Abstract
Introduction
Palmoplantar pustulosis (PPP) is a pruritic, painful, chronic dermatitis that greatly impacts functioning and quality of life and can be difficult to treat. Approved treatment options for PPP are limited, and many patients do not fully respond to current treatments.
Methods
This was a randomized, double-blind, placebo-controlled, phase 2 study in Japanese patients with moderate to severe PPP and inadequate response to topical treatment. Patients were randomized 1:1 to receive apremilast 30 mg twice daily or placebo for 16 weeks followed by an extension phase where all patients received apremilast through week 32. PPP Area and Severity Index (PPPASI), modified PPPASI (which evaluates pustules and vesicles separately), and Palmoplantar Severity Index (PPSI) total scores and subscores (erythema, pustules/vesicles, and desquamation/scales) were evaluated over 32 weeks of apremilast treatment. Achievement of ≥ 50% improvement in PPPASI (PPPASI-50) was evaluated at week 16 among baseline demographic and clinical characteristic subgroups.
Results
At week 16, improvements in total score and subscores for PPPASI, modified PPASI, and PPSI, as well as rates of PPPASI-50 were at least moderately greater with apremilast than placebo. Mean PPPASI total score decreased by − 68.3% from baseline to week 32 with continued apremilast treatment. At week 32, mean change from baseline in PPPASI/modified PPPASI subscores ranged from − 58.5% to − 77.0% with apremilast. At week 32, PPSI total score for physician and patient assessments decreased by − 51.3% and − 40.0%, respectively, with continued apremilast treatment. PPPASI-50 response at week 16 was greater with apremilast versus placebo in most demographic and baseline characteristic subgroups.
Conclusions
Improvements in all PPPASI and PPSI total scores and subscores observed with apremilast over 16 weeks were maintained through 32 weeks in patients with moderate to severe PPP and inadequate response to topical treatment. Rates of PPPASI-50 response at week 16 were mostly consistent across patient subgroups.
ClinicalTrials.gov
NCT04057937.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Approved treatment options for palmoplantar pustulosis (PPP) are limited. |
Improvements in PPP overall disease severity observed with apremilast over 16 weeks were maintained for 32 weeks in patients with moderate to severe PPP and inadequate response to topical treatment. |
Improvements in all signs and findings of PPP (i.e., erythema, pustules, vesicles, and desquamation/scales) were observed over 32 weeks of apremilast treatment as assessed by both the physician and the patient. |
Apremilast showed consistent benefit over placebo at week 16 across demographic and disease characteristic subgroups. |
Introduction
Palmoplantar pustulosis (PPP) is a pruritic, painful, chronic dermatitis [1]. It is characterized by a combination of intraepidermal vesicles, pustules, erythema, and scales/desquamation located on the palms and soles [2, 3]. As a result of the location of lesions, PPP can greatly limit a patient’s functional ability and can negatively impact quality of life [4]. PPP can also be difficult to treat. Topical treatments such as corticosteroids, active vitamin D3 ointments, and phototherapy are common treatments for PPP [1]. The efficacy of topical treatments is limited, however, because the thicker stratum corneum of the palms and soles acts as a barrier. There is an unmet need for improved treatments for PPP in patients whose disease is not adequately controlled by topical treatments.
Apremilast is an oral phosphodiesterase 4 inhibitor that has shown efficacy for the treatment of psoriatic disease, including palmoplantar psoriasis [5,6,7,8,9,10,11,12,13,14]. We previously reported improvements in disease severity and patient-reported symptoms after 16 and 32 weeks of apremilast treatment in Japanese patients with PPP [15]. Here we assess modified PPP Area and Severity Index (PPPASI) and Palmoplantar Severity Index (PPSI) total scores and subscores evaluated by the physician and the patient over 32 weeks of apremilast treatment as well as a subgroup analysis of achievement of ≥ 50% improvement in PPPASI (PPPASI-50) at week 16 among subgroups of patients stratified by baseline demographics and disease characteristics.
Methods
Study Design
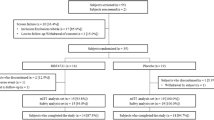
Study design and inclusion criteria have been reported in detail [15]. Briefly, this was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase 2 trial of apremilast in Japanese patients with PPP. Patients were randomized 1:1 to receive apremilast 30 mg twice daily or placebo for 16 weeks. After week 16, patients initially randomized to apremilast continued on apremilast and those initially randomized to placebo switched to apremilast (placebo/apremilast) through week 32 during the active treatment phase.
This study was conducted in accordance with International Council for Harmonization E6 and the ethical principals that are outlined in the Declaration of Helsinki. The study protocol and all amendments, the informed consent form, and any accompanying materials provided to the patients were reviewed and approved by an institutional review board or independent ethics committee at each study center (Online Resource 1). Patients provided written informed consent prior to study procedures.
Key Inclusion Criteria
Patients were adults (≥ 20 years of age) with a diagnosis of PPP for at least 24 weeks before screening. Key inclusion criteria were a PPPASI total score ≥ 12 at screening and baseline, moderate or severe pustules/vesicles on the palm or sole (PPPASI pustule/vesicle severity score ≥ 2) at screening and baseline, and an inadequate response to treatment with topical steroid and/or vitamin D3 derivative preparations.
Key Exclusion Criteria
Patients with plaque-type psoriasis or pustular psoriasis in any part of the body other than the palms or soles (excluding those derived from PPP, which have distinct characteristics [16]) were excluded. Patients were also excluded if they had received any procedures for focal infection (such as tonsillectomy and dental therapy) within 24 weeks of baseline. Additional exclusion criteria identified by dental examination at screening were dental focal infection, including periodontitis obviously requiring treatment (treatment includes any endodontic treatment for periapical pathosis [chronic periapical periodontitis] such as infected root canal treatment or tooth extraction, and periodontal surgery for moderate to severe periodontitis [chronic marginal periodontitis]) or chronic or recurring tonsilitis or sinusitis requiring any continuous treatment for a month or more at screening.
Assessments
The primary endpoint of this study (PPPASI-50 at week 16) and secondary endpoints (evaluated at weeks 16 and 32) have been previously reported [15]. Here we report exploratory endpoints assessed over 32 weeks, including percent change from baseline in PPPASI and its subscores (erythema, pustules/vesicles, and desquamation/scales), modified PPPASI and its subscores (erythema, pustules, vesicles, and desquamation/scales), PPSI total score (physician’s assessment), as well as a patient assessment of PPSI recording worst symptom between each visit through week 32. The PPPASI and modified PPPASI are differentiated by the pustules/vesicles subscore. The PPPASI evaluates both together whereas the modified PPPASI separates pustules and vesicles into separate subscores. In addition, we assessed the difference in PPPASI-50 response rates between apremilast and placebo groups stratified by baseline demographic and clinical characteristic subgroups.
Statistical Analysis
Efficacy endpoints were assessed in the intent-to-treat (ITT) population, defined as all randomized patients who received at least one dose of study drug. Endpoints measuring the percent change from baseline were assessed using data as observed. No imputation method was used for missing data. The subgroup analysis of PPPASI-50 response was assessed using non-responder imputation for missing data.
Results
Baseline Characteristics
Patient disposition and baseline characteristics for this population have been reported in detail [15]. Of 90 patients who were randomized, 87 (96.7%) completed the placebo-controlled phase; of the 87 who entered the active treatment phase, 84 (96.6%) completed this phase. Mean age was 54.8 years, 76.7% of patients were female, and mean body mass index (BMI) was 24.8 kg/m2. Most patients were current or past tobacco users (55.6% current users, 27.8% past users). Mean duration of PPP was 7.4 years, 34.4% had a PPPASI total score ≤ 20, 40.0% had a score ≥ 21 to ≤ 30, and 25.6% had a score ≥ 31. Most (71.1%) patients had presence of focal infection at randomization, 78.9% had a history of focal infection, and pustulotic arthro-osteitis (PAO) was present in 22.2%. Ongoing focal infections included periapical pathosis (28.9%), periodontitis (57.8%), tonsillitis (2.2%), and sinusitis (1.1%).
Clinical Outcomes
At baseline, mean PPPASI total score was 25.0 in the apremilast group and 24.9 in the placebo group. We previously reported that the percent change from baseline in PPPASI score was significantly greater with apremilast versus placebo at week 16 [15]. At week 32, these decreases in PPPASI total scores were maintained in apremilast-treated patients (− 68.3%), and decreases in the placebo/apremilast group were similar (− 72.1%) (Fig. 1a). Improvements were seen as early as week 2. Decreases in modified PPPASI total score were similar to decreases in PPPASI total score at week 16 (apremilast, − 65.5%; placebo, − 43.6%) and week 32 (apremilast, − 70.1%; placebo/apremilast, − 73.9%) (Fig. 1b). Greater decreases were seen in all PPPASI subscores for 3 signs/findings at week 16 with apremilast versus placebo (erythema, − 67.8% vs. − 46.0%; pustules/vesicles, − 60.1% vs. − 38.3%; and desquamation/scales, − 62.8% vs. − 41.0%), and further decreases were seen at week 32 with apremilast treatment (Fig. 2). At week 32, erythema decreased by 73.2% in the apremilast group and 75.7% in the placebo/apremilast group (Fig. 2a), pustules/vesicles decreased by 62.9% in the apremilast group and 70.8% in the placebo/apremilast group, and desquamation/scales decreased by 66.7% in the apremilast group and 70.1% in the placebo/apremilast group (Fig. 2d). The pustules and vesicles subscores were also evaluated separately in the modified PPPASI. The pustules subscore decreased by 77.0% in the apremilast group and 83.9% in the placebo/apremilast group at week 32 (Fig. 2b). Vesicles decreased by 58.5% in the apremilast group and 53.0% in the placebo/apremilast group at week 32 (Fig. 2c).
The physician’s assessment of PPSI total score evaluated on-site at each visit was 8.3 at baseline in the apremilast group and 8.2 in the placebo group. PPSI total score decreased by 49.0% with apremilast and 30.9% with placebo at week 16 (Fig. 3a). At week 32, PPSI total score decreased from baseline by 51.3% in the apremilast group and 56.7% in the placebo/apremilast group (Fig. 3a). The patient’s assessment of PPSI total score recording worst symptom between each visit was 7.9 at baseline in the apremilast group and 8.0 in the placebo group. Patient’s assessment of PPSI total score decreased by 39.5% with apremilast and 27.7% with placebo at week 16 (Fig. 3b). At week 32, decreases from baseline in patient’s assessment of PPSI total score were 40.0% in the apremilast group and 47.9% in the placebo/apremilast group (Fig. 3b). Decreases were seen in each subscore of the patient’s assessment of each sign/finding of PPP through week 32 (apremilast: 36.6%, 39.8%, and 39.0%; placebo/apremilast: 45.5%, 45.7%, and 46.2% for erythema, pustules/vesicles, and desquamation/scales, respectively; Fig. 4a–c).
We previously reported significantly higher rates of PPPASI-50 response with apremilast versus placebo at week 16 [15]. The overall difference between apremilast and placebo groups was 37.4% (Fig. 5). PPPASI-50 response rates were higher with apremilast than placebo across most baseline demographics and disease characteristics subgroups at week 16, including age, sex, BMI, tobacco use, duration of PPP, PPPASI total score at randomization, presence of focal infection, history of focal infection, and presence of PAO (Fig. 5); however, sample size was limited in some groups.
PPPASI-50 response rates at week 16 by baseline demographic and disease characteristic subgroups. *Data are limited by small sample size. †One case of tonsillitis is included in the presence of focal infection. Oral focal infection such as presence of periapical pathosis, periodontitis, and pericoronitis of wisdom tooth was determined by dental examination by a dentist during screening. Patients who had received any procedure for focal infection within 24 weeks of baseline, had periodontitis and periapical pathosis obviously requiring treatment at screening, or had chronic or recurrent tonsillitis or sinusitis requiring any continuous treatment for a month or more at screening were excluded from the study. Nonresponder imputation. BMI body mass index, CI confidence interval, PAO pustulotic arthro-osteitis, PPP palmoplantar pustulosis, PPPASI palmoplantar pustulosis area and severity index
Discussion
We have previously shown apremilast treatment improved primary and secondary outcomes at week 16, which were maintained at week 32 in patients with PPP [15]. Here we show that improvements in overall disease severity (as measured by PPPASI) as well as individual erythema, pustules, vesicles, and desquamation/scales subscores were maintained or even further improved after 32 weeks of apremilast treatment. In patients who switched from placebo to apremilast at week 16, changes from baseline at week 32 were similar to those in patients continuing on apremilast. Interleukin (IL)-8, IL-17, and IL-22 may be key cytokines driving pustulation and development of erythematous-squamous lesions in PPP [2, 17, 18]. Apremilast has been shown to reduce levels of inflammatory cytokines known to be involved in the pathogenesis of PPP, including IL-8, IL-17, and IL-22 [2, 19,20,21]. Downregulation of these cytokines may be one mechanism by which apremilast improves erythema, pustules, vesicles, and desquamation/scales.
Because PPPASI score has been shown to be directly correlated with quality of life [22], improving PPPASI scores may be considered a key treatment goal. However, there have been two proposed methods of evaluating PPPASI: evaluating pustules and vesicles together or evaluating them separately (modified PPPASI). Given that the development of vesicles followed by pustules on the palms and soles is distinctive pathophysiology in PPP [2], focusing on vesicles and pustules on the palms and soles may be a more sensitive assessment of PPP symptoms and the modified PPPASI may be a more appropriate assessment tool of PPP. In addition, evaluation of pustules and vesicles separately is important based on the mode of action of apremilast, which involves inhibition of migration and activation of neutrophils [20]. We found that changes in PPPASI total score were similar whether pustules and vesicles were grouped together or evaluated separately. Decreases in pustules were greater than decreases in the other subscores.
We evaluated the physician’s assessment of PPSI and the patient’s impression of worst symptoms between each visit to identify whether there are any gaps between the physician and patient perspectives. Because symptoms of PPP can cycle between flares and remission within a short period, the physician’s on-site assessment may not capture an accurate picture of the disease state. The patient-reported outcome of PPSI evaluation for worst status between visits indicated improvement with apremilast for PPSI total score and each subscore. The results were comparable to that of the physician’s assessment of PPSI at the study visit, suggesting no gaps between physician and patient assessment.
Achievement of PPPASI-50 was greater with apremilast than placebo regardless of age or sex. The only group that did not show a greater benefit with apremilast versus placebo was the group with BMI ≥ 30 kg/m2. However, sample size was extremely small (four patients in the apremilast group and seven in the placebo group), limiting conclusions. This will be further explored in a phase 3 study. Smoking has been reported to be associated with higher PPPASI scores [22]. Our analysis of PPPASI-50 rates suggested apremilast showed benefit over placebo regardless of current or past tobacco use, but sample size for the no tobacco use group was very small. Apremilast also showed efficacy regardless of baseline PPPASI score, even in patients with severe (PPPASI ≥ 31) disease, although the 95% CI for the difference between apremilast and placebo contained 0 for this group, which may be due to small sample size. Several reports have suggested a relationship between infectious bacterial foci and PPP [23]. In this study, dental focal infection was confirmed by dental examination at screening and patients were excluded if they had dental focal infection obviously requiring treatment such as periodontitis (treatments for periodontitis include any endodontic treatment for periapical pathosis [chronic periapical periodontitis] such as infected root canal treatment or tooth extraction, and periodontal surgery for moderate to severe periodontitis [chronic marginal periodontitis]) or chronic or recurrent tonsillitis or sinusitis requiring any continuous treatment for a month or more at screening. With these exclusion criteria in place, presence or history of focal infection did not appear to impact the efficacy of apremilast on achieving PPPASI-50 response. Although the difference in response rates favored apremilast, the 95% CI included 0 in the group without presence or history of focal infection. However, there were relatively few patients in this group, limiting the analysis. PAO is a common arthritic manifestation unique to PPP [24]. PPPASI-50 response rates favored apremilast over placebo regardless of the presence of PAO. Taken together, these subgroup analyses suggest patients may benefit from apremilast regardless of their individual traits.
This study is limited by small sample size in some subgroup analyses. Although data are reported as observed, not many patients were lost over time.
Conclusion
Apremilast treatment resulted in at least moderately greater improvements compared with placebo that were sustained over 32 weeks in patients with moderate to severe PPP and inadequate response to topical treatment. Similar results were seen with the PPPASI and modified PPPASI. Apremilast showed efficacy across all PPPASI subscores over 32 weeks, with the strongest effect on the pustules subscore. Improvements were seen in the patient assessment for each sign/finding of PPP using PPSI over 32 weeks and comparable to that of the physician’s assessment. Across all assessments, improvements were seen as early as 2 weeks after starting apremilast. Apremilast showed consistent benefit over placebo at week 16 across baseline demographic and disease characteristic subgroups. Overall, these results suggest apremilast is beneficial for the treatment of patients with PPP.
Data Availability
The datasets generated during and/or analyzed during the current study may be requested at http://www.amgen.com/datasharing.
References
Obeid G, Do G, Kirby L, Hughes C, Sbidian E, Le Cleach L. Interventions for chronic palmoplantar pustulosis. Cochrane Database Syst Rev. 2020;1: CD011628.
Murakami M, Terui T. Palmoplantar pustulosis: current understanding of disease definition and pathomechanism. J Dermatol Sci. 2020;98(1):13–9.
Misiak-Galazka M, Zozula J, Rudnicka L. Palmoplantar pustulosis: recent advances in etiopathogenesis and emerging treatments. Am J Clin Dermatol. 2020;21(3):355–70.
Trattner H, Blüml S, Steiner I, Plut U, Radakovic S, Tanew A. Quality of life and comorbidities in palmoplantar pustulosis—a cross-sectional study on 102 patients. J Eur Acad Dermatol Venereol. 2017;31(10):1681–5.
Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomized, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73(6):1020–6.
Cutolo M, Myerson GE, Fleischmann R, et al. A phase III, randomized, controlled trial of apremilast in patients with psoriatic arthritis: results of the PALACE 2 trial. J Rheumatol. 2016;43(9):1724–34.
Edwards CJ, Blanco FJ, Crowley J, et al. Apremilast, an oral phosphodiesterase 4 inhibitor, in patients with psoriatic arthritis and current skin involvement: a phase III, randomised, controlled trial (PALACE 3). Ann Rheum Dis. 2016;75(6):1065–73.
Wells AF, Edwards CJ, Kivitz AJ, et al. Apremilast monotherapy in DMARD-naive psoriatic arthritis patients: results of the randomized, placebo-controlled PALACE 4 trial. Rheumatology (Oxford). 2018;57(7):1253–63.
Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM 1]). J Am Acad Dermatol. 2015;73(1):37–49.
Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate to severe plaque psoriasis over 52 weeks: a phase III, randomized, controlled trial (ESTEEM 2). Br J Dermatol. 2015;173(6):1387–99.
Stein Gold L, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in systemic- and biologic-naive patients with moderate plaque psoriasis: 52-week results of UNVEIL. J Drugs Dermatol. 2018;17(2):221–8.
Strober B, Bagel J, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate plaque psoriasis (UNVEIL phase IV study) [poster 4892]. Presented at: Annual Meeting of the American Academy of Dermatology, Orlando, FL, March 3–7, 2017.
Stein Gold L, Papp K, Leonardi C, et al. Efficacy and safety of apremilast in patients with mild to moderate plaque psoriasis: results of a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2022;86(1):77–85.
Mrowietz U, Barker J, Conrad C, et al. Efficacy and safety of apremilast in patients with limited skin involvement, plaque psoriasis in special areas, and impaired quality of life: results from the EMBRACE randomized trial. J Eur Acad Dermatol Venereol. 2023;37(2):348–55.
Terui T, Okubo Y, Kobayashi S, et al. Efficacy and safety of apremilast for the treatment of Japanese patients with palmoplantar pustulosis: results from a phase 2, randomized, placebo-controlled study. Am J Clin Dermatol. 2023;24(5):837–47.
Yamamoto T. Extra-palmoplantar lesions associated with palmoplantar pustulosis. J Eur Acad Dermatol Venereol. 2009;23(11):1227–32.
Skov L, Beurskens FJ, Zachariae CO, et al. IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplantar pustulosis. J Immunol. 2008;181(1):669–79.
Hagforsen E, Hedstrand H, Nyberg F, Michaëlsson G. Novel findings of Langerhans cells and interleukin-17 expression in relation to the acrosyringium and pustule in palmoplantar pustulosis. Br J Dermatol. 2010;163(3):572–9.
Schafer PH, Chen P, Fang L, Wang A, Chopra R. The pharmacodynamic impact of apremilast, an oral phosphodiesterase 4 inhibitor, on circulating levels of inflammatory biomarkers in patients with psoriatic arthritis: substudy results from a phase III, randomized, placebo-controlled trial (PALACE 1). J Immunol Res. 2015. https://doi.org/10.1155/2015/906349.
Pincelli C, Schafer PH, French LE, Augustin M, Krueger JG. Mechanisms underlying the clinical effects of apremilast for psoriasis. J Drugs Dermatol. 2018;17(8):835–40.
Murakami M, Hagforsen E, Morhenn V, Ishida-Yamamoto A, Iizuka H. Patients with palmoplantar pustulosis have increased IL-17 and IL-22 levels both in the lesion and serum. Exp Dermatol. 2011;20(10):845–7.
Sarıkaya Solak S, Kara Polat A, Kilic S, et al. Clinical characteristics, quality of life and risk factors for severity in palmoplantar pustulosis: a cross-sectional, multicentre study of 263 patients. Clin Exp Dermatol. 2022;47(1):63–71.
Putra-Szczepaniak M, Maj J, Jankowska-Konsur A, Czarnecka A, Hryncewicz-Gwóźdź A. Palmoplantar pustulosis: factors causing and influencing the course of the disease. Adv Clin Exp Med. 2020;29(1):157–63.
Sonozaki H, Mitsui H, Miyanaga Y, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis. 1981;40(6):547–53.
Acknowledgements
We thank the participants of the study.
Medical Writing/Editorial Assistance
Writing support was funded by Amgen Inc. and provided by Rebecca Lane, PhD, of Peloton Advantage, LLC, an OPEN Health company.
Funding
This study was sponsored by Amgen Inc. Amgen will pay the Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Conceptualization: Tadashi Terui, Yukari Okubo, Masatomo Murakami, Satomi Kobayashi, Masafumi Yaguchi, Takeshi Kimura, Wendy Zhang. Methodology: Tadashi Terui, Yukari Okubo, Masatomo Murakami, Satomi Kobayashi, Masafumi Yaguchi, Takeshi Kimura, Wendy Zhang. Formal analysis and investigation: all authors. Writing—original draft preparation: Tadashi Terui, Masafumi Yaguchi, Takeshi Kimura, Wendy Zhang, Junichiro Shimauchi. Writing—review and editing: all authors. Supervision: Tadashi Terui, Masafumi Yaguchi, Takeshi Kimura, Wendy Zhang.
Corresponding author
Ethics declarations
Conflict of Interest
Yukari Okubo has received grants or contracts from AbbVie, Eisai, Jimro, Maruho, Shiseido, Sun Pharma, and Torii; consulting fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, LEO Pharma, Maruho, Pfizer, Sun Pharma, and UCB Pharma; and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eisai, Eli Lilly, Janssen Pharma, JIMRO, Kyowa Kirin, LEO Pharma, Maruho, Novartis Pharma, Pfizer, Sanofi, Sun Pharma, Taiho, Tanabe-Mitsubishi, Torii and UCB Pharma. Tadashi Terui has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Eisai, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Novartis, and Taiho Pharmaceutical. Satomi Kobayashi has received research grants from Kyowa Kirin; received honoraria from Janssen Pharma and Taiho Pharmaceutical. Shigetoshi Sano has received research grants from Kaken, Maruho, Nihon, Nippon Zoki, Sanofi, Taiho, and Torii; and honoraria from AbbVie, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, Maruho, Sun Pharma, Taiho, and UCB. Akimichi Morita has received research grants, consulting fees, and/or speaker’s fees from AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Eisai, Janssen, Kyowa Hakko Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, Ushio, and UCB Pharma. Shinichi Imafuku has received grants, consulting fees and/or speaker's fees from AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly, GSK, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Novartis, Pfizer, Sun Pharma, Taiho Pharmaceutical, Tanabe-Mitsubishi, Torii Pharmaceutical, Toyo seiyakukasei, and UCB Japan. Yayoi Tada has received honoraria and/or grants from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb KK, Amgen, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Novartis Pharma, Sun Pharma, Taiho Pharmaceutical, Torii Pharmaceutical, and UCB Pharma. Masatoshi Abe has received research grants, consulting fees, speaker fees, and/or participated in clinical trials for Celgene and Maruho. Masafumi Yaguchi, Takeshi Kimura, and Junichiro Shimauchi are employees of Amgen K.K. Wendy Zhang and Hamid Amouzadeh are employees and stockholders of Amgen Inc. Masamoto Murakami has received research grants from AbbVie, ARISTEA Therapeutics, Eisai, Eli Lilly, Kyowa Kirin, and Novartis Pharma; honoraria from AbbVie, Amgen Inc., Boehringer Ingelheim, Celgene, Eisai, Eli Lilly, Janssen Pharma, Kyowa Kirin, Maruho, Novartis Pharma, Taiho Pharmaceutical, and Torii Pharmaceutical; and participated in clinical trials for AbbVie, Amgen Inc., Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen Pharma, Maruho, and Novartis Pharma.
Ethical Approval
This study was conducted in accordance with International Council for Harmonization E6 and the ethical principals that are outlined in the Declaration of Helsinki. The study protocol and all amendments, the informed consent form, and any accompanying materials provided to the patients were reviewed and approved by an institutional review board or independent ethics committee at each study center (Online Resource 1). Patients provided written informed consent prior to study procedures.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Okubo, Y., Terui, T., Kobayashi, S. et al. Exploratory Efficacy Evaluation of Apremilast for the Treatment of Japanese Patients with Palmoplantar Pustulosis: 32-Week Results from a Phase 2, Randomized, Placebo-Controlled Study. Dermatol Ther (Heidelb) 14, 1863–1873 (2024). https://doi.org/10.1007/s13555-024-01195-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-024-01195-z