Abstract
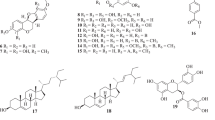
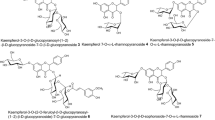
The ethyl acetate (EtOAc) layer of the hot water extracts of Camellia japonica flowers was found to have higher 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging activity than the other solvent layers. Nine phenolic compounds were isolated and purified from the EtOAc layer by Sephadex LH-20 column chromatography and octadecyl silane-high performance liquid chromatography using a guided DPPH radical-scavenging assay. The isolated compounds were identified as 3,4,5-trihydroxybenzoic acid (1), 3,4-dihydroxybenzoic acid (2), 4-hydroxybenzoic acid (3), 2,3-digalloyl-O-α-d-glucopyranoside (4), 2,3-digalloyl-O-β-d-glucopyranoside (5), quercetin 3-O-β-d-galactopyranoside (6), quercetin 3-O-β-d-glucopyranoside (7), kaempferol 3-O-β-d-galactopyranoside (8), and kaempferol 3-O-β-d-glucopyranoside (9), based on mass spectrometry and nuclear magnetic resonance. Four compounds (6–9) had been previously identified in the leaves of this plant, but other compounds (1–5) were newly isolated from this plant. Their DPPH radical-scavenging activities based on the 50% scavenging concentration decreased in the following order: 4 = 5 (4.7 μM) > 1 (9.8 μM) > 6 = 7 (8.2 μM) > α-tocopherol (24.7 M) > ascorbic acid (25.1 μM) > 2 (35.6 M) > 3 = 8 = 9 (> 250 μM). Quercetin glycosides (6, 7), gallic acid (1) and its glucosides (4, 5) showed higher DPPH radical-scavenging activities than other compounds. These results indicate that the antioxidant effect of C. japonica flowers may be attributable to quercetin glycosides and gallic acid derivatives. These isolated compounds will be useful in basic studies of plant physiology, food manufacturing, and biological function of C. japonica flowers.
Similar content being viewed by others
Literature Cited
Abe, N., A. Nemoto, Y. Tsuchiya, H. Hojo, and A. Hirota. 2000. Studies of the 1,1-diphenyl-2-picrylhydrazyl radical scavenging mechanism for a 2-pyrone compound. Biosci. Biotech. Biochem. 64:306–333.
Akihisa, T., K. Yasukawa, Y. Kimura, S. Takase, S. Yamanouchi, and T. Tamura. 1997. Triterpene alcohols from camellia and sasanqua oils and their anti-inflammatory effects. Chem. Pharm. Bull. 45: 2016–2023.
Arapitsas, P., S. Menichetti, F.F. Vincieri, and A. Romani. 2007. Hydrolyzable tannins with the hexahydroxydiphenoyl unit and the m-depsidic link: HPLC-DAD-MS identification and model synthesis. J. Agric. Food Chem. 55:48–55.
Burda, S. and W. Oleszek. 2001. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 49:2774–2779.
Cho, J.Y., J.H. Moon, K.Y. Seong, and K.H. Park. 1998. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotech. Biochem. 62:2273–2276.
Cho, J.Y., S.H. Ji, J.H. Moon, K.H. Lee, K.H. Jung, and K.H. Park. 2008. A novel benzyol glucoside and phenolic compounds from the leaves of Camellia japonica. Food Sci. Biotechnol. 17:1060–1065.
Cho, J.Y., H.J. Ryu, S.H. Ji, J.H. Moon, K.H. Jung, and K.H. Park. 2009. Phenolic compounds from the flower buds of Camellia japonica. Food Sci. Biotechnol. 18:766–770.
Chung, J.H., H.C. Shin, J.Y. Cho, S.K. Kang, H.J. Lee, S.C. Shin, K.H. Park, and J.H. Moon. 2009. Isolation and structural determination of free radical-scavenging compounds from Korean fermented red pepper paste (Gochujang). Food Sci. Biotechnol. 18:463–470.
Hahn, Y.S. 2005. Antimicrobial effects of Camellia japonica L. extract on food-borne pathogenic microorganisms. Korean J. Food Sci. Technol. 37:113–121.
Ito, S., M. Kodama, and M. Konoike. 1967. Structure of camelliagenins. Tetrahedron Lett. 8:591–596.
Hatano, T., S. Shida, L. Han, and T. Okuda. 1991. Tannins of theaceous plants. III. Camelliatannins A and B, two new complex tannins from Camellia japonica L. Chem. Pharm. Bull. 39:876–880.
Itokawa, H., N. Sawada, and T. Murakami. 1969. Structures of camelliagenins A, B, and C obtained from Camellia japonica. Chem. Pharm. Bull. 17:474–480.
Itokawa, H., H. Nakajima, A. Ikuta, and Y. Iitaka. 1981. Two triterpenes from the flowers of Camellia japonica. Phytochemistry 20:2539–2542.
Kim, J.H., S.Y. Lee, and S.I. Choi. 2003. Anti-proliferative effect of Camellia japonica leaves on human leukemia cell line. Korean J. Herbology 18:93–98.
Kwon, E.J., Y.C. Kim, M.S. Kwon, C.S. Kim, W.W. Kang, J.B. Lee, and S.K. Chung. 2001. Antioxidative activity of solvent fraction and isolation of antioxidative compound from chestnut husk. J. Korean Soc. Food Sci. Nutr. 30:726–731.
Lee, S.Y., E.J. Hwang, G.H. Kim, Y.B. Choi, C.Y. Lim, and S.M. Kim. 2005. Antifungal and antioxidant activities of extracts from leaves and flowers of Camellia japonica L. Korean J. Med. Crop Sci. 13:93–100.
Lu, Z.B., G.J. Nie, P.S. Belton, H.R. Tang, and B.L. Zhao. 2006. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 48:263–274.
Nakagawa, M. and Y. Sakamoto. 1967. Isolation of (−)-epicatechol and (+)-catechol from the leaf of Camellia japonica. Tea Industry and Technology Research, Japan 34:3472–3476.
Namba, T., M. Tsunezuka, Y. Takehana, S. Nunome, K. Takeda, Y.W. Shu, N. Kakiuchi, S. Takigi, and M. Hattori. 1984. Studies on dental caries prevention by traditional Chinese medicines. IV. Screening of crude drugs for anti-plaque action and effects of Artemisia capillaris spikes on adherence of Streptococcus mutans to smooth surface and synthesis of glucan. Japanese Soc. Pharm. 38:263–264.
Numata, A., A. Kitajima, T. Katsuno, K. Yamamoto, N. Nagahama, C. Takahashi, R. Fujiki, and M. Nabae. 1987. An antifeedant for the yellow butterfly larvae in Camellia japonica: A revised structure of camellidin II. Chem. Pharm. Bull. 35:3948–3951.
Onodera, K., K. Hanashiro, and T. Yasumoto. 2006. Camellianoside, a novel antioxidant glycoside from the leaves of Camellia japonica. Biosci. Biotech. Biochem. 70:1995–1998.
Rice-Evans, C.A., N.J. Miller, and G. Paganga. 1996. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad. Biol. Med. 20:933–956.
Takao, T., F. Kitatani, N. Watanabe, A. Yagi, and K. Sakata. 1994. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci. Biotech. Biochem. 58:1780–1783.
Yokozawa, T., C.P. Chen, E. Dong, T. Tanaka, G. Nonaka, and I. Nishioka. 1998. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 56:213–222.
Yoshikawa, M., E. Marada, T. Murakami, H. Matsuda, J. Yamahara, and N. Murakami. 1994. Camelliasaponins B1, B2, C1, and C2, new type inhibitors of ethanol absorption in rats from the seeds of Camellia japonica L. Chem. Pharm. Bull. 42:742–744
Yoshikawa, M., T. Murakami, S. Yoshizumi, N. Murakami, J. Yamahara, and H. Matsuda. 1996. Bioactive saponins and glycosides. V. Acylated polyhydroxyolean-12-ene triterpene oligoglycosides, camelliasaponins A1, A2, B1, B2, C1, and C2, from the seeds of Camellia japonica L.: structures and inhibitory activity on alcohol absorption. Chem. Pharm. Bull. 44:1899–1907.
Yoshikawa, M., T. Morikawa, Y. Asao, E. Fujiwara, S. Nakamura, and H. Matsuda. 2007. Medicinal flowers. XV. The structures of noroleanane- and oleanane-type triterpene oligoglycosides with gastroprotective and platelet aggregation activities from flower buds of Camellia japonica L. Chem. Pharm. Bull. 55:606–612.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HH., Cho, JY., Moon, JH. et al. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Hortic. Environ. Biotechnol. 52, 270–277 (2011). https://doi.org/10.1007/s13580-011-0157-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-011-0157-x