Abstract
Introduction
Deucravacitinib demonstrated superior efficacy to apremilast in patients with moderate to severe plaque psoriasis in the POETYK PSO-1 and PSO-2 clinical trials. In the study reported here, we aimed to determine the overall 52-week cumulative clinical benefit of treatment initiated with deucravacitinib versus apremilast and to compare the 52-week cumulative benefit of initiating and staying on deucravacitinib versus initiating apremilast and continuing or switching to deucravacitinib at week 24 of treatment.
Methods
This post hoc analysis of POETYK PSO-1 data (ClinicalTrials.gov identifier: NCT03624127) determined the cumulative clinical benefit of deucravacitinib 6 mg once daily and apremilast 30 mg twice daily in adults with moderate to severe plaque psoriasis. Patients treated with apremilast who did not achieve a 50% reduction in the Psoriasis Area and Severity Index (PASI 50) at week 24 were switched to deucravacitinib. The cumulative clinical benefit of deucravacitinib versus apremilast over 52 weeks was based on cumulative measures of ≥ 75% improvement from baseline in PASI score (PASI 75) and the proportion of patients with a static Physician Global Assessment score of 0 or 1 (sPGA 0/1). Ratios of area under the curve estimates between treatments were calculated and compared based on analysis of covariance regression models.
Results
Patients initiating deucravacitinib (N = 332) had a greater cumulative benefit as measured by the PASI 75 and sPGA 0/1 than those initiating apremilast (N = 168). Over 52 weeks, those initiating deucravacitinib experienced 50% more benefit as measured by PASI 75 and 58% more benefit as measured by sPGA 0/1 than those initiating apremilast. Results were consistent with the primary analysis when patients were classified by prior systemic and prior biologic therapy exposure.
Conclusion
Results from this analysis corroborate the primary efficacy analysis supporting the use of deucravacitinib compared with apremilast for moderate to severe plaque psoriasis, regardless of prior systemic or biologic use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Clinical trial results generally do not capture the cumulative clinical benefits of therapy as they are measured at fixed times during clinical trials; an area under the curve (AUC) analysis of clinical trial data can be performed to determine the cumulative clinical benefit of treatment |
For example, an AUC analysis would capture differences in cumulative benefit between a patient who experiences disease control at week 12 versus another who experiences disease control at week 48; response would look the same if solely measured at week 52 although the totality of benefit could vary substantially |
What was learned from this study? |
Patients initiating deucravacitinib (N = 332) had a greater cumulative benefit as measured by a 75% reduction in the Psoriasis Area and Severity Index (PASI 75) and obtaining a static Physician Global Assessment score of 0 or 1 (sPGA 0/1) over 52 weeks |
Patients initiating deucravacitinib experienced 50% more benefit as measured by PASI 75 and 58% more benefit as measured by sPGA 0/1 than those initiating apremilast |
Results were consistent with the primary analysis when patients were classified by prior systemic and prior biologic therapy exposure |
Introduction
Psoriasis is a chronic inflammatory skin disease that affects an estimated 3% of adults in the USA [1]; among patients with psoriasis, plaque psoriasis is the most common form, affecting 80–90% of patients [2]. Approximately one fifth of patients with psoriasis have moderate to severe disease [1, 2].
Systemic nonbiologic and biologic treatments are recommended for patients with moderate to severe psoriasis [3,4,5]. In the USA, targeted therapies approved for the treatment of moderate to severe psoriasis include a selective allosteric tyrosine kinase inhibitor (i.e., deucravacitinib), a phosphodiesterase 4 inhibitor (i.e., apremilast), tumor necrosis factor inhibitors, interleukin (IL)-12/23 inhibitors, IL-17 inhibitors, and selective IL-23p19 inhibitors [4, 5]. As the armamentarium of targeted therapies available for the treatment of moderate to severe psoriasis has increased, information on the sequencing of systemic treatments is needed to optimize patient outcomes.
The physical, psychological, social, and economic impacts of psoriasis and its associated comorbidities accumulate over time and may affect some patients’ ability to achieve their full life potential [6, 7]. However, it is difficult to capture the impact of this cumulative life course impairment in clinical trials [8]. Traditional measurements of treatment success in psoriasis trials generally capture improvements at fixed time points rather than accounting for a cumulative effect of treatment over time [9]. Evaluating the cumulative clinical benefit of a treatment is especially important to accurately determine patients' totality of clinical benefit due to a therapy administered over a period of time, rather than at a specific point in time.
An area under the curve (AUC) analysis of clinical trial data can be conducted to capture the cumulative clinical benefit of treatments for psoriasis longitudinally [9,10,11,12]. A patient-level AUC analysis evaluates the cumulative clinical benefit of treatment by capturing the total time spent at a certain clinical response level, the speed and magnitude of response onset, and response sustainability over a defined period [9, 10]. For example, an AUC analysis would capture differences in cumulative benefit between a patient who experiences disease control at week 12 versus another who experiences disease control at week 48. If the response for both patients was measured solely at week 52, the appearance of treatment success would be the same, but the totality of benefit that each patient derived during treatment could vary greatly [9].
Two orally administered targeted therapies, deucravacitinib and apremilast, are available for the treatment of moderate to severe plaque psoriasis. The phase 3 POETYK PSO-1 (ClinicalTrials.gov dentifier: NCT03624127) and POETYK PSO-2 (Clinicalrials.gov identifier: NCT03611751) clinical trials compared the efficacy and safety of deucravacitinib versus apremilast in adults with moderate to severe plaque psoriasis [13, 14]. In POETYK PSO-1, patients randomized to apremilast who did not achieve ≥ 50% reduction from baseline in Psoriasis Area and Severity Index scores (PASI 50) at week 24 switched to deucravacitinib, whereas those who achieved PASI 50 continued apremilast through week 52; deucravacitinib-randomized patients maintained their initial treatment through week 52 [13]. In both trials, deucravacitinib was well tolerated and demonstrated an efficacy that was superior to apremilast in multiple measures [13, 14].
Understanding the cumulative clinical benefit of certain psoriasis treatments or treatment sequences could be relevant to patients and healthcare providers and help identify treatments and treatment sequences with the greatest impact on disease burden [9, 10, 12]. Therefore, we aimed to determine the overall cumulative clinical benefit of treatment initiated with deucravacitinib versus apremilast over 52 weeks in patients with moderate to severe plaque psoriasis and to compare the 52-week cumulative benefit of initiating and staying on deucravacitinib versus initiating apremilast and continuing or switching to deucravacitinib at week 24 of treatment using POETYK PSO-1 trial data.
Methods
Data Source and Study Design
This study was a post hoc analysis of POETYK PSO-1 clinical trial data [13] over 52 weeks comparing two treatment arms: deucravacitinib 6 mg once daily versus apremilast 30 mg twice daily (Fig. 1). The POETYK PSO-1 trial included adults who had moderate to severe plaque psoriasis for at least 6 months before screening and were eligible for phototherapy or systemic therapy. Patients previously treated with deucravacitinib or apremilast were excluded. The primary efficacy endpoints in POETYK PSO-1 were evaluated at week 16; thereafter, endpoints were measured every 4 weeks. At week 24, patients treated with apremilast who had not achieved PASI 50 were switched to deucravacitinib. The POETYK PSO-1 trial was conducted in accordance with the requirements of the Declaration of Helsinki of 1964 and its later amendments and the International Council for Harmonisation Good Clinical Practice guideline. The study was reviewed and approved by an independent institutional review board, and all participants provided written informed consent.
Study Outcomes
The cumulative clinical benefit of initiating treatment with deucravacitinib compared with apremilast over 52 weeks was based on cumulative measures of response according to: (1) the proportion of patients achieving at least 75% improvement from baseline in PASI scores (PASI 75) and (2) the proportion of patients with a static Physician Global Assessment score of 0 or 1 (sPGA 0/1) with at least a 2-point improvement from baseline. Both the PASI 75 and the sPGA 0/1 were measured at weeks 0, 1, 2, 4, and then every 4 weeks thereafter through week 52.
Statistical Analysis
The cumulative clinical benefit was determined for patients randomized at baseline to deucravacitinib or apremilast. As individual patient-level data were available from the POETYK study, AUC estimates were calculated using adjusted analysis of covariance (ANCOVA) regression approaches. The baseline characteristics used for stratification included the use of prior biologic treatment (yes or no), region (USA, China, Japan, rest of the world), and body weight (< 90 kg or ≥ 90 kg) for the USA. The modification to a previous described methodology [9] was considered appropriate as individual patient-level data were available from the POETYK PSO-1 trial. The cumulative clinical benefit was based on the AUC of the percentage of responders calculated from baseline to week 52 using the trapezoidal rule and the following formula:
For each patient, \({R}_{i}\) denotes the response outcome (1 for responder or 0 otherwise) at each time point \({T}_{i}\). For the time points, \(i=0, 1, 2, 3,\dots , 15\) represents weeks 0, 1, 2, 4, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, and 52, and \(i+1\) represents the next time point.
The adjusted least squares means were calculated and interpreted as the total adjusted AUC at the population level; treatment differences between the cohorts were based on the least squares means with corresponding 2-sided 95% confidence intervals (CIs). Additionally, the resulting cumulative adjusted AUC from the least squares means estimate was standardized as a percentage (0–100%) of the maximum possible AUC over 52 weeks (5200 [% × weeks]; i.e., 52 weeks of 100% response in each week). The CI for the standardized value was computed from the CI of the least squares means estimate by applying the same multiplier (i.e., divided by 5200). Finally, relative ratios of the AUC estimates between treatment groups were calculated, using apremilast initiators as the reference group.
Results
Study Population
For this analysis, the population included 500 patients from POETYK PSO-1 (deucravacitinib, = 332; apremilast, = 168), with an observation duration of 52 weeks (Table 1). Among patients who initiated treatment with apremilast (N = 168), 87 patients continued apremilast and 54 discontinued apremilast and started deucravacitinib after week 24 owing to a lack of PASI 50 response; the remaining patients discontinued treatment and had no additional follow-up per the POETYK PSO-1 trial design [13]. Among patients who initiated treatment with deucravacitinib, 200 patients had previously received systemic therapy and 130 patients previously received biologic therapy. Among patients who initiated treatment with apremilast, 109 patients previously received a systemic therapy and 66 patients previously received biologic therapy.
Cumulative Clinical Benefit
Overall, patients initiating treatment with deucravacitinib had a greater cumulative benefit of treatment as measured by PASI 75 than patients initiating treatment with apremilast or patients who switched from apremilast to deucravacitinib at week 24 because of nonresponse (i.e., < PASI 50). Over 52 weeks, the adjusted standardized average cumulative response based on PASI 75 for patients initiating deucravacitinib was 57.3% compared with 38.2% for patients initiating apremilast. This difference corresponds to a cumulative clinical benefit ratio of 1.50 in favor of deucravacitinib compared with apremilast. The adjusted standardized average cumulative response based on achieving an sPGA 0/1 with deucravacitinib versus apremilast was 50.2% versus 31.9%, corresponding to a cumulative clinical benefit ratio of 1.58 in favor of deucravacitinib.
Cumulative Clinical Benefit by Prior Treatment
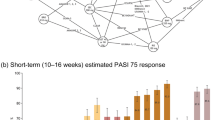
When patients were stratified based on prior treatment history, results were consistent with the primary analysis, showing a greater cumulative clinical benefit for deucravacitinib compared with apremilast based on PASI 75 and sPGA 0/1 regardless of previous systemic therapy exposure (Fig. 2) or specifically biologic therapy exposure. The cumulative clinical benefit ratios for deucravacitinib compared with apremilast based on PASI 75 and sPGA 0/1 for patients who were systemic therapy naive were 1.36 and 1.73, respectively, and for patients who were systemic therapy experienced, 1.57 and 1.55, respectively—all favoring deucravacitinib. The cumulative clinical benefit ratios for deucravacitinib compared with apremilast based on PASI 75 and sPGA 0/1 for patients who were biologic naive were 1.32 and 1.48, respectively, and for patients who were biologic experienced, 1.82 and 1.90, respectively—all favoring deucravacitinib.
Clinical benefit assessment in patients who were systemic therapy naive or experienced. AUC0–52 weeks Total area under the curve of the percentage of responders over 52 weeks, CI confidence interval, PASI Psoriasis Area and Severity Index, PASI 50 50% improvement from baseline in PASI score, PASI 75 75% improvement from baseline in PASI score. aAt week 24 (indicated by vertical dotted line), patients who initiated treatment with apremilast but did not achieve PASI 50 switched to deucravacitinib. Those who achieved PASI 50 continued apremilast. bAUC0–52 weeks ÷ maximum AUC0–52 weeks
Discussion
Results from this analysis show that patients who initiated treatment with deucravacitinib compared with apremilast had a higher cumulative benefit of treatment based on PASI 75 and sPGA 0/1 response during their first year of treatment. Specifically, across all patients, regardless of previous treatments received, patients who initiated treatment with deucravacitinib experienced 50% more benefit as measured by PASI 75 and 58% more benefit as measured by sPGA 0/1 than patients who initiated treatment with apremilast. These results were consistent in patients who were systemic and biologic therapy naive and in patients who were systemic and biologic therapy experienced. As the apremilast cohort included patients who received apremilast throughout the study and patients who initiated treatment with apremilast then crossed over to deucravacitinib at 24 weeks, greater clinical benefit potentially could be seen if deucravacitinib had been initiated as first-line treatment.
This study used valid and widely accepted measures to assess efficacy in a clinical trial population, and data quality was assured for the POETYK PSO 1 trial, which was reviewed and validated through quality control checks. Applying the AUC approach to evaluate cumulative clinical benefits can capture the continuous impact of treatment on patients with psoriasis versus using a “snapshot in time” approach. Capturing cumulative treatment benefits is particularly relevant for chronic disease states such as psoriasis. Throughout maintenance treatment, patients with psoriasis may experience a loss of treatment efficacy, see an improvement in treatment response, and regain lost efficacy [9]. By calculating the cumulative benefit of treatment, clinicians and researchers can measure the totality of an intervention’s effect over a defined period and not just a small portion of patients’ lives as is seen in clinical trials where capturing cumulative benefit is difficult to determine [8]. By using clinical trial results to calculate the cumulative benefit of treatment, we holistically captured the overall impact of treatment on a patient’s life over time [9].
A limitation of this study is that the results were obtained using clinical trial data from patients with moderate to severe plaque psoriasis. Therefore, these findings may not be generalizable to real-world effectiveness nor to patients with other forms of psoriasis.
Conclusions
Results from this cumulative benefit analysis support the primary efficacy analysis of deucravacitinib compared with apremilast in patients with moderate to severe psoriasis and provide a more comprehensive picture of deucravacitinib and apremilast benefit over time. A sustained treatment effect of deucravacitinib compared with apremilast was found over 52 weeks, with patients who initiated deucravacitinib having 1.5- to 1.6-fold higher average cumulative benefit as measured by the PASI 75 and sPGA 0/1. This benefit was seen in patients irrespective of prior systemic or biologic treatment use. These findings warrant further calculations of cumulative clinical benefits over follow-up periods exceeding 1 year, as well as in patients initiating deucravacitinib therapy earlier in the psoriasis treatment pathway.
Data Availability
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–6.
Wu JJ. Contemporary management of moderate to severe plaque psoriasis. Am J Manag Care. 2017;23(Suppl 21):S403–16.
Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81(3):775–804.
Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–86.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Kimball A, Gieler U, Linder D, Sampogna F, Warren R, Augustin M. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24(9):989–1004.
Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease-related impairment throughout the life course. Br J Dermatol. 2011;164(Suppl 1):1–14.
Mattei PL, Corey KC, Kimball AB. Cumulative life course impairment: evidence for psoriasis. Curr Probl Dermatol. 2013;44:82–90.
Armstrong AW, Feldman SR, Korman NJ, et al. Assessing the overall benefit of a medication: cumulative benefit of secukinumab over time in patients with moderate-to-severe plaque psoriasis. J Dermatolog Treat. 2017;28(3):200–5.
Blauvelt A, Gooderham M, Griffiths CEM, et al. Cumulative clinical benefits of biologics in the treatment of patients with moderate-to-severe psoriasis over 1 year: a network meta-analysis. Dermatol Ther (Heidelb). 2022;12(3):727–40.
Blauvelt A, Lomaga M, Burge R, et al. Greater cumulative benefits from ixekizumab versus ustekinumab treatment over 52 weeks for patients with moderate-to-severe psoriasis in a randomized, double-blinded phase 3b clinical trial. J Dermatolog Treat. 2020;31(2):141–6.
Warren RB, Gooderham M, Burge R, et al. Comparison of cumulative clinical benefits of biologics for the treatment of psoriasis over 16 weeks: results from a network meta-analysis. J Am Acad Dermatol. 2020;82(5):1138–49.
Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39.
Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51.
Acknowledgements
The authors thank all of the patients who participated in this study.
Medical Writing, Editorial, and Other Assistance
Medical writing and editorial assistance was provided by Catherine Mirvis, BA, and Beth Lesher, PharmD, BCPS, of OPEN Health Evidence & Access, and funded by Bristol Myers Squibb.
Funding
This study was sponsored by Bristol Myers Squibb. The rapid publication and open access fees were also funded by Bristol Myers Squibb.
Author information
Authors and Affiliations
Contributions
April W. Armstrong, Sang Hee Park, Vardhaman Patel, Matthew J. Colombo, and Viktor Chirikov contributed to the study design. Sang Hee Park, Pierre Nicolas, Wei-Jhih Wang, and Matthew J. Colombo participated in the collection and assembly of data. Sang Hee Park, Pierre Nicolas, Wei-Jhih Wang, Matthew J. Colombo, and Viktor Chirikov provided data analysis. All authors collaborated in the preparation of the manuscript, supported by two professional medical writers funded by Bristol Myers Squibb, and critically reviewed and provided revisions to the manuscript. All authors had access to the data and assume responsibility for the completeness and accuracy of the data and data analyses. All authors granted final approval of the manuscript for submission.
Corresponding author
Ethics declarations
Conflict of Interest
April W. Armstrong is a research investigator, scientific advisor, and/or speaker for AbbVie, Almirall, Arcutis, Aslan Pharmaceuticals, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Dermavant, Dermira, EPI Health, Incyte, Janssen, Leo Pharma, Lilly, Mindera Health, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Sang Hee Park, Vardhaman Patel, Pierre Nicolas, and Matthew J. Colombo are employees and may be shareholders in Bristol Myers Squibb. Wei-Jhih Wang and Viktor Chirikov are employees of OPEN Health Evidence & Access, which has received consulting fees from Bristol Myers Squibb.
Ethical Approval
The POETYK PSO-1 trial was conducted in accordance with the requirements of the Declaration of Helsinki of 1964 and its later amendments and the International Council for Harmonisation Good Clinical Practice guideline. The study was reviewed and approved by an independent institutional review board, and all participants provided written informed consent.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Armstrong, A.W., Park, S.H., Patel, V. et al. Cumulative Benefit Over 52 Weeks With Deucravacitinib Versus Apremilast in Moderate to Severe Plaque Psoriasis: POETYK PSO-1 Post Hoc Analysis. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01201-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13555-024-01201-4