and produce false alarms frequently, even in the absence of predators.
In this study, we showed that A. cerana japonica hissed every day and the time of occurrence was limited to the daytime in the natural environment. A. cerana japonica hissed more frequently at dawn and the hissing onset and offset times were close to sunrise and sunset times, respectively, and it is suggested that the timing of hissing onset and offset are determined by ambient light condition. These findings suggest that honey bee hissing behavior is related to environmental conditions, and question the significance of such behavior, which is typically considered to be a direct aposematic signal against a threatening stimulus. Our study suggests that A. cerana japonica use hissing more frequently than previous studies have expected and sheds a new light on the warning signals of honey bees.
References
Barton, K. (2018) MuMIn: multi-model inference. R package version 1.40.4 Available at: http://CRAN.R-project.org/package=MuMIn. Accessed 13 Nov 2018
Beauchamp, G. (2010) Determinants of false alarms in staging flocks of semipalmated sandpipers. Behav. Ecol. 21(3), 584–587. https://doi.org/10.1093/beheco/arq032
Bell, M. B. V., Radford, A. N., Rose, R., Wade, H. M., Ridley, A. R. (2009) The value of constant surveillance in a risky environment. Proc. R. Soc. B Biol. Sci. 276(1669), 2997–3005. https://doi.org/10.1098/rspb.2009.0276
Butler, CG, (1954) The world of the honey bee. London: Collins.
Cresswell, W., Hilton, G. M., Ruxton, G. D. (2000) Evidence for a rule governing the avoidance of superfluous escape flights. Proc. Biol. Sci. 267, 733–737. https://doi.org/10.1098/rspb.2000.1064
Fuchs, S., Koeniger, N. (1974) Schallerzeugung im Dienst der Verteidigung des Bienenvolkes (Apis cerana Fabr.). Apidologie 5(3), 271–287.
Fuchs, S., Tautz, J. (2011) Colony defence and natural enemies In Honeybees of Asia. (pp. 369–395). Berlin, Heidelberg: Springer Berlin Heidelberg. https://doi.org/10.1007/978-3-642-16422-4_17
Hamel, J. A., Cocroft, R. B. (2012) Negative feedback from maternal signals reduces false alarms by collectively signalling offspring. Proc. R. Soc. B Biol. Sci. 279(1743), 3820–3826. https://doi.org/10.1098/rspb.2012.1181
Henwood, K., Fabrick, A. (1979) A quantitative analysis of the dawn chorus: temporal selection for communicatory optimization Am. Nat. 114(2), 260–274. https://doi.org/10.1086/283473
Hrncir, M., Barth, F., Tautz, J. (2005) Vibratory and airborne-sound signals in bee communication (Hymenoptera). In Insect sounds and communication: physiology, behaviour, ecology, and evolution, pp. 421–436. CRC Press, Taylor & Francis Group, Boca Raton, FL
Johnson, P. C. D. (2014) Extension of Nakagawa & Schielzeth’s R2GLMM to random slopes models Methods Ecol. Evol. 5(9), 944–946. https://doi.org/10.1111/2041-210X.12225
Kahlert, J. (2006) Factors affecting escape behaviour in moulting Greylag Geese Anser anser. J. Ornithol. 147(4), 569–577. https://doi.org/10.1007/s10336-006-0081-5
Kawakita, S., Ichikawa, K., Sakamoto, F., Moriya, K. (2018) Hissing of A. cerana japonica is not only a direct aposematic response but also a frequent behavior during daytime. Insectes Soc. 65(2), 331–337. https://doi.org/10.1007/s00040-018-0617-8
Klein, B. A., Olzsowy, K. M., Klein, A., Saunders, K. M., Seeley, T. D. (2008) Caste-dependent sleep of worker honey bees. J. Exp. Biol. 211(18), 3028–3040. https://doi.org/10.1242/jeb.017426
Klein, B. A., Stiegler, M., Klein, A., Tautz, J. (2014) Mapping sleeping bees within their nest: spatial and temporal analysis of worker honey bee sleep. PLoS One 9(7), e102316. https://doi.org/10.1371/journal.pone.0102316
Koeniger, N., Fuchs, S. (1972) Kommunikative Schallerzeugung vonApis cerana Fabr. im Bienenvolk. Naturwissenschaften 59(4), 169–169. https://doi.org/10.1007/BF00637365
Kovac, H., Stabentheiner, A., Schmaranzer, S. (2010) Thermoregulation of water foraging honeybees – balancing of endothermic activity with radiative heat gain and functional requirements. J. Insect Physiol. 56, 1834–1845
Mellanby, K. (1939) Low temperature and insect activity. Proc. R. Soc. B Biol. Sci. 127(849), 473–487. https://doi.org/10.1098/rspb.1939.0035
Nakagawa, S., Schielzeth, H. (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4(2), 133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Narendra, A., Reid, S. F., Hemmi, J. M. (2010) The twilight zone: ambient light levels trigger activity in primitive ants. Proceedings. Biol. Sci. 277(1687), 1531–1538. https://doi.org/10.1098/rspb.2009.2324
Papachristoforou, A., Sueur, J., Rortais, A., Angelopoulos, S., Thrasyvoulou, A., Arnold, G. (2008) High frequency sounds produced by Cyprian honeybees Apis mellifera cypria when confronting their predator, the Oriental hornet Vespa orientalis. Apidologie 39(4), 468–474. https://doi.org/10.1051/apido:2008027
Sakagami, S. (1960) Preliminary report on the specific difference of behaviour and other ecological characters between European and Japanese honeybees. Acta Hymenopterologica 1, 171–198.
Sarma, M. Sen, Fuchs, S., Werber, C., Tautz, J. (2002) Worker piping triggers hissing for coordinated colony defence in the dwarf honeybee Apis florea. Zoology 105(3), 215–223. https://doi.org/10.1078/0944-2006-00064
Schlegel, T., Visscher, P. K., Seeley, T. D. (2012) Beeping and piping: characterization of two mechano-acoustic signals used by honey bees in swarming. Naturwissenschaften 99(12), 1067–1071. https://doi.org/10.1007/s00114-012-0990-5
Seeley, T. D. (1989) The honey bee colony as a superorganism. Am. Sci. 77(6), 546–553
Seeley, T., Tautz, J. (2001) Worker piping in honey bee swarms and its role in preparing for liftoff. J. Comp. Physiol. A Sensory, Neural, Behav. Physiol. 187(8), 667–676. https://doi.org/10.1007/s00359-001-0243-0
Seeley, T. D., Seeley, R. H., Akratanakul, P. (1982) Colony defense strategies of the honeybees in Thailand. Ecol. Monogr. 52(1), 43–63. https://doi.org/10.2307/2937344
Seeley, T. D., Visscher, P. K., Schlegel, T., Hogan, P. M., Franks, N. R., Marshall, J. A. R. (2012) Stop signals provide cross inhibition in collective decision-making by honeybee swarms. Science 335, 108–111. https://doi.org/10.1126/science.1210361
Taylor, L. R. (1963) Analysis of the effect of temperature on insects in flight. J. Anim. Ecol. 32(1), 99. https://doi.org/10.2307/2520
Wehmann, H.-N., Gustav, D., Kirkerud, N. H., Galizia, C. G. (2015) The sound and the fury—bees hiss when expecting danger. PLoS One 10(3), e0118708. https://doi.org/10.1371/journal.pone.0118708
Wiley, R. H., Richards, D. G. (1978) Physical constraints on acoustic communication in the atmosphere: implications for the evolution of animal vocalizations. Behav. Ecol. Sociobiol. 3(1), 69–94. https://doi.org/10.1007/BF00300047
Acknowledgements
We are grateful to all those who helped with fieldwork and gave us constructive suggestions. We also thank the Field Science Education and Research Center, Kitashirakawa Experimental Station, Kyoto University, for supporting our experiments.
Author information
Authors and Affiliations
Contributions
All authors conceived the study. S.K performed analysis. S.K wrote the manuscript with contributions from K.I, F.S, and K.M.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Manuscript editor: Monique Gauthier
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Des enregistrements sonores de colonies D' apis cerana japonica pendant 24 heures révèlent des schémas de sifflement quotidiens uniques
Comportement acoustique vibratoire / Apis cerana japonica / sifflement / abeilles domestiques / chatoyant
Tonaufnahmen in Apis cerana japonica Völkern mit 24-Stundenperiodik zeigen einzigartige Tagesmuster von Zischlauten
Akustisches Verhalten / Apis cerana japonica / Zischlaute / Honigbiene / Schimmer
Rights and permissions
About this article
Cite this article
Kawakita, S., Ichikawa, K., Sakamoto, F. et al. Sound recordings of Apis cerana japonica colonies over 24 h reveal unique daily hissing patterns. Apidologie 50, 204–214 (2019). https://doi.org/10.1007/s13592-018-0631-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-018-0631-x