Abstract
Vegetative propagation of sweet potato lead to the accumulation of diseases from generation to generation, which represents a threat to both productivity and conservation of genetic resources. In vitro techniques can help to overcome phytosanitary problems by applying plant material cleaning strategies. The objective of this study was to develop in vitro micropropagation strategies for the production of high-quality plant material of an orange-fleshed variety of sweet potato recently released in Colombia. Molecular identification of contaminating microorganisms was performed by sequencing the 16S rRNA gene for bacteria and ITS for fungi. Five disinfection protocols were evaluated, three of which were previously developed for sweet potato and included disinfection with 0.5, 1, and 2% sodium hypochlorite respectively, while two protocols are proposed in this work and included washing with povidone-iodine, disinfection with sodium hypochlorite 2%; one of these two new protocols also contains acetic acid and quaternary ammonium. For the evaluation of the viability of in vitro plants after disinfection, they were acclimatized in a greenhouse, reintroduced, and a molecular testing by PCR of 16S rRNA gene and ITS was carried out to verify the phytosanitary status of the material. The contaminating microorganisms found were filamentous fungi of the genera Fusarium, Sarocladium, Cladosporium and Aspergillus, yeasts of the genera Pseudozyma and Moesziomyces, and the actinobacterium Curtobacterium sp. The results indicated that washing with povidone-iodine and disinfection with 2% sodium hypochlorite, acetic acid and quaternary ammonium was the most efficient disinfection protocol, reducing the number of contaminated cultures by up to 10% and eradicating 70% of contaminants. The in vitro plants established in the greenhouse remained healthy and, after reintroduction, the molecular test for bacteria and fungi was negative. These results allowed the generation of an optimized protocol that can be incorporated into the in vitro micropropagation process to generate contamination-free sweet potato seeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Sweet potato (Ipomoea batatas (L.) Lam; Convolvulaceae) is the third most important root crop after potato and cassava (Chandrasekara and Josheph Kumar 2016; Gobena et al. 2022), and it is recognized as a functional food with nutraceutical properties, given its high content of fiber, carbohydrates, vitamins and antioxidant compounds (Salawu et al. 2015; Grace et al. 2015; Amagloh et al. 2021). These characteristics make the sweet potato a crop with growing demand in international markets, and can generate business opportunities in those developing countries that have a high potential for sweet potato production (Mu and Singh 2019). A new sweet potato variety, named “Agrosavia–Aurora”, was released for cultivation in the Caribbean zone of Colombia by the Corporación Colombiana de Investigación Agropecuaria–Agrosavia. This variety exhibits high beta carotene (214 ± 21 µg g−1) and total carotene (246 ± 25 µg g−1) content and is phenotypically characterized by an intense orange-flesh color. In the field, it showed a yield of more than 20 Ton fresh tuberous roots ·ha−1 year−1.
The plant material typically used for sweet potato propagation is represented by cuttings obtained from previous commercial crops, multiplied mainly by vegetative propagule propagation (Kim et al. 2015; Qiao et al. 2019; Ssamula et al. 2020), exchanged from farmer to farmer and from one cycle to another, with multiple rooting and without renewal (Rajendran et al. 2017). This vegetative propagation without proper management has resulted in the accumulation of diseases from generation to generation (Kim et al. 2017; Wanjala et al. 2020). This low phytosanitary quality is mainly due to bacteria, fungi, and viruses that affect both production and quality of tuberous roots (Mwanga et al. 2017; Makokha et al. 2020; Abrham et al. 2021). Thus, the use of high-quality planting material is one of the keys to increase sweet potato productivity (Wanjala et al. 2020; Abrham et al. 2021).
Phytopathogenic fungi, especially species of the genera Fusarium, Alternaria, Sphaceloma, and Sclerotium (Aguoru and Amuzie 2009; Ekman and Lovatt 2015; Paul et al. 2017; Samiyarsih et al. 2018), as well as bacteria of the genera Streptomyces, Pseudomonas and Ralstonia (Jena and Samal 2011; Ekman and Lovatt 2015; Mwanga et al. 2017; Zhang et al. 2018), are among the major constraints for sustainability of sweet potato production and tuber quality (Mwanga et al. 2017; Makokha et al. 2020). Virus diseases, spread through infected propagation material, also represent an important phytosanitary problem in sweet potato (Wanjala et al. 2020; Jo et al. 2020): Sweet potato chlorotic stunt virus (genus Crinivirus, family Closteroviridae), Sweet potato feathery mottle virus (genus Potyvirus, family Potyviridae) and Sweet potato leaf curl virus (genus Begomovirus, family Geminiviridae) are some major viral pathogens of sweet potato responsible for substantial damage to the world’s sweet potato industry (Kim et al. 2017; Mwanga et al. 2017; Mulabisana et al. 2018; Zhang et al. 2020).
The production of high-quality sweet potato plant material is an effective and widely used strategy to improve productivity and prevent disease spreading. One of the techniques used to generate high quality plant material is the micropropagation, which is based on obtaining in vitro cultures from apical meristems and introduces several steps for cleaning the plant material. In vitro techniques have a positive impact on overcoming contaminants such as microorganisms and viruses (Yang 2010; Delgado-Paredes et al. 2016; Alula et al. 2018). The micropropagation of plants has the following stages: selection of stock plants, establishment of aseptic culture, multiplication of explants, rooting of regenerated shoots or somatic embryo germination, acclimatization or transfer of plantlets to the soil (Bhatia 2015; Singh 2015). In seed systems, these processes are accompanied by cleaning procedures and diagnostics, to finally get indexed (clean) plant material. The first two stages of the micropropagation process (selection of stock plants and establishment of aseptic cultures) are decisive to eliminate phytopathogens associated with the culture through disinfection strategies in order to obtain pathogen-free plant material (Aguoru and Amuzie 2009; Jena and Samal 2011; Hammond et al. 2014).
In sweet potato, the fungi Aspergillus spp., Penicillum spp., Fusarium spp., Alternaria spp. (Aguoru and Amuzie 2009) and the bacteria Pseudomonas spp., Klebsiella spp. and Corynebaterium spp. (Jena and Samal 2011), have been reported as frequent contaminants in micropropagation processes. There are disinfection protocols evaluated for sweet potato that allow obtaining uncontaminated explants in in vitro conditions (Yang 2010; Hammond et al. 2014; Delgado-Paredes et al. 2016; Alula et al. 2018); these protocols generally involve a washing step with detergent and a disinfection step mainly with sodium hypochlorite, a compound with known microbicidal effects (Lazo-Javalera et al. 2016; Carmello and Cardoso 2018). Considering that the plant microbiome depends on variety, growth conditions and environment (Compant et al. 2019; Santos and Olivares 2021), the disinfection protocols require to be re-evaluated and adjusted when working with new varieties that will be introduced for the first time. Therefore, the objective of this work was to produce high-quality plant material, since significant losses caused by rotting problems and incidence of microorganisms were reported in different sweet potato accessions in the Colombian Caribbean zone; this has been done through i) evaluation of different protocols for plant material disinfection; ii) identification of main contaminating bacterial and fungal species associated with plant material; iii) determination of viability and phytosanitary quality of in vitro sweet potato plants under greenhouse conditions. The results of this research will be of primary importance for the establishment of an optimized disinfection protocol in the new orange-fleshed sweet potato variety for seed system establishment.
2 Materials and methods
2.1 Assessment of losses caused by rotting problems and incidence of microorganisms in eight accessions of sweet potato grown in the Colombian Caribbean
Eight accessions of sweet potato (0113-634VAL, 0113-656COR, 0113-660VAL, 0113-664VAL, 0113-668VAL, 0113-671VAL, Criolla, and Agrosavia–Aurora) were established in four localities of the Caribbean zone of Colombia in the Departments of Córdoba (Cereté), Sucre (Corozal and Tolú) and Cesar (Jagua). The selected genotypes were planted in plots with five rows of five meters length (total area: 25 m2; inter-row: one meter; inter-plant: 30 cm). At 100 days after planting (DAP), percentage and yield (t ha−1) of tuberous roots affected by rot were recorded. Damage symptoms caused by pathogens were evaluated according to Rosero et al. (2019) at 15, 30, 45, 60, 75 and 90 DAP, and percentages of average damage throughout the crop cycle were obtained.
2.2 Plant material
The new cultivar “Agrosavia–Aurora” presented a high risk of tuberous root yield loss due to rotting and, therefore, it was selected for scheme implementation to produce high-quality planting material through micropropagation process. Two-hundred apical cuttings of Agrosavia–Aurora variety, obtained from the Agrosavia field germplasm bank, were sown in germination trays in a mixture of alluvium and sand (2:1 v/v), previously solarized for 8 days and disinfected with 2 mL L−1 poloxamer iodine (Agrodyne® SL, Santander—Colombia). Plants were grown for a week in a greenhouse at 29 ± 4 °C temperature, 75 ± 10% relative humidity and 300–600 μmol m−2 s−1 luminosity, with 12/12 h light/dark photoperiods.
2.3 Thermotherapy and virus testing for the selection of stock plants
For the thermotherapy treatment of the greenhouse counters, a 2 m (length) × 1 m (width) × 1.5 m (height) plastic chamber was used. Relative humidity and air temperature inside of the plastic chamber were monitored with an Extech RHT10 USB-type Datalogger (Extech Industries, Nashua, USA). The highest air temperature (50 ± 4 °C) and relative humidity (73 ± 5%) occurred between 11:30 am and 12:30 pm. Luminosity was 300–600 μmol m−2 s−1, with 12/12 h light/dark photoperiods. Plant material grown for one week was then subjected to thermotherapy for the three following weeks. During thermotherapy process, 30–40 cc of irrigation water per plant were supplied manually twice a day. Pests and diseases were controlled by applications of both 2 g L−1 axil-metal-based fungicide mancozeb (Ridomil® Gold MZ 68 WP) and 2 mL L−1 cypermethrin (Cypermon® 20 EC) at 15 and 30 DAP.
Virus diagnosis was performed on the 3 top youngest leaves of 30 plants. Leaf samples were wrapped in kimwipes to protect them from mechanical damage, stored in Ziploc bags containing fresh silica gel, and sent for analysis to the Centro Internacional de Agricultura Tropical (CIAT) for the analysis of two types of virus: Sweet potato feathery mottle virus (SPFMV, genus Potyvirus, family Potyviridae) and Sweet potato chlorotic stunt virus (SPCSV, genus Crinivirus, family Closteroviridae), according to Li et al. (2012) and Kwak et al. (2014), respectively. Plants that survived the thermotherapy process and were negative for the presence of viruses were then selected as stock plants for the micropropagation process.
2.4 Disinfection protocols for in vitro initiation of cuttings
Cuttings, approximately 3–5 cm long and containing at least 3 axillary buds, were harvested from the greenhouse stock plants, and submitted to different surface disinfection treatments (Table 1). A completely randomized design was established. For each protocol, 10 apical explants were used and four replicates per each apical explant were established, for a total of 40 plants per protocol evaluated.
After the different disinfection processes, explants were placed in MS culture medium (Murashige & Skoog, 1962) (30 g L−1 sucrose, 100 mg L−1 myoinositol, 1 mg L−1 thiamine, 2.5 g·L−1 Phytagel™—Sigma-Aldrich, pH 5.6–5.8; Delgado-Paredes et al. 2016) and cultivated in a bioclimatic chamber Caron 7300–50 (Caron Products & Services Inc, Marietta, USA). Light intensity was 500 µmol s−1 m−2, under 16/8 h light/dark photoperiod. Air temperature was set at 27/25 °C during the light/dark cycle, respectively, and relative humidity was maintained at 65 ± 10%. Explants were monitored every two days during the first 10 days of in vitro culture, and number of contaminated explants was recorded for each protocol. After 10 days, contaminated material was discarded. Between 10 and 30 days after cultivation, no contamination was observed. After four weeks, the number of successfully introduced in vitro plants was registered.
2.5 Characterization of contaminating microorganisms associated to in vitro initiation
Microorganisms were isolated in solid culture media. For filamentous fungi, a portion of mycelium was removed with a straight loop and cultured by puncture on potato dextrose agar (PDA; Oza et al. 2020) plates. For non-filamentous microorganisms, a small quantity was taken with a ring loop and streaked on Sabouraud dextrose agar (SDA; Kačániová et al. 2020) to isolate yeast, and King’s B medium (Egamberdieva et al. 2017) to isolate bacteria. Petri dishes were incubated at 25 °C for fungi and 30 °C for bacteria. Subcultures were done until pure cultures were obtained.
A macroscopic and microscopic characterization of pure cultures was performed at 24 h for bacteria and 96 h for fungi. Macroscopic characteristics included coloration, texture, edge and surface. For microscopic characterization, a Leica DM750 microscope (Leica microsystems, Wetzlar, Germany) was used. Lacto phenol cotton blue staining (Wanger et al. 2017) was used for fungi, while Gram staining (Tripathi and Sapra 2021) was used for yeasts and bacteria. Structures in fungi visualized by microscope, were used to identify them at the genus level (Dugan 2017; Germain & Summerbell 2010).
The isolated microorganisms were characterized molecularly by the universal direct PCR amplification system from pure cultures (Ben-Amar et al. 2017), using a PCR Master mix 2X K0171 (Thermo Fisher Scientific). For fungi and yeasts, the amplification of the ITS region was performed using primers ITS-1F and ITS-4 (Gardes and Bruns 1993), with an initial denaturation of 95 °C for 3 min, followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s and 72 °C for 45 s, and a final extension at 72 °C for 7 min. For bacteria, amplification of 16S rRNA gene was done using primers 27F and 1492R, with an initial denaturation of 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, 52 °C for 30 s and 72 °C for 90 s, and a final extension at 72 °C for 10 min. PCR products were sequenced using an ABI 3500 capillary electrophoresis equipment (Applied Biosystems®, USA). The sequences obtained were submitted to Genbank repository (www.ncbi.nlm.nih.gov) under the accession numbers MZ343564 to MZ343570. Alignment with reference sequences from GenBank® database of the National Center for Biotechnology Information (NCBI), using the Basic Local Alignment Search Tool (BLAST; Altschul et al. 1990) was performed, and a phylogenetic analysis was carried out using the MEGAX software v. 10.2.4 (Kumar et al. 2018). The sequences were aligned using MUSCLE program (Multiple Sequence Comparison by Log-Expectation; Edgar 2004). Phylogenetic trees were inferred using the maximum likelihood method (Felsenstein 1981), and evolutionary distances were calculated using the 2-parameter Kimura model (Kimura 1980). Initial trees for heuristic search were obtained with the maximum parsimony method. A discrete gamma distribution was used to model differences on evolution rate between sites [2 categories (+ G, parameter = 0.8710)]. All positions that contained gaps and missing data were removed. The tree evaluation was performed using Bootstrap method with 1000 permutations to assign confidence levels to the nodes of the tree (Felsenstein 1985).
2.6 Determination of the percentage of incidence of microorganisms after the disinfection process
To determine the incidence percentage of microorganisms after explants disinfection using the evaluated protocols, during first 10 days of in vitro culture, microorganisms and their appearance frequency in each protocol were recorded. For each protocol, 10 apical explants were used and four replicates per each apical explant were established, for a total of 40 plants per protocol evaluated.
2.7 Determination of viability and phytosanitary quality of in vitro sweet potato plants under greenhouse conditions
In vitro plants obtained from the best disinfection protocol were grown in bioclimatic chambers for four weeks; afterword, in vitro plants were established under greenhouse conditions to determine both development and phytosanitary quality. In vitro plants were carefully removed from the test tube and roots were disinfected with 58% copper oxychloride solution (2 g L−1) for five minutes, and further transferred in germinating trays in 60% peat, 20% vermicompost and 20% rice husk. A total of 40 in vitro plants were established under greenhouse conditions at Turipaná research center, under semi-controlled condition (28–34 °C temperature, 60–95% relative humidity, 300–600 μmol m−2 s−1 luminosity, 12/12 h light/dark photoperiod), with plant material isolated to minimize contamination. During the first week, the light was regulated at 20% with 80% commercial shade cloth; later, seedlings were moved to an area with 35% commercial shade cloth. Irrigation water (30–40 mL per plant) was manually supplied each morning.
Monitoring was carried out once a week for four weeks, recording the presence of symptoms related to fungi and/or bacteria (Rosero et al. 2019). After four weeks, the number of plants with and without symptoms was recorded, and a destructive sampling of 15 individuals was done to both verify the phytosanitary quality of seedlings and obtain photographic records.
Twenty plants acclimatized in the greenhouse were reintroduced to the laboratory to revalidate the phytosanitary quality of the plant material. These plants were disinfected and grown for four weeks in MS medium, and subsequently the DNA was extracted from ten plants using the DNeasy Plant Mini Kit extraction kit (Qiagen, Germany). Extracted DNA was used to amplify 16S rRNA gene and ITS, in order to detect the presence of bacterial and fungal DNA, respectively. DNA from Curtobacterium sp. and Sarocladium subulatum was used as positive controls for 16S rRNA gene and ITS, respectively, while ultrapure sterile water was used as negative control. PCR products were visualized on 2% agarose gels after stained with Syber Safe (1µL per 100 mL of gel); one µL of each amplicon was mixed with five µL of loading buffer, and then run in horizontal electrophoresis for 45 min at 90 V.
2.8 Statistical analysis
To determine the best in vitro disinfection protocol, the software SAS (Khattree and Naik 1999) was used to perform one-way analysis of variance (ANOVA), followed by Tukey's honestly significant difference (HSD) post-hoc test, at p ≤ 0.05.
3 Results
3.1 Assessment of losses caused by rotting problems and incidence of microorganisms in eight sweet potato accessions grown in the Colombian Caribbean
The results obtained for different varieties of sweet potato in the Colombian Caribbean area indicated that the accessions 0113-671VAL in the site Tolú and 0113-634VAL in the site Cereté presented a rot percentage of 30–35%, while the variety Agrosavia–Aurora registered the highest percentage (> 45%) of rot in Tolú. For the rest of the accessions, in all the evaluated localities, a percentage of tuberous root rot < 20% occurred (Fig. 1a). The Agrosavia–Aurora variety presented the highest values of rotting tuberous roots per hectare in three of the four localities (Cereté, Corozal and Tolú), reaching losses greater than 16 t ha−1 in Cereté (Fig. 1b), which suggests that for this variety there is a high incidence of rot incidence.
Record of losses of tuberous roots due to rot in sweet potato varieties grown in different areas of the Colombian Caribbean. a Percentage of tuberous roots affected by rot. b Tuberous roots affected by rot (ton ha−1). c Percentage of damage caused by bacteria. d Percentages of damage caused by fungi. e Percentage of damage caused by bacteria and fungi
Incidence of bacterial-caused damage for the accession 0113-660VAL was 23% and for the Agrosavia–Aurora variety was 7% in the town of Cereté, while for the accession 0113-668VAL it was 20%the locality of Cereté, 15% in Corozal and 59% in Tolú (Fig. 1c). Regarding the percentage of fungal damage, it was found that in the Jagua locality all the varieties presented percentages lower than 4%. The Criolla variety presented a percentage of fungal damage of 46% in Corozal, while accession 0113-668VAL showed a percentage of 7% in Cereté, 59% in Tolú, and 100% in Corozal (Fig. 1d). The percentage of damage caused by a bacteria/fungi mixture in the locality of Cereté was 15% for the accessions 0113-660VAL, 38% for 0113-664VAL, 51% for 0113-668VAL and 38% for Criolla; for the Agrosavia–Aurora variety, percentages of damage were recorded in Cereté (15%) and Corozal (7%) (Fig. 1e).
3.2 Disinfection protocols for in vitro initiation of cuttings
The protocols P4 and P5, which included both a washing step with detergent plus povidone iodine (20 min) and a disinfection step with 2% sodium hypochlorite (NaOCl) plus tween 80 (10 min), were the most effective for the surface disinfection of sweet potato cuttings. Using P4 and P5 disinfection protocols, it was observed that contamination appeared at the sixth day of in vitro culture, presenting percentages of contamination of explants less than 25% (Fig. 2a). The highest average percentage of successfully established in vitro plants (90%) was produced with the P4 protocol, which incorporates a complementary disinfection with acetic acid (1 min) and quaternary ammonium (1 min) (Fig. 2b). In contrast, the cuttings treated with protocols P2 [washing with detergent (30 min) and disinfection with NaOCl 0.5% plus tween 80 (3 min)] and P3 [washing with detergent (10 min) disinfection with hypochlorite 1% plus tween 80 (12 min)], lead to microbial contamination at the fourth day of in vitro culture and reached the highest percentages of contamination of the explants already after six days of in vitro culture (Fig. 2a). Moreover, the lowest percentage (< 50%) of successfully established in vitro plants was observed with the P2 and P3 disinfection protocols (Fig. 2b).
Status of the sweet potato plant material after the evaluation of different disinfection protocols in the in vitro initiation process. a Percentage of contaminated explants during the first 10 days after in vitro culture. The error bars indicate the standard error. b Percentage of successfully introduced in vitro plants four weeks after in vitro culture. Different letters indicate significant differences between protocols (Tukey test, p < 0.05)
3.3 Characterization of contaminating microorganisms associated to in vitro initiation
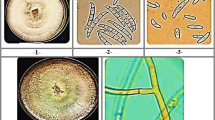
Seven contaminating microorganisms were isolated and characterized (Fig. 3a). The sequences obtained from the microorganisms were compared with the databases to identify the most similar species (Table 2). The phylogenetic trees from the sequences obtained, indicated that four strains, namely H1 to H4, corresponded to filamentous fungi of the Ascomycota division distributed in the orders Hypocreales, Capnodiales and Eurotiales; two strains, L1 and L2, were affiliated to yeasts of the Basidiomycota division, order Ustilaginales; and a prokaryotic microorganism (B1) belonged to the phylum Actinobacteria, order Actinomycetales (Fig. 3).
For the description at the genus level of the isolated filamentous fungi, in addition to the molecular characterization, the macro and microscopic characteristics were considered (Table S1). The H1 strain presented a percentage of identity greater than 97% and a coverage of 93% with S. subulatum (Table 2). Microscopy allowed the identification of phialids with pericline thickening and spindle-shaped conidia arranged in viscous heads (Fig. S1a–d), structures reported for S. subulatum (Giraldo et al. 2015).
The H2 strain under microscope presented typical oval or reniform microconidia characteristic of Fusarium (Fig. S1e–h), while the molecular characterization related it to species of the Fusarium fujikuroi complex (Moussa et al. 2017; Montoya-Martínez et al. 2019; Bashyal et al. 2019) with a percentage of identity higher than 99%.
The H3 strain showed a relationship with species of the genus Cladosporium (Cladosporium cladosporioides complex; Bensch et al. 2010; Amirmijani et al. 2014; Sandoval-Denis et al. 2016) with an identity percentage greater than 99% (Table 2); conidiophores were observed forming acropetal chains and conidia with the presence of a thick refractive scar to obscured cladosporioid or crowned characteristic of these species (Fig. S1i-l). The H4 strain (Fig. S1m-p) showed a percentage of identity greater than 99% with several species of the genus Aspergillus (Table 2), namely A. assiutensis and A. brunneoviolaceus (Fig. 3), species of the Nigri section (Varga et al. 2011; Frisvad et al. 2014; Hussein et al. 2017).
Unicellular microorganisms differentiated as yeasts and bacteria. The macroscopic and microscopic characterization are reported in supplementary material (Table S2). In the case of yeast encoded as L1, the molecular characterization associated it with the genus Pseudozyma (Fig. S2); large ovoid and elongated grouped or individual cells, pseudohyphae and blastoconidia were observed under the microscope (Fig. S2a, d). Similar structures have been reported for this genera (Boekhout 1995; Wang et al. 2016). L2 yeast strain was identified as Moesziomyces parantarcticus with 99.6% identity percentage (Table 2). The morphology was creamy and rough beige colonies (Fig. S2b) and under microscope the presence of pseudohyphae and blastoconidia was observed (Fig. S2e). Similar characteristics were reported for this species (Liu et al. 2019). Bacterial strain B1 was found associated with species of the genus Curtobacterium with an identity percentage higher than 98%; considering that its macro- and microscopic morphologies were did relate it to any species (Fig. 2S c, f), it was identified as Curtobacterium sp.
3.4 Determination of the percentage of incidence of microorganisms after the disinfection process
Explants subjected to washing with detergent (30 min) and disinfection with 0.5% NaOCl plus tween 80 (3 min) (protocol P2) showed the presence of all microorganisms identified (Fig. 4). The explants disinfected with protocol P3 [washing with detergent (10 min), disinfection with 1% NaOCl plus tween 80 (12 min)] and P1 [washing with detergent (5 min), disinfection with 2% NaOCl (7 min)] showed the presence of five of the seven identified microorganisms, presenting the highest incidences of microorganisms with P3 (Fig. 4).
Percentage of incidence of contaminating microorganisms associated with the evaluated disinfection protocols. P1: Washing with detergent (WD) (5 min), disinfection with NaClO 2% (7 min). P2: WD (30 min), 0.5% NaClO + tween 80 (T80) (3 min). P3: WD (5 min), 1% NaClO + T80 (12 min). P4: WD and povidone iodine (20 min), NaClO 2% + T80 (10 min), acetic acid (1 min) and quaternary ammonium (1 min). P5: WD and povidone iodine (20 min), 2% NaClO + T80 (10 min)
The disinfection of explants with a washing with detergent plus povidone iodine (20 min) and disinfection with 2% NaOCl plus tween 80 (10 min) (P5) allowed to eliminate 43% of the contaminating microorganisms, presenting incidences of less than 8% (Fig. 4). The eradication of 72% of the contaminating microorganisms with incidences of 5% was achieved by incorporating an additional disinfection with acetic acid and quaternary ammonium (1 min) (P4). The microorganisms Fusarium sp. and Pseudozyma were present in all protocols after disinfection processes (Fig. 4).
3.5 Determination of viability and phytosanitary quality of in vitro sweet potato plants under greenhouse conditions
The in vitro plants (Fig. 5a) that were acclimatized and hardened under greenhouse conditions (Fig. 5b) did not report any affection by fungi or bacteria, did not present anomalies in stems, leaves or roots (Fig. 5c), and showed adequate average growth parameters (Table 3).
Determination of the viability of plants in vitro through the establishment in greenhouse conditions. a State of in vitro plants of 4 weeks of culture in the laboratory. b Seeding of plants in vitro under greenhouse conditions. c In vitro plants after 4 weeks of acclimatization under greenhouse conditions
For the validation of the phytosanitary quality, it was possible to obtain DNA of good quality from the reintroduced in vitro plants (Fig. 6a). The results of the amplification of the 16S rRNA gene and ITS showed no bands, except for the positive controls at 1500 and 615 bp, respectively (Fig. 6b-c).
Verification by PCR of molecular markers of the presence of bacteria and fungi in the DNA of plants reintroduced after the greenhouse acclimatization phase. P1–P10 correspond to in vitro plants. C + and C − indicate positive and negative controls, respectively. a In vitro plant DNA. b 16S gene amplification. c ITS amplification
4 Discussion
4.1 Assessment of losses caused by rotting problems caused by microorganisms
The results obtained in this work indicate that the sweet potato varieties present percentages of damage by bacteria and fungi that cause losses at the productive level. For the Agrosavia–Aurora variety, which is of main interest for this research, a high percentage of tuberous root affectation was found, generating low yields due to the notable loss in the harvest. In Agrosavia–Aurora, phytosanitary alterations have been reported caused by Fusarium sp., and Pseudomonas solanacearum; these phytopathogens produce blockages of the vascular system and deterioration of the foliage, decrease the photosynthetic capacity of the plant and affect the accumulation of reserve substances in the tuberous roots to the point of causing the death of the plant (Rosero et al. 2019). Considering these results, it is very important for this new variety to establish a phytosanitary cleaning scheme for plant material in order to mitigate the effects of pathogens on yield; for this reason, the use of strategies such as plant micropropagation is essential to obtain plants free of pathogens and of high phytosanitary quality.
4.2 Disinfection protocols for in vitro initiation of cuttings and characterization of contaminating microorganisms associated to in vitro initiation
In this work, we tested the efficacy of five different disinfection protocol to achieve the highest quality plant material of a new variety of orange pulp sweet potato. The lowest average contamination percentage (20%) was obtained with the use of the P4 and P5 protocols; additionally, it was observed that they suppressed the proliferation of Cladosporium sp., Curtobacterium sp. and Aspergillus sp. These protocols, unlike P1, P2 and P3, incorporate povidone iodine in the detergent washing process, which has a broad-spectrum bactericidal, fungicidal and virucidal effect (Bigliardi et al. 2017). The incorporation of this compound has been reported in Rosa canina (Shirdel et al. 2017), Curcuma aeruginosa (Khumaida et al. 2019) and Sargassum polycystum (Muhamad et al. 2018). For these plant species, it has been favorable to use povidone iodine to reduce the percentages of contamination in processes of plant material introduction, similarly to the results obtained in this work.
In relation to the disinfection process, the protocols with concentrations of NaOCl lower than 2% (P2 and P3), lead to a higher percentage of contaminated seedlings as well as the presence of a higher incidence of contaminating microorganisms. A NaOCl concentration of 2% and longer exposure time to the disinfectant (20 min), as used in protocols P4 and P5, allowed obtaining a higher percentage of seedlings without contamination and reducing the presence or incidence of contaminating microorganisms. A percentage of 2% of NaOCl is found in the typical range considered as efficient for the reduction of microbial contamination in plant tissue cultures (Lazo-Javalera et al. 2016; Carmello and Cardoso 2018).
Although there was no statistically significant difference between P4 and P5 protocols in the percentages of contaminated and uncontaminated seedlings, P4 increased the quality values obtained, and there was no growth of five out of the seven isolated contaminating microorganisms. This is possibly related to the incorporation of acetic acid and quaternary ammonium in the disinfection stage of P4, widely reported as disinfectants with bactericidal, fungicidal and virucidal effects (McDonnell 2017; Obłąk et al. 2019; Becker et al. 2019; Chowdhury et al. 2019; Kwaśniewska et al. 2020). Considering that even after applying the best P4 protocol, the microorganisms Fusarium sp. and Pseudozyma sp. persist as contaminants, possibly these microorganisms are low-abundance endophytes of sweet potato, as recently reported for tomato (Imazaki and Kadota 2015). Pseudozyma is a genus already reported as endophyte in other plants as well (Sadeghi et al. 2019; Talukdar et al. 2020).
Sarocladium subulatum, Cladosporium sp., Pseudozyma sp., Moesziomyces parantarcticus, and Curtobacterium sp. were reported here for the first time during the in vitro initiation process of the orange pulp sweet potato plant material of the Agrosavia–Aurora variety. Other microorganisms reported in this investigation were Fusarium sp. and Aspergillus sp., which have also been reported in initiation processes in sweet potatoes (Aguoru and Amuzie 2009), and recently reported as contaminants in in vitro initiation of Musa textiles (Cobrado and Fernandez 2016, 2017), Bambusa balcooa (Tyagi et al. 2017), Citrus sinensis (Yeasmin et al. 2018) and Chlorophytum borivilianum (Kumar et al. 2019). Cladosporium has been found as a contaminant in Phoenix dactylifera (Singh 2018) and Chlorophytum borivilianum (Kumar et al. 2019). Species of the genus Sarocladium have been reported as endophytes in Citrus (S. subulatum; Nicoletti 2019) and Coffea arabica (S. bacillisporum; Oliveira et al. 2014). In addition, yeast-like microorganisms of the genus Pseudozyma and Moesziomyces have been reported as epiphytic fungi associated with plant leaves in Magnolia denudata and Zea mays, respectively (González-Teuber et al. 2014; Wang et al. 2016; Into et al. 2020). The bacterial genus Curtobacterium has been reported as a contaminant in micropropagation processes (Tekielska et al. 2019) and associated with Ipomoea aquatica (Rahayu et al. 2021) and Pancratium maritimum (Tumbarski et al. 2018).
4.3 Determination of viability and phytosanitary quality of in vitro sweet potato plants under greenhouse conditions
The in vitro plants obtained after the micropropagation process using the disinfection protocol (P4), which lead to > 90% of plants without contamination, were acclimatized under greenhouse conditions. The plants in greenhouse developed leaves and roots without the presence of disease symptoms, which suggests that the process of in vitro disinfection was efficient allowing to eliminate pathogens such as fungi or bacteria. Ex vitro acclimatization is a very important phase in the in vitro micropropagation process. At this stage, the plants that are in controlled laboratory conditions are transferred to ex vitro conditions where they are exposed to different types of stress. The survival of the plants in greenhouse condition will depend mainly on the conditions of the mother in vitro plants. If the plant material is not vigorous, abnormal stomatal development, absence of epicuticular waxes and few functional roots may occur. An effective micropropagation protocol is essential to achieve high levels of survival (Rescalvo-Morales et al. 2019; Monja-Mio et al. 2020).
The verification of the phytosanitary quality of the acclimatized plants was carried out through the reintroduction of the plant material and subsequent determination at molecular level of the presence of bacteria and fungi, by means of 16S rRNA and ITS amplification, respectively. These molecular markers have been widely used to determine the presence of microorganisms in the DNA of plants; for example, in Dendrobium officinale the primers 27R and 1492R that amplify the 16S rRNA gene were effective for the amplification of plant endophytes in both in vitro and potted plants (Yu et al. 2013). Likewise, amplification with specific markers such as ITS are widely used strategies for molecular diagnosis of pathogenic fungi in plants (McCartney et al. 2003; Mancini et al. 2016). The results indicated that in the DNA of the in vitro plants the PCR of these molecular markers did not produce any amplicon, demonstrating the absence of DNA of bacterial or fungal origin and consequently validating the efficacy of the newly established disinfection protocol P4 used in the micropropagation process.
5 Conclusion
The Agrosavia–Aurora sweet potato variety established in the field presented percentages of damage by bacteria and fungi that caused losses of tuberous roots at harvest, indicating a quality deficiency of the plant material. The evaluation of disinfection protocols in the Agrosavia–Aurora micropropagation processes allowed obtaining a disinfection protocol (P4) in which a percentage of in vitro plants without contamination of 90% is obtained.
As a result of this research, the microorganisms Sarocladium subulatum, Cladosporium sp, Pseudozyma sp., Moesziomyces parantarcticus and Curtobacterium sp. are reported as main contaminants for the new Ipomoea batatas orange pulp variety Agrosavia–Aurora.
The plants obtained after the in vitro disinfection and micropropagation process presented good growth characteristics and no presence of pathogens in the acclimatization phase. Verification with molecular markers, 16S rRNA gene and ITS, allowed establishing the absence of bacteria and fungi in the DNA of the in vitro plants reintroduced after the acclimatization process in the greenhouse.
Data availability
New sequences produced in this work were submitted to NCBI under the accession numbers MZ343564- MZ343570.
References
Abrham T, Beshir HM, Haile A (2021) Sweetpotato production practices, constraints, and variety evaluation under different storage types. Food Energy Secur 10:e263. https://doi.org/10.1002/FES3.263
Aguoru C, Amuzie U (2009) Associated microbial contaminants in in-vitro micropropagation of sweet potato (Ipomoea batatas L.). Int J Nat Appl Sci 5:49964. https://doi.org/10.4314/ijonas.v5i2.49964
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Alula K, Zeleke H, Manikandan M (2018) In vitro propagation of sweet potato Ipomoea batatas (L.) Lam) through apical meristem culture. J Pharmacogn Phytochem 7:2386–2392
Amagloh FC, Yada B, Tumuhimbise GA, Amagloh FK, Kaaya AN (2021) The potential of sweetpotato as a functional food in sub-Saharan Africa and its implications for health: a review. Molecules 26:2971. https://doi.org/10.3390/MOLECULES26102971
Amirmijani A, Khodaparast SA, Zare R (2014) Contribution to the identification of Cladosporium species in the North of Iran. Rostaniha 15:133–145. https://doi.org/10.22092/BOTANY.2014.101237
Bashyal BM, Yadav J, Gupta AK, Aggarwal R (2019) Understanding the secondary metabolite production of Gibberella fujikuroi species complex in genomic era. Indian Phytopathol 72:607–617
Becker B, Henningsen L, Paulmann D, Bischoff B, Todt D, Steinmann E, Steinmann J, Brill FHH, Steinmann J (2019) Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrob Resist Infect Control 8:121. https://doi.org/10.1186/s13756-019-0569-4
Ben-Amar A, Oueslati S, Mliki A (2017) Universal direct PCR amplification system: a time- and cost-effective tool for high-throughput applications. 3 Biotech 74(7):1–7. https://doi.org/10.1007/S13205-017-0890-7
Bensch K, Groenewald JZ, Dijksterhuis J, Starink-Willemse M, Andersen B, Summerell BA, Shin HD, Dugan FM, Schroers HJ, Braun U, Crous PW (2010) Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Stud Mycol 67:1–94. https://doi.org/10.3114/sim.2010.67.01
Bhatia S (2015) Plant tissue culture. In: Bhatia S, Sharma K, Dahiya R, Bera T (eds) Modern applications of plant biotechnology in pharmaceutical sciences, 1st edn. Academic Press, Cambridge, pp 31–10
Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, Pyon JK, Wa CTC, Villa MA (2017) Povidone iodine in wound healing: a review of current concepts and practices. Int J Surg 44:260–268. https://doi.org/10.1016/j.ijsu.2017.06.073
Boekhout T (1995) Pseudozyma Bandoni emend. Boekhout, a genus for yeast-like anamorphs of Ustilaginales. J Gen Appl Microbiol 41:359–366. https://doi.org/10.2323/jgam.41.359
Carmello CR, Cardoso JC (2018) Effects of plant extracts and sodium hypochlorite on lettuce germination and inhibition of Cercospora longissima in vitro. Sci Hortic 234:245–249. https://doi.org/10.1016/j.scienta.2018.02.056
Chandrasekara A, Josheph Kumar T (2016) Roots and tuber crops as functional foods: a review on phytochemical constituents and their potential health benefits. Int J Food Sci. https://doi.org/10.1155/2016/3631647
Chowdhury D, Rahman A, Hu H, Jensen SO, Deva AK, Vickery K (2019) Effect of disinfectant formulation and organic soil on the efficacy of oxidizing disinfectants against biofilms. J Hosp Infect 103:e33–e41. https://doi.org/10.1016/j.jhin.2018.10.019
Cobrado JS, Fernandez AM (2016) Common fungi contamination affecting tissue-cultured Abaca (Musa textiles Nee) during initial stage of micropropagation. Asian Res J Agric 1:1–7. https://doi.org/10.9734/ARJA/2016/28353
Cobrado JS, Fernandez AM (2017) Bioefficacy test of different chemotherapeutic substances against Aspergillus sp. and Chrysosporium sp. contaminants of tissue-cultured Abaca (Musa textiles NEE.) during initial stage of micropropagation. J Adv Microbiol 4:1–12. https://doi.org/10.9734/JAMB/2017/33289
Compant S, Samad A, Faist H, Sessitsch A (2019) A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J Adv Res 19:29–37. https://doi.org/10.1016/J.JARE.2019.03.004
Delgado-Paredes GE, Idrogo CR, Chanamé-Céspedes J, Floh EI, Walter H (2016) In vitro direct organogenesis in roots of Ipomoea batatas. Asian J Plant Sci Res 6:17–27
Dugan FM (2017) The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature. In The identification of fungi: an illustrated introduction with keys, glossary, and guide to literature (3rd ed.). The American Phytopathological Society. https://doi.org/10.1094/9780890545041
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Egamberdieva D, Wirth SJ, Shurigin VV, Hashem A, AbdAllah EF (2017) Endophytic bacteria improve plant growth, symbiotic performance of chickpea (Cicer arietinum L.) and induce suppression of root rot caused by Fusarium solani under salt stress. Front Microbiol 8:1887. https://doi.org/10.3389/FMICB.2017.01887
Ekman J, Lovatt J (2015) Pests, diseases and disorders of sweetpotato: a field identification guide. Horticulture Innovation Australia Limited, North Sydney
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17:368–376. https://doi.org/10.1007/BF01734359
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.2307/2408678
Frisvad JC, Petersen LM, Lyhne EK, Larsen TO (2014) Formation of sclerotia and production of indoloterpenes by Aspergillus niger and other species in section nigri. PLoS ONE 9:e94857. https://doi.org/10.1371/journal.pone.0094857
Gardes M, Bruns TD (1993) ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol 2:113–118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x
Germain GS, Summerbell R (2010) Identifyng fungi - A Clinical Laboratory Handbook. Star Publishing Company. http://www.identifyingfungi.com/actual-pages-from-the-book.html
Giraldo A, Gené J, Sutton DA, Madrid H, de Hoog GS, Cano J, Decock C, Crous PW, Guarro J (2015) Phylogeny of Sarocladium (Hypocreales). Persoonia 34:10–24. https://doi.org/10.3767/003158515X685364
Gobena TL, Asemie MM, Firisa TB (2022) Evaluation of released sweet potato [Ipomoea batatas (L.) Lam] varieties for yield and yield-related attributes in Semen-Bench district of Bench-Sheko-Zone South-Western Ethiopia. Heliyon 8:e10950. https://doi.org/10.1016/J.HELIYON.2022.E10950
González-Teuber M, Jiménez-Alemán GH, Boland W (2014) Foliar endophytic fungi as potential protectors from pathogens in myrmecophytic Acacia plants. Commun Integr Biol 7:e970500. https://doi.org/10.4161/19420889.2014.970500
Grace MH, Truong AN, Den TV, Raskin I, Lila MA (2015) Novel value-added uses for sweet potato juice and flour in polyphenol- and protein-enriched functional food ingredients. Food Sci Nutr 3:415–424. https://doi.org/10.1002/fsn3.234
Hammond R, Buah JN, Asare PA, Acheampong S (2014) Optimizing sterilization condition for the initiation of sweet potato (Ipomoea batatas) culture in vitro. Asian J Biotechnol 6:25–37. https://doi.org/10.3923/ajbkr.2014.25.37
Hussein N, Abdel-Hafez SI, Abdel-Sater M, Ismail M, Al-Amrey E (2017) Aspergillus homomorphus, a first global record from millet grains. Curr Res Environ Appl Mycol 7:82–89. https://doi.org/10.5943/cream/7/2/4
Imazaki I, Kadota I (2015) Molecular phylogeny and diversity of Fusarium endophytes isolated from tomato stems. FEMS Microbiol Ecol 91:fiv098. https://doi.org/10.1093/femsec/fiv098
Into P, Pontes A, Sampaio JP, Limtong S (2020) Yeast diversity associated with the phylloplane of corn plants cultivated in Thailand. Microorganisms 8:80. https://doi.org/10.3390/microorganisms8010080
Jena RC, Samal KC (2011) Endogenous microbial contamination during in vitro culture of sweet potato [Ipomoea batatas (L.) Lam]: identification and prevention. J Agric Technol 7:1725–1731
Jo Y, Kim SM, Choi H, Yang JW, Lee BC, Cho WK (2020) Sweet potato viromes in eight different geographical regions in Korea and two different cultivars. Sci Rep 10:2588. https://doi.org/10.1038/s41598-020-59518-x
Kačániová M, Kunová S, Sabo J, Ivanišová E, Žiarovská J, Felsöciová S, Terentjeva M (2020) Identification of yeasts with mass spectrometry during wine production. Fermentation 6:5. https://doi.org/10.3390/fermentation6010005
Khattree R, Naik DN (1999) Applied multivariate statistics with SAS software, 2nd edn. John Wiley & Sons, New York
Khumaida N, Ardie SW, Setiadi A, Artiningsih LN (2019) In vitro multiplication and acclimatization of black galingale (Curcuma Aeruginosa Roxb.). J Appl Pharm Sci 9:110–116. https://doi.org/10.7324/JAPS.2019.90414
Kim J, Kil E-J, Kim S, Seo H, Byun H-S, Park J, Chung M-N, Kwak H-R, Kim M-K, Kim C-S, Yang J-W, Lee K-Y, Choi H-S, Lee S (2015) Seed transmission of Sweet potato leaf curl virus in sweet potato (Ipomoea batatas). Plant Pathol 64:1284–1291. https://doi.org/10.1111/PPA.12366
Kim J, wook Yang J, Kwak H-R, Kim M-K, Seo J-K, Chung M-N, Lee H, Lee K-B, Nam SS, Kim C-S, Lee G-S, Kim J-S, Lee S, Choi H-S (2017) Virus incidence of sweet potato in Korea from 2011 to 2014. Plant Pathol J 33:467. https://doi.org/10.5423/PPJ.OA.08.2016.0167
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120. https://doi.org/10.1007/BF01731581
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Kumar S, Yadav AK, Prabha C (2019) Microbial contamination in tissue culture of Chlorophytum borivilianum, a rare medicinal herb: identification and prevention. J Plant Pathol 101:991–995. https://doi.org/10.1007/s42161-019-00327-1
Kwak HR, Kim MK, Shin JC, Lee YJ, Seo JK, Lee HU, Jung MN, Kim SH, Choi HS (2014) The current incidence of viral disease in Korean sweet potatoes and development of multiplex RT-PCR assays for simultaneous detection of eight sweet potato viruses. Plant Pathol J 30(4):416–424. https://doi.org/10.5423/PPJ.OA.04.2014.0029
Kwaśniewska D, Chen YL, Wieczorek D (2020) Biological activity of quaternary ammonium salts and their derivatives. Pathogens 9:459. https://doi.org/10.3390/pathogens9060459
Lazo-Javalera MF, Troncoso-Rojas R, Tiznado-Hernández ME, Martínez-Tellez MA, Vargas-Arispuro I, Islas-Osuna MA, Rivera-Domínguez M (2016) Surface disinfection procedure and in vitro regeneration of grapevine (Vitis vinifera L.) axillary buds. Springerplus 5:453. https://doi.org/10.1186/s40064-016-2081-0
Li F, Zuo R, Abad J, Xu D, Bao G, Li R (2012) Simultaneous detection and differentiation of four closely related sweet potato potyviruses by a multiplex one-step RT-PCR. J Virol Meth 186(1–2):161–166. https://doi.org/10.1016/j.jviromet.2012.07.021
Liu Y, Zou Z, Hu Z, Wang W, Xiong J (2019) Morphology and molecular analysis of Moesziomyces antarcticus isolated from the blood samples of a Chinese patient. Front Microbiol 10:254. https://doi.org/10.3389/fmicb.2019.00254
Makokha P, Ssali RT, Wanjala BW, Rajendran S, McEwan MA, Low JW (2020) Yield potential of sandponically produced sweetpotato (Ipomoea batatas (L.) Lam) pre-basic seed for selected genotypes. Open Agric 5:236–242. https://doi.org/10.1515/opag-2020-0025
Mancini V, Murolo S, Romanazzi G (2016) Diagnostic methods for detecting fungal pathogens on vegetable seeds. Plant Pathol 65:691–703. https://doi.org/10.1111/PPA.12515
McCartney HA, Foster SJ, Fraaije BA, Ward E (2003) Molecular diagnostics for fungal plant pathogens. Pest Manag Sci 59:129–142. https://doi.org/10.1002/PS.575
McDonnell GE (2017) Antisepsis, Disinfection, and sterilization: types, action, and resistance, 2nd edn. ASM Press, Washington, D.C.
Monja-Mio KM, Olvera-Casanova D, Herrera-Herrera G, Herrera-Alamillo MÁ, Sánchez-Teyer FL, Robert ML (2020) Improving of rooting and ex vitro acclimatization phase of Agave tequilana by temporary immersion system (BioMINT™). In Vitro Cell Dev Biol-Plant 56:662–669. https://doi.org/10.1007/s11627-020-10109-5
Montoya-Martínez AC, Rodríguez-Alvarado G, Fernández-Pavía SP, Proctor RH, Kim HS, O’Donnell K (2019) Design and validation of a robust multiplex polymerase chain reaction assay for MAT idiomorph within the Fusarium fujikuroi species complex. Mycologia 111:772–781. https://doi.org/10.1080/00275514.2019.1649956
Moussa TAA, Al-Zahrani HS, Kadasa NMS, Ahmed SA, de Hoog GS, Al-Hatmi AMS (2017) Two new species of the Fusarium fujikuroi species complex isolated from the natural environment. Antonie Van Leeuwenhoek 110:819–832. https://doi.org/10.1007/S10482-017-0855-1
Mu TH, Singh J (2019) Sweet potato: chemistry, processing and nutrition. Elsevier, New Zealand
Muhamad SNS, Ling APK, Wong CL (2018) Effect of plant growth regulators on direct regeneration and callus induction from Sargassum polycystum C. Agardh. J Appl Phycol 30:3299–3310. https://doi.org/10.1007/s10811-018-1649-1
Mulabisana MJ, Cloete M, Mabasa KG, Laurie SM, Oelofse D, Esterhuizen LL, Rey MEC (2018) Surveys in the Gauteng, Limpopo and Mpumalanga provinces of South Africa reveal novel isolates of sweet potato viruses. South Afr J Bot 114:280–294. https://doi.org/10.1016/j.sajb.2017.11.022
Mwanga ROM, Andrade MI, Carey EE, Low JW, Yencho GC, Grüneberg WJ (2017) Sweetpotato (Ipomoea batatas L.). In: Campos H, Caligari PD (eds) Genetic improvement of tropical crops, 1st edn. Springer, Cham, pp 181–218
Nicoletti R (2019) Endophytic fungi of citrus plants. Agriculture 9:247. https://doi.org/10.3390/agriculture9120247
Obłąk E, Piecuch A, Rewak-Soroczyńska J, Paluch E (2019) Activity of gemini quaternary ammonium salts against microorganisms. Appl Microbiol Biotechnol 103:625–632. https://doi.org/10.1007/s00253-018-9523-2
Oliveira R, Souza R, Lima T, Cavalcanti M (2014) Endophytic fungal diversity in coffee leaves (Coffea arabica) cultivated using organic and conventional crop management systems. Mycosphere 5:523–530. https://doi.org/10.5943/mycosphere/5/4/4
Oza K, Jain B, Maitreya B (2020) Isolation and identification of fungi from Kalipati variety of Sapota fruits. Int J Bot Stud 5:264–266
Paul NC, Hwang EJ, Nam SS, Lee HU, Lee JS, Yu GD, Kang YG, Lee KB, Go S, Yang JW (2017) Phylogenetic placement and morphological characterization of Sclerotium rolfsii (Teleomorph: Athelia rolfsii) associated with blight disease of Ipomoea batatas in Korea. Mycobiology 45:129–138. https://doi.org/10.5941/MYCO.2017.45.3.129
Qiao Q, Zhang Z, Zhao X, Wang Y, Wang S, Qin Y, Zhang D, Tian Y, Zhao F (2019) Evidence for seed transmission of sweet potato symptomless virus 1 in sweet potato (Ipomoea batatas). J Plant Pathol 1022(102):299–303. https://doi.org/10.1007/S42161-019-00427-Y
Rahayu RS, Ramadhani I, Masrukhin M, Riastiwi I, Prawestri AD, Yuliani Y (2021) Confirmation of endophytic microbes causing contamination in water spinach (Ipomoea aquatica Forssk.) tissue culture. J Bioteknol Biosains Indones 7:234–249. https://doi.org/10.29122/jbbi.v7i2.4381
Rajendran S, Kimenye LN, McEwan M (2017) Strategies for the development of the sweetpotato early generation seed sector in eastern and southern Africa. Open Agric 2:236–243. https://doi.org/10.1515/OPAG-2017-0025
Rescalvo-Morales A, Monja-Mio KM, Robert ML, Sánchez-Teyer LF (2019) Telomere length in Agave tequilana Weber plants during the in vitro to ex vitro transition. Plant Cell Tissue Organ Cult 136:133–140. https://doi.org/10.1007/s11240-018-1499-1
Rosero A, Pastrana Vargas IJ, García Peña JA, Espitia Montes AA, Sierra Naranjo CM, Sierra Monroy JA, Martínez Botello DH, Santana Rodríguez MO, Pérez Gamero JL, Regino Hernández SM, Espitia Negrete LB, Araújo Vásquez HA, Martínez R, García Herazo JL (2019) AGROSAVIA Aurora. Variedad de batata de pulpa anaranjada para el Caribe colombiano. Corporación Colombiana de Investigación Agropecuaria (Agrosavia), Monteria
Sadeghi F, Samsampour D, Seyahooei MA, Bagheri A, Soltani J (2019) Diversity and spatiotemporal distribution of fungal endophytes associated with Citrus reticulata cv. Siyahoo. Curr Microbiol 76:279–289. https://doi.org/10.1007/s00284-019-01632-9
Salawu SO, Udi E, Akindahunsi AA, Boligon AA, Athayde ML (2015) Antioxidant potential, phenolic profile and nutrient composition of flesh and peels from Nigerian white and purple skinned sweet potato (Ipomea batatas L.). Pelagia Res Libr Asian J Plant Sci Res 5:14–23
Samiyarsih S, Juwarno J, Muljowati JS (2018) The structural resistance’s anatomy of sweet potato leaves to fungal pathogen sphaceloma batatas. Biosaintifika J Biol Biol Educ 10:131–137. https://doi.org/10.15294/biosaintifika.v10i1.12116
Sandoval-Denis M, Gené J, Sutton DA, Wiederhold NP, Cano-Lira JF, Guarro J (2016) New species of Cladosporium associated with human and animal infections. Persoonia 36:281–298. https://doi.org/10.3767/003158516X691951
Santos LF, Olivares FL (2021) Plant microbiome structure and benefits for sustainable agriculture. Curr Plant Biol 26:100198. https://doi.org/10.1016/J.CPB.2021.100198
Shirdel M, Motallebi-Azar AR, Matloobi M, Mokhtarzadeh S, Ozdemir FA (2017) In Vitro establishment procedures of dog rose (Rosa canina). J Appl Biol Sci 11:06–09
Singh A (2015) Micropropagation of plants. In: Bahadur B, Rajam MV, Sahijram L, Krishnamurthy KV (eds) Plant biology and biotechnology: volume II: plant genomics and biotechnology, 1st edn. Springer, New Delhi, pp 329–346
Singh CR (2018) Review on problems and its remedy in plant tissue culture. Asian J Biol Sci 11:165–172. https://doi.org/10.3923/ajbs.2018.165.172
Ssamula A, Okiror A, Avrahami-Moyal L, Tam Y, Gaba V, Gibson RW, Gal-On A, Mukasa SB, Wasswa P (2020) Factors influencing reversion from virus infection in sweetpotato. Ann Appl Biol 176:109–121. https://doi.org/10.1111/AAB.12551
Talukdar R, Wary S, Mili C, Roy S, Tayung K (2020) Antimicrobial secondary metabolites obtained from endophytic fungi inhabiting healthy leaf tissues of Houttuynia cordata Thunb., an ethnomedicinal plant of Northeast India. J Appl Pharm Sci 10:99–106. https://doi.org/10.7324/JAPS.2020.10912
Tekielska D, Peňázová E, Kovács T, Křižan B, Čechová J, Eichmeier A (2019) Bacterial contamination of plant in vitro cultures in commercial production detected by high-throughput amplicon sequencing. Acta Univ Agric Silv Mendel Brun 67:1005–1014. https://doi.org/10.11118/actaun201967041005
Tripathi N, Sapra A (2021) Gram staining. StatPearls, Tampa
Tumbarski Y, Georgiev V, Nikolova R, Pavlov A (2018) Isolation, identification and antibiotic susceptibility of Curtobacterium flaccumfaciens strain Pm_Yt from sea daffodil (Pancratium Maritimum L.) shoot cultures. J Microbiol Biotechnol Food Sci 7:623–627. https://doi.org/10.15414/jmbfs.2018.7.6.623-627
Tyagi B, Tewari S, Dubey A (2017) Biochemical characterization of fungus isolated during in vitro propagation of Bambusa balcooa. Pharmacogn Mag 13:S775–S779. https://doi.org/10.4103/pm.pm_20_17
Varga J, Frisvad JC, Kocsubé S, Brankovics B, Tóth B, Szigeti G, Samson RA (2011) New and revisited species in Aspergillus section Nigri. Stud Mycol 69:1–17. https://doi.org/10.3114/sim.2011.69.01
Wang Q-M, Jia J-H, Bai F-Y, Bai Y (2016) Pseudozyma hubeiensis sp. nov. and Pseudozyma shanxiensis sp. nov., novel ustilaginomycetous anamorphic yeast species from plant leaves. Int J Syst Evol Microbiol 56:289–293. https://doi.org/10.1099/ijs.0.63827-0
Wanger A, Chavez V, Huang RSP, Wahed A, Actor JK, Dasgupta A (2017) Biochemical tests and staining techniques for microbial identification. In A Wanger, RSP Huang, JK Actor, V Chavez, A Wahed, A Dasgupta (eds), Microbiology and Molecular Diagnosis in Pathology (pp 61–73). Elsevier. https://doi.org/10.1016/b978-0-12-805351-5.00005-3
Wanjala BW, Srinivasulu R, Makokha P, Ssali RT, McEwan M, Kreuze JF, Low JW (2020) Improving rapid multiplication of sweetpotato (Ipomoea batatas L. (Lam) pre-basic seed using sandponics technology in East Africa. Exp Agric 56:347–354. https://doi.org/10.1017/S0014479719000413
Yang X (2010) Rapid production of virus-free plantlets by shoot tip culture in vitro of purple-coloured sweet potato (Ipomoea batatas (L.) Lam.). Pakistan J Bot 42:2069–2075
Yeasmin S, Samiul Islam M, Sujon T, Sultana R, Shah Alam M, Sikdar B, Asadul Islam M, Khalekuzzaman M (2018) Molecular and microscopic identification of fungi in micropropagation of nodal and shoot tip culture of orange. Int J Pure Appl Biosci 6:6–19. https://doi.org/10.18782/2320-7051.7081
Yu J, Zhou XF, Yang SJ, Liu WH, Hu XF (2013) Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in Dendrobium officinale using nested PCR-DGGE. Appl Microbiol Biotechnol 97:9825–9836. https://doi.org/10.1007/s00253-013-5294-y
Zhang Y, Jiang G, Ding Y, Loria R (2018) Genetic background affects pathogenicity island function and pathogen emergence in Streptomyces. Mol Plant Pathol 19:1733–1741. https://doi.org/10.1111/mpp.12656
Zhang K, Lu H, Wan C, Tang D, Zhao Y, Luo K, Li S, Wang J (2020) The spread and transmission of sweet potato virus disease (SPVD) and Its effect on the gene expression profile in sweet potato. Plants 9:492. https://doi.org/10.3390/plants9040492
Funding
Open access funding provided by Università del Salento within the CRUI-CARE Agreement. Funding was provided by the Ministerio de Agricultura y Desarrollo Rural de Colombia (MADR), Project code 1000745, TV18 AV17 Agreement.
Author information
Authors and Affiliations
Contributions
JPP performed the establishment of the experiments, data collection, statistical analysis and wrote the manuscript. RG contributed to the conception of the study, supervised the experiments, analysis of results, writing the manuscript and directed the research. AR contributed to the conception of the study, analysis of results and writing of the manuscript. MC contributed to the interpretation of the results and supervised the writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Cecile Segonzac.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Pazos, J., Rosero, A., Cardinale, M. et al. Development of control strategies for bacteria and fungi associated with a micropropagated new cultivar of orange-fleshed sweet potato (Ipomoea batatas cv. Agrosavia–Aurora). Hortic. Environ. Biotechnol. 64, 859–875 (2023). https://doi.org/10.1007/s13580-023-00521-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-023-00521-2