Abstract
Objective
The role of post-mastectomy radiation therapy (PMRT) for male breast cancer (MBC) is not well defined. Because of the rarity of male breast cancer, large randomized clinical trials are not possible.
Methods
Using the Surveillance Epidemiology and End Results database, we identified MBC patients diagnosed between 1988 and 2007 and treated with mastectomy and axillary lymph node dissection. Risk groups were broadly assigned based on the female breast cancer literature: low risk (LR; T1-2N0), intermediate risk (IR; T1-2N1), and high risk (HR; T3-4N0-3, T1-2N2-3). Kaplan–Meier and Cox regression analyses were used to compare overall survival (OS) and breast-cancer-specific survival (BCSS) for all patients.
Results
We identified 2,382 patients who met the selection criteria, of whom 1,888 (79.3 %) received mastectomy alone and 494 (20.7 %) received mastectomy followed by PMRT. For patients with LR disease there were no differences in OS or BCSS. For IR disease, there were no differences in OS but BCSS was worse for those who received PMRT (p = 0.008). For the HR group, median survival and 5-year OS was 72 months and 56.3 % for those who underwent surgery alone and 99 months and 67.4 % for those receiving PMRT respectively (p = 0.002). However, there was no difference in BCSS between the two groups (p = 0.52).
Conclusion
In this large population-based study, there is a significant OS benefit without any change in BCSS associated with PMRT for MBC patients with HR disease. Further prospective studies are needed to confirm and validate these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer in men represents 0.7 % of all breast cancer cases in the USA. The American Cancer society estimates that there will be approximately 2,190 new cases of male breast cancer (MBC) diagnosed in 2012, with an estimated 410 deaths attributed to the disease [1]. While the overall incidence of breast cancer appears to be decreasing, the incidence of MBC appears to be rising. On average, MBC is diagnosed 5 to 10 years later than female breast cancer and usually presents with a higher stage, with 40 % of patients presenting with stage III or IV disease. However, MBC patients are more likely to be of a lower grade and be estrogen receptor positive [2]. More recent studies show little difference in outcome when matched stage for stage with their female counterparts [3].
Most MBC are treated with mastectomy [4]. Because of the rarity of MBC, large-scale randomized clinical trials are not possible and treatment recommendations are based on retrospective evidence, as well as evidence derived from the treatment of female breast cancer. Several studies have shown a local control benefit to post-mastectomy radiation therapy (PMRT) similar to female breast cancer, but no overall survival benefit [5–8], unlike female breast cancer [9].
In this study, we queried the Surveillance, Epidemiology, and End Results Database (SEER) in order to obtain a large cohort of patients from which to analyze the demographics of MBC as well as the impact of PMRT on survival.
Methods
Study cohort
After waiver from the NY Harbor Department of Veterans Affairs institutional review board, we queried the SEER database to collect individual patient data for MBC patients who were diagnosed between 1988 and 2007 and were treated with a mastectomy and axillary lymph node dissection. We excluded patients who were found to have metastatic disease or who had incomplete information regarding staging. We also excluded patients who did not have removal of any lymph nodes for evaluation, in order to remove possible bias favoring radiation due to the radiation compensating for inadequate surgery. Patients who refused radiation therapy were coded as unknown regarding radiation treatment and who had neoadjuvant radiation, or any radiation other than postoperative external beam radiation, were excluded. Only patients who survived six or more months post-surgery were included in the cohort. This was done to allow inclusion only of patients who survived long enough to undergo radiation and remove potential selection bias in favor of those who received PMRT. Please see Table 1 for an outline of the exclusion criteria and patients excluded from this study.
Outcome
The primary endpoint was all-cause mortality. We also analyzed breast-cancer-specific mortality as a secondary endpoint. Breast-cancer-specific mortality was defined by SEER as death from “breast cancer.” Follow-up time was calculated from the month and year of initial diagnosis.
Statistical analysis
Comparisons of the characteristics between the PMRT and surgery alone groups were made using Pearson chi square. Actuarial overall survival (OS) curves and breast-cancer-specific survival (BCSS) curves were generated using the Kaplan–Meier method and compared using the log-rank test. The 5- and 10-year actuarial OS and BCSS rates, as well as median survival, were analyzed. Based on previously published guidelines for PMRT use in the female breast literature [10] patients were grouped into low risk (LR; T1-2N0), intermediate risk (IR; T1-2N1), and high risk (HR; T3-4N0-3, T1-2N2-3), and their survival was analyzed according to risk group. Univariate and multivariate Cox regression analysis was also used to study the adjusted and unadjusted impact of PMRT on OS. The covariates analyzed included delivery of PMRT (yes or no), age, tumor stage (T1, T2, T3, or T4), number of pathologically positive nodes (0, 1–3, 4–9, and >9), race (white, black, or other), estrogen receptor (ER) status (positive, negative, or unknown), and year of diagnosis (continuous). Multivariate analysis was repeated for each risk group. The multivariate model was tested for interactions between PMRT and the other covariates and did not find significant interactions. Statistical analysis was performed using SPSS version 20.0 (SPSS Inc., Chicago, Illinois). Statistical significance was defined as a two-sided p value of 0.05 or less.
Results
A total of 2,382 patients met the selection criteria for this study. Of these, 1,888 (79.3 %) received mastectomy alone and 494 (20.7 %) received mastectomy followed by PMRT. The median age of all patients was 67 years and the median follow-up time for all patients was 53 months (range 6–235 months). A complete description of patient characteristics, as well as a comparison between the PMRT and surgery alone groups is available in Table 2.
Survival analysis
For the entire cohort of patients, there was no difference in OS for those who were treated with surgery alone compared to PMRT. The median survival was 126 months (95 % CI 115.9–136.0) for those receiving surgery alone compared with 110 months (95 % CI 90.4–129.6) for those receiving PMRT, p = 0.43. The corresponding 5- and 10-year OS were 74.7 and 50.8 % for those receiving surgery alone and 72.4 and 46.3 % for those receiving PMRT, respectively.
Overall survival by risk group
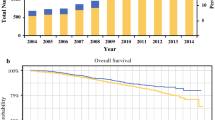
There were a total of 1,148 patients with LR disease, of which 85 (7.4 %) received PMRT (Fig. 1). There were 526 patients with IR disease, of which 121 (23 %) received PMRT. When comparing the OS between those receiving surgery alone and those who received PMRT in these two risk groups, there were again no significant differences in survival (p = 0.36 and 0.55, respectively) (Fig. 2).
There were 708 patients with HR disease, of which 288 (40.7 %) received PMRT. There was a significant improvement in OS for those patients who received PMRT compared to surgery alone (p = 0.002). Please see Table 3 for a detailed outline of the survival data for each risk group (Fig. 3).
Breast-cancer-specific survival
For LR disease, there were 61 (5.3 %) breast cancer deaths out of 1,148 patients. There were no significant differences in BCSS (p = 0.74). The median BCSS was not reached in either arm. The 5-year BCSS was 95.6 % for surgery alone and 95.6 % for PMRT.
For IR disease, there were 70 (13.3 %) breast cancer deaths out of 526 patients. Patients who received surgery alone were associated with improved BCSS (p = 0.008). The median survival was not reached for patients receiving surgery alone and the 5- and 10-year BCSS was 91.4 and 76.2 %, compared with a median survival of 144 months and a 5- and 10-year BCSS of 84.9 and 60.3 % for PMRT.
For HR disease, there were 118 (16.7 %) breast cancer deaths out of 708 patients. Of these, 107 patients had positive lymph nodes. There were no significant differences in BCSS by treatment group (p = 0.52). The median BCSS was not reached in either arm. The 5- and 10-year BCSS was 81.7 and 66.4 % for those undergoing surgery alone compared to 81.8 and 71.8 % for those undergoing PMRT. Since most of the breast cancer deaths were in those with positive nodes, we repeated the BCSS analysis just in these patients. There was still no difference in BCSS. The median BCSS was 194 months for those receiving PMRT compared to 136 months in those undergoing surgery alone (p = 0.17).
Univariate and multivariate analysis for survival
The use of PMRT was not associated with any difference in OS on UVA or MVA for LR disease (HR 0.88, 95%CI 0.58–1.34, p = 0.54) or IR disease (HR 1.23, 95%CI 0.87–1.75, p = 0.23).
On univariate analysis for HR disease, the use of PMRT was associated with improved OS, with a hazard ratio of 0.68 (95 % CI 0.54–0.87, p = 0.002). Increasing T-category, 10 or more positive lymph nodes, Black race, and ER-negative disease were all associated with decreased survival. Further details are available in Table 4.
On multivariate analysis for HR disease, the use of PMRT continued to be associated with improved overall survival, with a hazard ratio of 0.70 (95 % CI 0.54–0.90, p = 0.01). Increasing T-category, four or more positive lymph nodes, Black race, and increasing age were associated with worse survival, while more recent year of diagnosis was associated with improved survival. Further details are available in Table 5.
OS and BCSS accounting for potential selection biases in HR patients
The OS and BCSS survival analyses were also reanalyzed after excluding patients who survived 12 months or less and again after excluding patients who survived 24 months or less. There were no changes in the survival outcomes. OS remained significantly better for those receiving PMRT surviving more than 12 months (p = 0.003) and 24 months (p = 0.009), without any differences noted in BCSS for those surviving beyond 12 months (p = 0.65) and 24 months (p = 0.332). Similarly, these findings remained consistent when excluding patients 65 or older (p = 0.03 for OS, p = 0.40 for BCSS).
Discussion
The results of this large population-based study suggest that there may be a significant OS benefit but not BCSS benefit to post-mastectomy radiation therapy for MBC patients with T3-4N0-3 or T1-2N2-3 disease. However, there are numerous limitations to this SEER study, which will be discussed in detail. Therefore, the findings of this study need confirmation from prospective databases with more complete datasets.
In this study, the addition of PMRT was associated with an absolute improvement in OS of 11 % at 5 years and 10 % at 10 years. These OS findings seem to mirror the benefits of post-mastectomy radiation therapy in the female breast cancer literature [11–13]. A recent retrospective study from Moffitt Cancer Center showed that MBC patients treated with current female standards had comparable outcomes to female breast cancer patients, further strengthening our findings here [14]. Others have proposed different indications for PMRT, which include less advanced disease. At the European Institute of Oncology in Milan, RT for MBC is proposed for tumors >1 cm or with more than one positive lymph node [15]. The National Cancer Care Network strongly recommends consideration of PMRT for any amount of lymph node involvement in female and MBC patients [10].
This is the first study to show a survival benefit for PMRT for MBC in a large cohort of patients. The findings in this study are in contrast to a recently reported study of MBC by Crew et al. using the SEER-Medicare database [4]. In this study, there was no impact on overall survival associated with the addition of radiation therapy. However, there are several differences between this study and the aforementioned study by Crew et al. That SEER-Medicare study looked at the impact of radiation on all stages but did not further breakdown the patients into risk groups. Therefore, the finding that there was no difference in OS does, in fact, correlate well with the current study, in which there was also no survival benefit when analyzing all patients as one large group. In addition, the SEER-Medicare analysis was limited to patients ages 65 and older. This would eliminate >40 % of the patients in the current study. Therefore, these two studies should probably not be directly compared to one another.
Though a survival benefit has not previously been reported, other studies have shown improved local control with the addition of PMRT [5, 8, 16]. The SEER database unfortunately does not provide information on local control. However, it is reasonable to assume the local control benefit seen in these studies would translate to a survival benefit in a much larger cohort of patients, as can be derived from the female breast cancer literature. This latter finding has been reported in a large meta-analysis of over 40,000 women, which reported that a 5-year improvement in local control of 20 % translated to a 5.9 % improvement in survival at 15 years [17].
Never the less, the finding of a significant OS benefit in the face of no BCSS benefit calls into question whether there are unmeasured confounding factors resulting in healthier patients being selected for PMRT and therefore surviving longer. This is a potential limitation that we cannot discount. However, in an attempt to adjust for this, we performed three additional analyses excluding patients who were 65 or older, survived <13 months or survived <25 months. This would have more likely excluded patients who were not referred for PMRT due to poor overall health or poor performance status. We found that there were no changes in the significance of our OS and BCSS findings. Furthermore, when we limited our BCSS analysis in patients with HR disease to those with positive lymph nodes, there was a trend towards improved BCSS with an improvement in median BCSS from 136 to 194 months, p = 0.17. This does lend some support to the notion that there were not enough breast-cancer-specific deaths to identify a significant difference.
The finding of improved BCSS for the intermediate risk patients in the setting of no OS difference is also confusing as it contradicts the findings in the HR patients where there was an OS benefit without any change in BCSS. If the high-risk patients who received radiation were found to have improved OS but no BCSS due to an unmeasured selection bias favoring the radiation patients, it is unclear why then the unmeasured biases would favor the surgery alone patients in the intermediate risk group. Perhaps different unmeasured confounders are present in this intermediate risk group which could portend worse outcomes. These could include traditional indications for radiation therapy such as positive surgical margins, extensive lymphovascular invasion, or extracapsular extension in lymph nodes. Again, this uncertainty makes definitive interpretations of the survival outcomes, specifically the BCSS, difficult.
Finally, one should use caution when measuring cancer-specific mortality in the SEER database as they are generally considered less reliable than OS endpoints. SEER itself states on their website, “Cancer registries use algorithms to process causes of death from death certificate in order to identify a single, disease-specific, underlying cause of death. In some cases, attribution of a single cause of death may be difficult and misattribution may occur. For example a death may be attributed to the site of metastasis instead of the primary site.” [18] Some have reported that cancer-specific mortality endpoints are in fact not reliable [19], while others have suggested several methods to attempt to reduce this disparity [20, 21].
The role of systemic therapy is not well defined in MBC. Prior reports indicate that chemotherapy has been used in nearly 30 % of male breast cancer patients [4]. However, its impact on survival is uncertain [22]. SEER does not offer information on systemic therapy and therefore we are unable to comment on its impact on survival or whether the decision to offer or withhold treatment was related to the decision to offer PMRT.
In this study, 77 % of all patients had ER-positive disease. In the female breast cancer literature, this subset of patients is routinely treated with an anti-estrogen such as Tamoxifen or Arimidex. However, the role of Tamoxifen in MBC remains unclear. A prospective study of Tamoxifen therapy for stages II and III operable MBC has been performed. Survival in this cohort of 39 patients was 61 % at 5 years, which was significantly greater than the 44 % 5-year survival observed in historical controls (P = 0.006) [23]. A retrospective study from M.D. Anderson cancer center of MBC revealed that adjuvant hormonal therapy was associated with a lower risk of death, with a hazard ratio of 0.45 [22]. However, several studies have suggested that Tamoxifen is relatively underutilized in MBC [21, 24] compared to female breast cancer. Unfortunately, while SEER does identify patients with ER-positive disease, it does not provide information on who was treated with Tamoxifen, and therefore we are unable to comment on its impact in this study.
On multivariate analysis, Black race was associated with inferior survival, with a hazard ratio of 2.06 (1.47–2.90), p < 0.001. This correlates with prior reports of inferior survival in black MBC patients. Not surprisingly, increasing T-category and N-category were also associated with decreased survival in this study, which also correlates well to prior studies [8].
In addition to the limitations to this study noted above, there are several other significant limitations to this study. These include the lack of information regarding margin status, radiation techniques, radiation doses used, as well as underascertainment of radiation use by the SEER database [25]. All of these factors have added unmeasured bias that limit us drawing definitive treatment recommendations from this study.
Conclusion
Despite the flaws inherent in the SEER database, this study provides a very large cohort of patients from which to study this relatively rare disease. We report a significant improvement in OS associated with the addition of PMRT for patients with HR disease without an apparent impact on BCSS. Further prospective studies are needed to confirm and validate these findings, as well as to determine whether there is a BCSS benefit to the addition of PMRT.
References
“Breast Cancer in Men.” American Cancer Society. Web. 16 Oct. 2011. <http://www.cancer.org/Cancer/BreastCancerinMen/DetailedGuide/index>
Gnerlich JL, Deshpande AD, Jeffe DB et al (2011) Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 18:1837–1844
Foerster R, Foerster FG, Wulff V et al (2011) Matched-pair analysis of patients with female and male breast cancer: a comparative analysis. BMC Cancer 11(1):335
Crew KD, Neugut AI, Wang X et al (2007) Racial disparities in treatment and survival of male breast cancer. J Clin Oncol 25(9):1089–1098
Yu E, Suzuki H, Younus J et al (2012) The impact of post-mastectomy radiation therapy on male breast cancer patients—a case series. Int J Radiat Oncol Biol Phys 82:696–700
Donegan WL, Redlich PN, Lang PJ et al (1998) Carcinoma of the breast in males: a multi-institutional survey. Cancer 83:498–509
Yildirim E, Berberoglu U (1998) Male breast cancer: a 22-year experience. Eur J Surg Oncol 24:548–552
Cutuli B, Lacroze M, Dilhuydy JM et al (1995) Male breast cancer: results of the treatments and prognostic factors in 397 cases. Eur J Cancer 31A(12):1960–1964
Whelan TJ, Julian J, Wright J et al (2000) Does locoregional radiation therapy improve survival in breast cancer? A meta-analysis. J Clin Oncol 18(6):1220–1229
National Comprehensive Cancer Care Guidelines for Breast Cancer. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed 9/1/11)
Overgaard M, Hansen PS, Overgaard J et al (1997) Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 337(14):949–955
Overgaard M, Jensen MB, Overgaard J et al (1999) Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 353(9165):1641–1648
Ragaz J, Olivotto IA, Spinelli JJ et al (2005) Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 97(2):116–126
Kiluk JV, Lee MC, Park CK et al (2011) Male breast cancer: management and follow-up recommendations. Breast J 17(5):503–509
Zurrida S, Nole F, Bonanni B et al (2010) Male breast cancer. Future Oncol 6(6):985–991
Erlichman C, Murphy KC, Elhakim T et al (1984) Male breast cancer: a 13-year review of 89 patients. J Clin Oncol 2(8):903–909
Clarke M, Collins R, Darby S et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106
Surveillance, Epidemiology and End Results Database. Available at http://seer.cancer.gov/causespecific/ and also at http://surveillance.cancer.gov/survival/measures.html
Bach PB, Guadagnoli E, Schrag D et al (2002) Patient demographic and socioeconomic characteristics in the SEER-Medicare database, applications and limitations. Med Care 40(IV):19–25
Gioradno SH, Kuo YF, Duan Z et al (2008) Limits of observational data in determining outcomes from cancer therapy. Cancer 112:2456–2466
Howlader N, Ries LA, Mariotto AB et al (2010) Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 102(20):1584–1598
Giordano SH, Perkins GH, Broglio K et al (2005) Adjuvant systemic therapy for male breast carcinoma. Cancer 104(11):2359–2364
Ribeiro G, Swindell R (1985) Male breast carcinoma: a review of 301 cases from the Christie Hospital and Holt Radium Institute, Manchester. Br J Cancer 51:115–119
Anelli TF, Anelli A, Tran KN (1994) Tamoxifen administration is associated with a high rate of treatment-limiting symptoms in male breast cancer patients. Cancer 74(1):74
Jagsi R, Abrahamse P, Hawley ST et al (2012) Underascertainment of radiotherapy receipt in surveillance, epidemiology, and end results registry data. Cancer 118:333–341
Conflicts of interest
The authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sroufe, R.L., Schwartz, D., Rineer, J. et al. A population-based study of the impact of post-mastectomy radiation on survival for male breast cancer. J Radiat Oncol 1, 337–345 (2012). https://doi.org/10.1007/s13566-012-0062-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13566-012-0062-7