Abstract
Background
The efficacy and safety of secukinumab in patients with psoriasis has been established in randomised clinical trials. However, data on effectiveness and safety of secukinumab in Latin American real-world settings are scarce.
Objectives
To evaluate the effectiveness and safety of secukinumab in real-world settings in patients with psoriasis in Latin America.
Methods
PURE is an ongoing multinational, prospective, observational study in patients with moderate-to-severe chronic plaque psoriasis in Canada and Latin America assessing the real-world safety and effectiveness of secukinumab and other approved therapies. The study enrolled (1:1) patients treated with secukinumab versus other approved therapies (other Tx) per local standard of care from 81 community- and hospital-based speciality sites (21 in Latin America). Here, we report effectiveness and safety outcomes with secukinumab and other Tx for plaque psoriasis for up to 12 months in a Latin American population.
Results
Overall, 187 patients were included in the analysis, 89 of whom initiated secukinumab treatment and 98 of whom received other Tx. At month 12, 84.4%, 71.1% and 53.3% of patients treated with secukinumab achieved Psoriasis Area and Severity Index (PASI) 75/90/100, respectively, compared with 66.7%, 47.9% and 29.2% of patients who received other Tx. Investigator Global Assessment (IGA) 0/1 responders in secukinumab versus other Tx were 78.3% versus 36.7% at month 3 and 81.8% versus 66.7% at month 12, respectively. Overall, the proportion of patients achieving Dermatology Life Quality Index (DLQI) 0/1 improved from 6.9% at baseline to 76.5% at month 12 in patients treated with secukinumab versus 5.6% at baseline to 54.5% at month 12 in patients on other Tx. No unexpected adverse events were reported during the 12-month observation period.
Conclusion
Secukinumab demonstrated real-world effectiveness and improved dermatology quality-of-life in chronic plaque psoriasis patients from Latin America.
Trial Registration
PURE: NCT02786186.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Secukinumab has shown rapid and sustained effectiveness with a favourable safety profile in the treatment of moderate-to-severe plaque psoriasis and its manifestations in real-world studies |
There is limited information on clinical data pertaining to outcomes with secukinumab use for psoriasis in Latin America. In addition, variation exists between patients (severity of disease and other patient characteristics), physician perspectives (drug prescription patterns) and health care systems (clinical practice, local guideline–driven management of disease and reimbursement policies) in routine clinical practice |
This study was conducted to assess the effectiveness and safety of secukinumab in a Latin American population |
What was learned from the study? |
The current study provides data on the real-world effectiveness of secukinumab in Latin American patients with moderate-to-severe plaque psoriasis |
The 12-month follow-up data from the PURE registry demonstrated improvements in psoriasis disease severity and a positive impact on quality of life with secukinumab treatment in a clinical setting |
The findings from this interim analysis are likely to support an evidence-based framework for clinical decisions on treating patients with chronic plaque psoriasis in Latin America |
Introduction
Psoriasis is a chronic inflammatory condition characterised by well-defined erythematous plaques with scales that can be itchy and that vary in severity [1]. Prevalence of psoriasis in Latin America has been estimated to be 1.13–2.9% [2]. However, owing to scant literature evidence for psoriasis in Latin American countries, the true burden of psoriasis remains largely unknown. Treatment options for chronic plaque psoriasis vary from topical agents and conventional systemic treatment to biologic therapies. Tumour necrosis factor alpha (TNF-α) inhibitors, interleukin (IL)-12/23 and IL-23 inhibitors, and IL-17 cytokine inhibitors are the three main classes of biologic agents approved for the treatment of moderate-to-severe psoriasis [3, 4].
Secukinumab, a fully human monoclonal antibody that directly inhibits IL-17A, a cornerstone cytokine involved in the pathophysiology of psoriasis, has demonstrated long-lasting efficacy and safety in the complete spectrum of psoriasis manifestations [5,6,7,8,9]. Efficacy of secukinumab in patients with moderate-to-severe plaque psoriasis has been well established in the pivotal phase 3 trials [7, 10, 11]. Real-world evidence (RWE) studies on secukinumab have demonstrated its long-term effectiveness and favourable safety profile in several key registries, such as CorEvitas (in North America), BADBIR (in the UK), PROSE (across 17 European countries) and PROSPECT (in Germany) [12,13,14,15]. However, clinical data regarding outcomes with secukinumab use for psoriasis in Latin America are still limited. PURE is the first real-world registry to evaluate the clinical effectiveness, safety profile and impact on the quality of life (QoL) of secukinumab in the management of patients with moderate-to-severe psoriasis from Canada and Latin America [16]. Here, we report the effectiveness and safety profiles of secukinumab in patients from Latin America who completed a 12-month follow-up in the PURE registry.
Methods
Study Design
PURE is an ongoing, prospective, multinational, observational, two-cohort registry of adult Patients with moderate to severe chronic plaqUe psoRiasis in Latin AmErica and Canada (NCT02786186) [16]. There were 21 research sites in seven countries across Latin America (Argentina, Brazil, Guatemala, Costa Rica, Mexico, Dominican Republic and Panama), including public hospitals and private clinics. Patients were divided into two cohorts, those who received secukinumab treatment and those who received other approved therapies (other Tx) (Fig. 1). The study includes a 5-year follow-up at completion, with recommended assessments at enrollment, at months 3 and 6, and every 6 months thereafter (Fig. 1). Treatment switch can occur because of the longitudinal and observational nature of the study.
Inclusion and Exclusion Criteria
Patients (aged ≥ 18 years) who were diagnosed with chronic moderate-to-severe plaque psoriasis by a specialist were included in the study. The decision to start psoriasis treatment (secukinumab, other biologics, systemic treatments or phototherapy) must have been reached prior to and independent of the enrollment visit. Other inclusion criteria were prescription of treatments in accordance with the product monograph as per standard of care and regional regulatory and reimbursement policies, and the ability of patients to understand and communicate with the investigator and comply with the requirements of the study. Patients who were involved in a clinical trial of an investigational drug, concurrently or within the last 30 days, were not eligible for enrollment in the PURE registry.
Baseline Assessments
Patient demographic characteristics, including age, gender, race and medical history, were recorded at enrollment in the registry. Severity of the disease at baseline was assessed using Investigator’s Global Assessment (IGA), Psoriasis Area and Severity Index (PASI), and body surface area (BSA) measurements. In addition, prior and current psoriasis therapies and psoriasis diagnoses were also captured in the study.
Outcome Measures and Variables
Outcome measures were assessed at baseline, 3, 6, and 12 months. Effectiveness assessments for psoriasis included physician-reported outcomes (PASI and IGA). The impact of psoriasis on patient’s health-related QoL (HR-QoL) was assessed by patient-reported outcomes (PROs) and the Dermatology Life Quality Index (DLQI). Safety was evaluated by the incidence of adverse events (AE). The safety outcomes assessed included incidence of total AEs, total serious AEs (SAE) and infections defined by the proportion of patients with at least 1 event, the mean number of events per patient, and the mean number of events per person-year of follow-up. All AEs were mapped on the basis of the hierarchy of exposures: secukinumab > other biologic agents > non-biologic agents.
Statistical Analysis
All analyses were per protocol based on the modified intent-to-treat population (mITT). The mITT population includes all enrolled patients with an assigned cohort at baseline who had at least one post-baseline visit or electronic PRO (ePRO) submission, or a post-baseline AE evaluation. Patients with major protocol violations were excluded from the mITT population. For both treatment cohorts, AEs and SAEs were assessed using the total number and proportion of patients who experienced at least one event within each system organ class (SOC) and within each preferred term (PT), and the number of patients who experienced the specific event by 100 patient-years (PY). All AEs are coded by Medical Dictionary for Regulatory Activities (MedDRA Version 23.1). In each treatment cohort, patients who experienced the same event multiple times were counted only once for the corresponding SOC and PT. All statistical analyses were descriptive in nature.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from all eligible patients before any data were collected. Wherever required, the study was reviewed and approved by ethics committees or institutional review boards.
Results
Patient Population
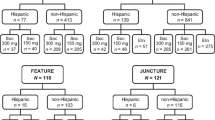
As of 24 January 2020, a total of 233 patients from Latin America were enrolled in the study, and data from 187 patients qualified under mITT population (Fig. 2). Overall, 16 patients (6.9%) prematurely discontinued treatment. The most common reasons for discontinuation were withdrawal of informed consent [7 patients (3.0%)] and termination of study by sponsor [3 patients (1.3%)]; (Fig. 2). Among the patients analysed, 89 initiated secukinumab treatment and 98 initiated other Tx during the 12-month observation period, on the basis of physician’s decision.
Patient disposition in 12-month data from the Latin American population of the PURE registry. AE adverse event, mITT modified intent-to-treat population, N total number of patients, n number of patients, Other Tx other approved therapies SEC secukinumab. aThe mITT includes all enrolled patients with an assigned cohort at baseline who had at least 1 post-baseline visit or ePRO submission, or a post-baseline AE evaluation. Patients with major protocol violations were excluded from the mITT population
Demographics and Baseline Disease Characteristics
Patients’ baseline demographics were comparable across the two treatment groups (Table 1). Of the 187 patients, 99 (53.0%) were male, the mean age was 47.2 years, and the mean body mass index (BMI, overall population) was 28.0 kg/m2. Overall, the proportion of patients who received biologic systemic therapy and non-biologic systemic therapy at baseline was 62.6% and 21.9%, respectively. Each biologic and non-biologic systemic therapy has been detailed in Supplementary Table S1. The mean baseline PASI score was 16.0, and the mean baseline total BSA involvement was 24% in the overall population. The baseline IGA score was moderate in 61.5% of patients and severe in 32.4% of patients. Overall, the mean time since diagnosis of psoriasis was 12.3 years (Table 1).
Treatment Effectiveness
The respective mean (standard deviation [SD]) absolute PASI score improved in patients treated with secukinumab versus other Tx from 16.6 (9.51; n = 88) versus 15.5 (7.59; n = 95) at enrollment to 2.3 (3.84; n = 82) versus 5.6 (5.70; n = 88) at month 3, 2.5 (6.53; n = 64) versus 4.4 (6.14; n = 72) at month 6, and 2.0 (4.38; n = 45) versus 3.7 (5.11; n = 48) at month 12 (Fig. 3a). The corresponding median (range) PASI score for patients who received secukinumab versus other Tx was 14.5 (1.5–50.5) versus 14.6 (2.7–48.0), 1.1 (0.0–24.9) versus 4.2 (0.0–31.6), 0.3 (0.0–39.1) versus 2.4 (0.0–30.6) and 0.0 (0.0–20.7) versus 1.5 (0.0–22.2) at baseline, month 3, month 6 and month 12, respectively. PASI 90/100 response was achieved by (61.7%/43.2%; N = 81), (71.9%/48.4%; N = 64) and (71.1%/53.3%; N = 45) patients treated with secukinumab at month 3, month 6 and month 12, respectively (Fig. 3b). At month 12, the proportion of secukinumab-treated patients who achieved absolute PASI ≤ 5 and PASI ≤ 3 was 88.9% and 84.4%, respectively, and the corresponding values for patients treated with other Tx were 77.1% and 64.6%, respectively (Fig. 3c). The proportion of patients who achieved an IGA 0/1 (clear to almost clear skin) response in the secukinumab versus other Tx groups was 78.3% versus 36.7% at month 3, 80.0% versus 54.1% at month 6, and 81.8% versus 66.7% at month 12, respectively (Fig. 4).
Efficacy over time as assessed by PASI. a Mean PASI scores, b Proportion of patients with PASI75/90/100 responses, and c proportion of patients with PASI ≤ 5 and PASI ≤ 3 responses through month 12. n number of patients, Other Tx other approved therapies, PASI Psoriasis Area and Severity Index, SEC secukinumab
Disease severity (IGA) of patients at enrollment and up to 12 months follow-up. IGA is a five-point scale that provides a global clinical assessment of disease severity ranging from 0 to 4, where 0 indicates clear, 1 almost clear, 2 mild, 3 moderate and 4 indicates severe. IGA Investigator’s Global Assessment, n number of patients, Other Tx other approved therapies, SEC secukinumab
Skin Disease-Related Quality of Life
The mean (SD) DLQI score in patients treated with secukinumab versus other Tx was 11.5 (7.12; N = 87) versus 11.5 (7.63; N = 90) at baseline, 3.5 (4.33; N = 70) versus 5.7 (6.35; N = 76) at month 3, 3.5 (5.47; N = 52) versus 4.1 (4.92; N = 64) at month 6, and 1.8 (3.29; N = 34) versus 3.7 (6.07; N = 33) at month 12, respectively (Fig. 5a). The proportion of patients with DLQI 0–1 in the secukinumab group versus patients treated with other Tx was 52.9% (N = 70) versus 31.6% (N = 76) at month 3, 46.2% (N = 52) versus 40.6% (N = 64) at month 6, and 76.5% (N = 34) versus 54.5% (N = 33) at month 12, respectively (Fig. 5b).
Safety
The percentage of patients reporting any AEs in the overall population was 36.9% (Table 2). The exposure-adjusted incidence rate (IR) of AEs with secukinumab treatment was 49.6/100 PY and 48.7/100 PY in the patients who received other Tx. The IR of SAEs in patients treated with secukinumab was 6.7/100 PY and 3.7/100 PY in patients who received other Tx. The IR of AEs leading to permanent discontinuation of study dose was 5.4/100 PY in the secukinumab-treated patients and 2.5/100 PY in the patients treated with other Tx. Infections and infestations (17.1%) were the most commonly reported SOC AEs, followed by gastrointestinal disorders (5.9%) and skin and subcutaneous tissue disorders (5.3%) (Table 2). The percentage of infections and infestations reported in patients treated with secukinumab and patients who received other Tx was 14.6% and 19.4%, respectively (Table 2). Percentages of SAEs for patients treated with secukinumab were 5.1% and 4.1% for patients who received other Tx (Table 2). Further, AEs categorised by SOC and PT have been detailed in supplementary table S2. The fulminant hepatitis reported in the secukinumab group was not related to the study drug. One death was reported among the patients who received secukinumab, which was not related to study treatment as assessed by the investigator.
Discussion
In randomised controlled trials (RCT), secukinumab has demonstrated good efficacy and an overall consistent safety profile in the treatment of patients with moderate to severe psoriasis [7, 10, 11]. Complementing the efficacy of secukinumab, real-world studies conducted in various countries across Europe and the USA have reported comparable effectiveness of secukinumab in patients with plaque psoriasis [12, 17,18,19,20,21]. However, there remains insufficient data regarding treatment outcomes and effectiveness of secukinumab in real-world clinical practice in Latin America.
The ongoing PURE registry generated real-world evidence in patients from Latin America that fill gaps in our understanding of treatment outcomes with secukinumab therapy as part of routine clinical practice in Latin America. Thus far, in the 187 patients analysed from the study, secukinumab demonstrated effective and rapid clinical improvement with most patients achieving PASI 75/90 response. Improvement was also observed in the total mean PASI scores from baseline up to 12 months. Furthermore, the PASI 75 and PASI 90 response rates achieved at month 6 were maintained at month 12 of secukinumab treatment. These results are in accordance with the efficacy outcomes reported in clinical trials of secukinumab [9, 22]. In the CLEAR study, secukinumab demonstrated superior efficacy to ustekinumab in the proportion of patients who achieved PASI 90 at week 12 (76.0% versus 61.0%; P < 0.0001) which was sustained up to week 52 (73.2% versus 59.8%; P < 0.0001). In the SCULPTURE extension study, PASI 75/90/100 responses at week 52 (88.9%, 68.5% and 43.8%, respectively) were maintained up to year 5 (88.5%, 66.4% and 41.0%) on continued therapy with secukinumab [22]. Data from the real-world study by Galluzzo et al. demonstrated rapid improvement with secukinumab treatment at week 16 (PASI 75/90/100: 90.0%, 79.0% and 64.0%, respectively), which was maintained up to week 136 (PASI 75/90/100: 79.0%, 72.0% and 55.0%, respectively) [23]. However, it is important to practice caution when comparing PASI 90 parameters from RWE with those observed in RCT as the populations differ, and often the baseline disease severity measures in RWE are not precise. A real-world retrospective analysis from Italy demonstrated a decrease in mean PASI scores from 15.3 at baseline to 0.5, BSA scores from 21.4 at baseline to 0.7, and DLQI of 11.7 at baseline to 0.2 after 84 weeks of treatment with secukinumab [21].
In this PURE registry analysis, consistent and sustained improvement was observed in a high proportion of patients who reported almost clear or clear skin from month 3 onwards. A higher proportion of patients on secukinumab achieved IGA 0/1 response at month 6 which was maintained until month 12 compared with those on other Tx. These findings agree with a prior publication of a real-world study by Strober et al. where significant improvement in IGA 0/1 was observed with secukinumab treatment at a 6-month follow-up versus baseline (50.0% versus 5.9%) [12]. Long-term benefits of secukinumab at clearing skin of patients with moderate-to-severe psoriasis have also been well established in previous studies [9, 22, 24, 25].
Results from this registry also showed that treatment with secukinumab improved patients’ HR-QoL measured by DLQI. Similar improvements in PROs have been observed in previous studies [12]. In the CLARITY study, patients treated with secukinumab demonstrated greater improvement in DLQI 0–1 responses compared with those treated with ustekinumab (69.9% versus 61.2%; P = 0.0028) at week 52 [24]. In the CorEvitas (CORRONA) psoriasis registry, significant improvement in DLQI score [mean (SD) DLQI score at baseline: 7.7 (6.0) versus at month 6: 2.9 (4.1)] was observed in patients at the 6-month follow-up visit [12]. In the PROSE study, 70.8% patients achieved DLQI 0/1 response at week 16, which was sustained up to week 52 [26]. In addition, the PROSE study showed greatest improvement in DLQI 0/1 response in naive patients (74.7%), compared with those received conventional systemic therapy (71.3%) and biologic therapy (61.7%) [26].
No new safety signal for secukinumab has been observed in this study. AEs in SOC of “infections and infestations” were reported most frequently, with most infections being mild to moderate in severity. The overall safety profile of secukinumab in this study has been consistent with that of the pivotal phase 3 trials [7, 9, 27].
Limitations of this study included its non-interventional nature; hence, missing or incomplete data, selection bias and inclusion bias may be expected, and the population size is relatively small. Further studies with a larger number of patients may be required to better assess the efficacy of individual or class of therapies in this group. These findings are purely descriptive and not all patients who initiated secukinumab at baseline had a 12-month follow-up visit during the study duration, which resulted in a smaller sample size for analysis. Further analyses with a larger patient population from the ongoing PURE registry may provide more robust RWE on psoriasis treatment outcomes in Latin America.
Conclusions
PURE registry is the first prospective, international, observational study to report measured improvements in psoriasis disease severity and HR-QoL outcomes, demonstrating the real-world effectiveness of secukinumab in the Latin American population, with safety consistent with that reported in previous studies.
References
Fry L. An atlas of psoriasis. 2nd ed. London: Taylor & Francis; 2005.
Hernández-Vásquez AML, Larrea N, Ciapponi A. Psoriasis in Latin America and the Caribbean: a systematic review. J Eur Acad Dermatol Venereol. 2017;31:1991–8.
Sbidian E, Chaimani A, Garcia-Doval I, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12;CD011535.
Sbidian E, Chaimani A, Afach S, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2020;1;CD011535.
Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials. J Dermatolog Treat. 2020;33:1–9.
Baeten D, Sieper J, Braun J, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–48.
Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med. 2014;371:326–38.
McInnes IB, Mease PJ, Kirkham B, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–46.
Thaçi D, Blauvelt A, Reich K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol. 2015;73:400–9.
Adsit SZE, Sofen H, et al. Secukinumab is efficacious and safe in Hispanic patients with moderate-to-severe plaque psoriasis: pooled analysis of four phase 3 trials. Adv Ther. 2017;34:1327–39.
Bissonnette R, Luger T, Thaci D, et al. Secukinumab sustains good efficacy and favourable safety in moderate-to-severe psoriasis after up to 3 years of treatment: results from a double-blind extension study. Br J Dermatol. 2017;177:1033–42.
Strober BE, Germino R, Guana A, et al. US real-world effectiveness of secukinumab for the treatment of psoriasis: 6-month analysis from the Corrona Psoriasis Registry. J Dermatolog Treat. 2020;31:333–41.
Yiu ZZN, Mason KJ, Hampton PJ, et al. Drug survival of adalimumab, ustekinumab and secukinumab in patients with psoriasis: a prospective cohort study from the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR). Br J Dermatol. 2020;183:294–302.
DE Augustin M, Mrowietz U, et al. Secukinumab treatment leads to normalization of quality of life and disease symptoms in psoriasis patients with or without prior systemic psoriasis therapy: the PROSE study results. J Eur Acad Dermatol Venereol. 2021;35:431–40.
Thaçi DKA, von Kiedrowski R, et al. Secukinumab is effective in treatment of moderate-to-severe plaque psoriasis: real-life effectiveness and safety from the PROSPECT study. J Eur Acad Dermatol Venereol. 2020;34:310–8.
Papp KA, Gooderham M, Beecker J, et al. Rationale, objectives and design of PURE, a prospective registry of patients with moderate to severe chronic plaque psoriasis in Canada and Latin America. BMC Dermatol. 2019;19:9.
Körber ATD, von Kiedrowski R, et al. Secukinumab treatment of moderate to severe plaque psoriasis in routine clinical care: real-life data of prior and concomitant use of psoriasis treatments from the PROSPECT study. J Eur Acad Dermatol Venereol. 2018;32:411–9.
Momose M, Asahina A, Umezawa Y, Nakagawa H. Long-term clinical efficacy and safety of secukinumab for Japanese patients with psoriasis: a single-center experience. J Dermatol. 2018;45:318–21.
Galluzzo M, Talamonti M, De Simone C, et al. Secukinumab in moderate-to-severe plaque psoriasis: a multi-center, retrospective, real-life study up to 52 weeks observation. Expert Opin Biol Ther. 2018;18:727–35.
Notario J, Deza G, Vilarrasa E, et al. Treatment of patients with plaque psoriasis with secukinumab in a real-life setting: a 52-week, multicenter, retrospective study in Spain. J Dermatolog Treat. 2019;30:424–9.
Megna M, Di Costanzo L, Argenziano G, et al. Effectiveness and safety of secukinumab in Italian patients with psoriasis: an 84 week, multicenter, retrospective real-world study. Expert Opin Biol Ther. 2019;19:855–61.
Bissonnette RLT, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32:1507–14.
Galluzzo M, D’Adamio S, Silvaggio D, Lombardo P, Bianchi L, Talamonti M. In which patients the best efficacy of secukinumab? Update of a real-life analysis after 136 weeks of treatment with secukinumab in moderate-to-severe plaque psoriasis. Expert Opin Biol Ther. 2020;20:173–82.
Bagel J, Blauvelt A, Nia J, et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY). J Eur Acad Dermatol Venereol. 2021;35:135–42.
Blauvelt A, Reich K, Tsai TF, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: Results from the CLEAR study. J Am Acad Dermatol. 2017;76(60–69): e69.
Augustin M, Dauden E, Mrowietz U, et al. Secukinumab treatment leads to normalization of quality of life and disease symptoms in psoriasis patients with or without prior systemic psoriasis therapy: the PROSE study results. J Eur Acad Dermatol Venereol. 2021;35:431–40.
Mrowietz U, Leonardi CL, Girolomoni G, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. 2015;73(27–36): e21.
Acknowledgements
The authors thank the clinical investigators, site staff and participants of the study.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Novartis Pharma Logistics Inc., Panama.
Medical Writing, Editorial and Other Assistance
We thank the following for their contribution: Antonio Vieira (Novartis Pharmaceutical Canada), for critical feedback on the manuscript; Syreon Corporation, Canada for providing operational management/data management and statistical analysis services/other, which was paid by Novartis Pharmaceutical Canada. The authors thank Amrita Dubey and Mohammad Fahad Haroon (Novartis Health Care Pvt. Ltd., Hyderabad, India) for providing medical writing support and editorial support, which was funded by Novartis Pharma Logistics Inc., Panama, in accordance with the Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Kim A. Papp, Melinda Gooderham and Ignacio Dei-Cas contributed to manuscript concept and design. Material preparation and data collection were performed by Kim A. Papp, Melinda Gooderham, Ignacio Dei-Cas, Adriana LopezTello, Juan C. Garcia-Rodriguez, Carmen Yris Taveras, Azucena Hernández Rousselin, Alberto Lavieri, Mónica Maiolino and Delfina Guadalupe Villanueva Quintero. Data analysis was performed by Syreon Corporation, Canada. All authors provided critical feedback on the manuscript, approved the final manuscript for submission and are accountable for the accuracy and integrity of the manuscript.
Disclosures
Kim A. Papp serves as a consultant for AbbVie, Akros, Amgen, Arcutis, Atellas, Avillion, Bausch Health/Valeant, Baxalta, Boehringer Ingelheim, Can-Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly and Company, Evelo, Galapagos, Galderma, Genentech, Incyte, Janssen, Kyowa Hakko Kirin, Leo Pharma A/S, Merck (MSD), Merck Serono, Novartis, Pfizer, PRCL Research, Regeneron, Roche, Sanofi-Aventis/Genzyme, Takeda, and UCB; received research grants from Anacor, Gilead, GSK, MedImmune, Moberg Pharma and Sun Pharmaceuticals; is a scientific officer for Akros, Anacor, Arcutis, Dice Pharmaceuticals and Kyowa Hakko Kirin; is a consultant for Dice Pharmaceuticals, Meiji Seika Pharma and Mitsubishi Pharma; is a speaker, received honoraria and participated in steering committees and advisory boards for AbbVie, Amgen, Bausch Health/Valeant, Celgene, Eli Lilly and Company, Janssen, Merck (MSD), Novartis, Pfizer and Sanofi-Aventis/Genzyme. Speaker for Astellas, Galderma, Incyte, Kyowa Hakko Kirin and Leo Pharma A/S; participated in steering committees for Boehringer Ingelheim, Kyowa Hakko Kirin, Merck Serono and Regeneron; participated in advisory boards for Astellas, Boehringer Ingelheim, Bristol-Myers Squibb, Dow Pharma, Galderma, Regeneron, Sun Pharmaceuticals and UCB; received honoraria from Akros, Boehringer Ingelheim, Coherus, Galderma, Kyowa Hakko Kirin, Merck Serono, Mitsubishi Pharma, PRCL Research, Takeda and UCB. Melinda Gooderham serves as speaker, investigator or advisory board member for AbbVie, Amgen, Akros, AnaptysBio, Arcutis, Arena, Aslan, Bausch, Boehringer Ingelheim, BMS, Celgene, Coherus, Dermira, Dermavant, Eli Lilly, Galderma, GSK, Incyte, Janssen, Kyowa Kirin, LEO Pharma, Medimmune, Merck, Meiji, Moonlake, Novartis, Pfizer, Regeneron, Reistone, Sanofi Genzyme, Sun Pharmaceuticals, Takeda, and UCB. Ignacio Dei-Cas and Adriana LopezTello have no conflict of interest to declare. Juan C. Garcia Rodriguez has received honorariums as a speaker, researcher and advisor from Abbvie, Boehringer-Ingelheim, Lilly, Novartis, Janssen, Sanofi, Pfizer. Also, serves as principal investigator of the PURE study. Carmen Yris Taveras serves as speaker, investigator or advisory board member for AbbVie, Janssen, Novartis, Roche. Also, she serves as principal investigator for PURE study. Azucena Hernández Rousselin reports no conflicts of interest in this work. She serves as principal investigator for PURE study. Alberto Lavieri and Monica Maiolino have no conflict of interest to declare. Delfina Guadalupe Villanueva Quintero serves as director of the CATEI Clinical Group (Center for Attention in Inflammatory Diseases); is a speaker and advisor to Novartis, Abbvie, Janssen, Eli Lilly, UCB. Lenka Rihakova was an employee of Novartis Pharmaceuticals Canada Inc., Dorval, QC, Canada, until submission draft development of this manuscript. Mariano Salibe is an employee of Novartis Argentina S.A., Ciudad Autónoma de Buenos Aires, Argentina. Wilfran Pertuz is an employee of Novartis Pharma Logistics, Inc., Panama, Costa del Este, Panama City, Panama.
Compliance with Ethics Guidelines
The study was conducted in accordance with the Declaration of Helsinki principle. Informed consent was obtained from all eligible patients before any data were collected. Wherever required the study was reviewed and approved by ethics committees/institutional review boards.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Papp, K.A., Gooderham, M., Dei-Cas, I. et al. Effectiveness and Safety of Secukinumab in Latin American Patients with Moderate to Severe Plaque Psoriasis: PURE Registry 12-Month Data. Dermatol Ther (Heidelb) 13, 269–283 (2023). https://doi.org/10.1007/s13555-022-00849-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-022-00849-0