Abstract
Introduction
Patients with psoriasis (PsO) are at increased risk of herpes zoster (HZ), but recent data on the incidence of HZ among patients with PsO and the impact of HZ on healthcare resource use (HRU) and costs for patients with PsO have not been described.
Methods
This retrospective, longitudinal, cohort study estimated HZ incidence in cohorts of adults with vs without PsO (PsO + vs PsO–) and HRU and costs among those with PsO, with vs without HZ (PsO + /HZ + vs PsO + /HZ–) using Optum’s de-identified Clinformatics Data Mart Database during 2015–2020. Patients with psoriatic arthritis were excluded from all four cohorts. Comparisons between cohorts used generalized linear models to adjust outcomes based on various baseline characteristics.
Results
The incidence rate of HZ was significantly higher in the PsO + (n = 144,115) vs PsO– (n = 23,837,237) cohorts at 11.35 vs 7.67 per 1000 patient-years; adjusted incidence rate ratio (aIRR): 1.21, 95% confidence interval (CI): 1.16–1.25. HRU (outpatient, emergency department, and inpatient) was significantly higher in the PsO + /HZ + (n = 1859) vs PsO + /HZ– (n = 78,664) cohorts during 1 month and 3 months after HZ diagnosis (e.g., outpatient visits during month: 2.83 vs 1.30 per patient; aIRR: 1.96; 95% CI 1.86–2.06). Mean all-cause costs were also significantly higher in the PsO + /HZ + vs PsO + /HZ– cohort during both month ($5020 vs $2715 per patient; adjusted cost difference: $1390; 95% CI $842–$1964) and 3 months ($12,305 vs $8256; adjusted cost difference: $1422; 95% CI $280–$2889) after HZ diagnosis.
Conclusion
These findings show the increased incidence of HZ among patients with PsO and the clinical and economic burdens of HZ in this population. Considering the high prevalence of PsO, insights into the impact of HZ in these patients provide valuable evidence to inform clinical decision-making.
Graphical abstract

Plain Language Summary
Psoriasis is an inflammatory condition that causes flaky, scaly skin. Herpes zoster (shingles) causes a painful rash, usually on the abdomen. However, recent data on the proportion of patients with psoriasis who develop herpes zoster is lacking. Furthermore, little is known about the healthcare resources that are used or the costs of care for patients with psoriasis who develop herpes zoster. We found that patients with psoriasis were 21% more likely to have herpes zoster than patients without psoriasis. Among patients with psoriasis, those who developed herpes zoster had twice as many doctor’s visits, 3 times as many emergency department visits, and twice as many inpatient hospital stays during the month after a herpes zoster diagnosis as patients without herpes zoster. This resulted in an additional cost of $1390 per patient with psoriasis and herpes zoster compared with those with psoriasis but without herpes zoster. Overall, patients with psoriasis are at increased risk of developing herpes zoster and the healthcare resource use and associated cost of treating herpes zoster in patients with psoriasis is substantial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with psoriasis (PsO) are at increased risk of herpes zoster (HZ), but the recent incidence of HZ in patients with PsO and the healthcare resource use (HRU) and costs in patients with both conditions are unknown. |
What was learned from the study? |
The incidence rate of HZ was higher in patients with vs without PsO (11.35 vs 7.67 per 1000 patient-years; adjusted incidence rate ratio [aIRR]: 1.21, 95% confidence interval [CI] 1.16–1.25). |
HRU was significantly higher in patients with PsO and HZ (PsO + /HZ +) vs PsO without HZ (PsO + /HZ–) during 1 and 3 months after HZ (e.g., outpatient visits during month: 2.83 vs 1.30 per patient; aIRR: 1.96; 95% CI 1.86–2.06). |
Mean all-cause costs were significantly higher in the PsO + /HZ + vs PsO + /HZ– cohort during month ($5020 vs $2715 per patient; adjusted cost difference: $1390; 95% CI $842–$1964) and 3 months ($12,305 vs $8256; adjusted cost difference: $1422; 95% CI $280–$2889) after HZ. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.23684547.
Introduction
The prevalence of psoriasis (PsO) — a chronic, inflammatory, immune-mediated skin disease [1] — among people aged ≥ 20 years in the United States (US) has been estimated to be 3%, resulting in over 7 million affected adults [2]. Treatment options for patients with mild/moderate PsO are generally topical, such as corticosteroids and calcineurin inhibitors [3]. If these are insufficient, options include phototherapy [4], systemic immunosuppressants (e.g., methotrexate and cyclosporine) [5], and biologics (e.g., tumor necrosis factor [TNF]-alpha inhibitors and interleukin inhibitors) [6]. Since many of these treatments target the immune system, patients are at increased risk of infection [5,6,7], including herpes zoster (HZ). Various studies have reported that patients with PsO are at increased risk of HZ [8,9,10,11], and that this risk varies by medication class [12,13,14,15,16].
HZ is a disease that occurs when varicella zoster virus reactivates after remaining latent in the sensory ganglia following a varicella (chickenpox) infection [17]. HZ is characterized by a painful blistering skin rash, and patients may subsequently develop complications such as postherpetic neuralgia or HZ ophthalmicus [17]. HZ and its complications can result in considerable quality of life impairment [18] and substantial healthcare resource use (HRU) and costs [19]. In the US, approximately 99.5% of people born before 1980 have been infected with varicella zoster virus and are therefore at risk of developing HZ; around 1/3 will experience HZ over the course of their lifetime [20]. The risk of HZ increases with age, from around 5 cases per 1000 people aged 50–54 years to around 11 cases per 1000 people aged ≥ 85 years [21]. The risk of HZ also increases in patients with immunosuppressive conditions/treatments, including cancer, human immunodeficiency virus (HIV), hematopoietic stem cell transplantation, oral corticosteroids, methotrexate, and cyclosporine [22]; and various autoimmune and chronic diseases, including rheumatoid arthritis [23], inflammatory bowel disease [24], chronic obstructive pulmonary disease (COPD) [25], and PsO [8,9,10,11].
In the US, the Advisory Committee on Immunization Practices first recommended HZ vaccination for adults aged ≥ 60 years in June 2008, at which time only live zoster vaccine was available [26]. Since January 2018, recombinant zoster vaccine has been recommended for immunocompetent adults aged ≥ 50 years [27]. In January 2022, an additional recommendation was published for the use of recombinant zoster vaccine for the prevention of HZ in immunodeficient/immunosuppressed adults aged ≥ 19 years [28]. This includes hematopoietic cell transplantation or solid organ transplant recipients and patients with cancer, HIV, or autoimmune/inflammatory conditions/treatments [29], which may include patients with PsO on immunosuppressive treatments.
As previous studies have reported a 1.1–1.7-fold increased risk of HZ among patients with PsO [8,9,10,11], but the HRU and costs of treating HZ among patients with PsO are unknown, the objectives of the current study were to estimate: (1) the recent incidence of HZ among adults in the US with vs without PsO (PsO + vs PsO– cohorts) and (2) the burden of HZ in terms of additional HRU and costs among patients with PsO (PsO + /HZ + vs PsO + /HZ– cohorts). For patients in the PsO + /HZ + cohort, HZ-related HRU and medical costs are also reported.
Methods
This retrospective, longitudinal, cohort study (GSK study identifier: VEO-000258) estimated HZ incidence among patients with vs without PsO (PsO + vs PsO– cohorts). Also, HRU and costs among patients with PsO with vs without HZ (PsO + /HZ + vs PsO + /HZ– cohorts) were estimated. Patients with psoriatic arthritis (PsA) were excluded from all four cohorts due to previous evidence demonstrating higher HRU and costs in this population [30, 31], which would make the populations more heterogeneous in terms of HRU and costs.
Data Source
Optum’s de-identified Clinformatics Data Mart Database (CDM) was used for this study. This database contains administrative claims data for individuals with commercial and Medicare Advantage health plans. The database includes medical and pharmacy claims, member eligibility, and inpatient admissions from a geographically diverse population. The claims data also include standard pricing for all medical and pharmacy claims and inpatient admissions. Further details can be found in Text S1 in the Supplementary Materials.
The study complied with all applicable patient privacy laws and there was no direct patient contact or primary collection of individual subject data. As the results are aggregate analyses without any patient identification, ethics committee approval, institutional review board approval, and informed consent were not required.
Variables
The following variables were retrieved or derived: demographic characteristics such as length of observation, age, sex, race and ethnicity, geographic location, insurance type; clinical characteristics such as number of PsO diagnoses before index, Charlson comorbidity index (CCI) and component conditions, comorbidities potentially associated with HZ, additional immunosuppression conditions, PsO-related medications, and all-cause direct costs.
HZ Incidence Assessment Inclusion Criteria
Patients in the database during October 1, 2015 through February 28, 2020 were divided into those with ≥ 1 claim associated with a diagnosis of PsO (but no diagnosis of PsA) and those with no claim associated with a diagnosis of PsO or PsA (see Table S1 for International Classification of Diseases, 10th Revision, Clinical Modification [ICD-10-CM] codes). Patients included in the PsO + cohort additionally had a confirmed diagnosis of PsO, defined as ≥ 2 medical claims ≥ 30 days apart, with a diagnosis of PsO.
Inclusion criteria for both the PsO + and PsO– cohorts included: ≥ 6 months of continuous enrolment before the index date, which was defined as the later of the date of first PsO diagnosis or 6 months after the start of continuous enrolment for the PsO + cohort, and after 6 months of continuous enrollment for the PsO– cohort; no HZ diagnosis, including HZ complications, before or on the index date (see Table S1 for ICD-10-CM codes); no HZ vaccine before or on the index date; and age ≥ 18 years on the index date.
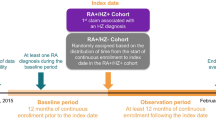
For the portion of the study that assessed HZ incidence, baseline was the 6 months before index, and no minimum follow-up time was required (Fig. 1a).
Study designs for the assessment of (a) HZ incidence (PsO + and PsO– cohorts) and (b) HRU and costs (PsO + /HZ + and PsO + /HZ– cohorts). aRandomly assigned based on the distribution of the pre-index eligibility (i.e., time between beginning of continuous enrolment and index date) in the PsO + /HZ + cohort. bPatients had to have ≥ 1 claim with a PsO diagnosis during baseline. HRU healthcare resource use, HZ herpes zoster, PsO psoriasis
HZ Incidence Outcomes
Both unadjusted incidence rates of HZ and adjusted incidence rate ratios (aIRRs) are reported for the overall population and by patient age categories at index: 18–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years. In the PsO + cohort, unadjusted HZ incidence rates are reported by PsO therapy type (phototherapy, systemic biologic therapies [IL-17, -23, and -12/23 inhibitors, TNF-alpha inhibitors, and T-cell inhibitors], systemic non-biologics, Janus kinase [JAK] inhibitors, and topical/systemic corticosteroid/no therapy/other) used prior to incident HZ, HZ vaccination, or end of data availability.
HZ Incidence Statistical Analyses
Various demographics (at index), clinical characteristics, and healthcare costs (during 6-month baseline) are reported in the PsO + and PsO– cohorts. Comparisons of these measures were conducted using standardized differences. Thresholds of standardized differences of 20%, 50%, and 80% were assumed to represent small, medium, and large differences, respectively [32].
Multivariable comparative analyses were performed using doubly robust propensity score adjustment to account for differences between the cohorts. The propensity score was calculated using patients’ demographic and clinical characteristics, the selection of which depended on the cohorts and objective. Covariates with no variation between cohorts were excluded. The propensity score was then used in multivariable models that regressed the outcome on the propensity score, cohort status, and covariates that were clinically relevant or imbalanced between the cohorts, following on a doubly robust estimation approach.
Unadjusted incidence rates of HZ were estimated by multiplying the number of HZ cases by 1000 and dividing by the total patient-years (PY) of observation from the index date to an incident HZ (event), HZ vaccination (censor), or end of data availability, i.e., end of continuous enrolment or end of the study period (censor). Incidence rates are reported per 1000 PY. aIRRs with 95% confidence intervals (CIs) were calculated using generalized linear models (GLMs) assuming a Poisson distribution and log link. Statistical significance was assumed for aIRRs when the 95% CIs did not include 1. The propensity score of being diagnosed with PsO and relevant baseline characteristics were accounted for. When over-dispersion was observed, negative binomial regression models were used.
HRU and Cost Assessment Inclusion Criteria
Patients with ≥ 1 claim associated with a diagnosis of PsO (but no diagnosis of PsA) were divided into those with and without ≥ 1 claim with a diagnosis of HZ. Inclusion criteria for the PsO + /HZ + and PsO + /HZ– cohorts were: a confirmed diagnosis of PsO; continuous eligibility for ≥ 12 months before and ≥ 1 month after the index date; no claim for an HZ vaccine before or on the index date; and age ≥ 18 years on the index date. A confirmed diagnosis of PsO was defined as ≥ 2 medical claims ≥ 30 days apart associated with a diagnosis of PsO between the beginning and end of continuous enrolment (including ≥ 1 during the 12-month baseline period). The index date was defined as the first HZ diagnosis during October 1, 2016 through January 28, 2020 in the PsO + /HZ + cohort or was randomly assigned based on the distribution of the pre-index eligibility in the PsO + /HZ + cohort for the PsO + /HZ– cohort. Patients in the PsO + /HZ + cohort had to have a first HZ diagnosis during October 1, 2016 through January 28, 2020, and the first observed HZ diagnosis could not be for HZ with other nervous system involvement (ICD-10-CM: B02.2).
For the portion of the study that assessed HRU and costs, baseline was the 12 months before index and the minimum follow-up time was 1 month (Fig. 1b).
HRU and Cost Outcomes
HRU outcomes include: (1) all-cause HRU (outpatient visits, emergency department [ED] visits, and inpatient admissions) in the PsO + /HZ + vs PsO + /HZ– cohorts; (2) all-cause costs (medical [outpatient visits, ED visits, inpatient admissions, and other] and pharmacy) in the PsO + /HZ + vs PsO + /HZ– cohorts; and (3) HZ-related HRU and medical costs in the PsO + /HZ + cohort, which were identified using claims with an HZ diagnosis in any position; these were only available for medical claims, not pharmacy claims. HRU and cost outcomes are reported during 1, 3, and 12 months after the index date among patients with ≥ 1, ≥ 3, and ≥ 12 months of observation, respectively. All-cause HRU and costs at 1 and 3 months are also reported among patients with ≥ 12 months of observation.
Medications administered in a medical setting during a medical encounter and coded with a procedure code were considered a medical cost.
HRU and Cost Statistical Analyses
Various demographics (at index), clinical characteristics, and healthcare costs (during 12-month baseline) are reported in the PsO + /HZ + and PsO + /HZ– cohorts. Comparisons of these cohorts’ baseline characteristics were conducted using standardized differences.
Propensity score adjustment was carried out as detailed in the HZ Incidence Statistical Analyses Section.
HRU outcomes are reported as the mean number of each encounter type per patient during 1, 3, or 12 months after the index date and were compared using aIRRs with 95% CIs. aIRRs were calculated using GLMs assuming a negative binomial distribution and log link. The propensity score of being in the PsO + /HZ + cohort and relevant baseline characteristics were accounted for.
Costs are reported as the mean total, medical, and pharmacy costs per patient during 1, 3, and 12 months following the index date. A two-part modeling approach was used to estimate adjusted cost differences. Firstly, logistic regression was used to model the probability of a positive cost. Secondly, a GLM with a gamma distribution and log link was used to predict costs among patients who had positive costs. Both of the models included the patients’ propensity scores and relevant baseline characteristics. The GLM adjusted for a propensity score based on baseline characteristics in a doubly robust approach similar to the HRU model described above. The 95% CIs were estimated from nonparametric bootstrap procedures with 499 replications. Statistical significance was assumed for adjusted cost differences when the 95% CIs did not include 0. All costs were adjusted to 2021 US dollars (USD) using the medical care component of the Consumer Price Index.
All statistical analyses were conducted using the statistical software SAS Enterprise Guide 7.1 and SAS Studio (SAS Institute Inc., Cary, North Carolina, US).
Results
Higher HZ Incidence Among Patients with PsO
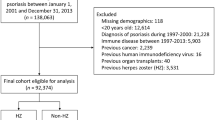
Among 38,113,848 patients identified in the CDM from October 1, 2015 through February 28, 2020, 144,115 were included in the PsO + cohort and 23,837,237 in the PsO– cohort (Fig. S1). Patients in the PsO + vs PsO– cohort were older (mean age 57.2 vs 49.7 years), more frequently White (73.0% vs 62.8%), more often had Medicare Advantage (41.8% vs 28.1%), had a higher comorbidity burden (mean CCI 0.6 vs 0.4), had more comorbidities potentially associated with HZ (7.8% vs 4.6%), more frequently used systemic PsO-related treatments or phototherapy (27.4% vs 11.5%), and had higher mean all-cause healthcare costs during baseline ($12,171 vs $6102) (Table 1). The mean lengths of the observation periods in the PsO + and PsO– cohorts were 24.1 ± 14.1 and 21.7 ± 16.0 months.
There were 3105 HZ events during 273,683 PY of follow-up in the PsO + cohort and 318,864 HZ events during 41,557,883 PY in the PsO– cohort. The unadjusted incidence rate of HZ was higher in the PsO + vs PsO– cohorts, overall (11.35 vs 7.67 per 1000 PY) and in each age group (Fig. 2). After adjustment for baseline covariates, the incidence rate of HZ was significantly higher in the PsO + vs PsO– cohorts, overall (aIRR: 1.21; 95% CI 1.16–1.25) and in each age group. The incidence rate of HZ increased with age, from 3.51 to 17.35 per 1000 PY for those aged 18–29 to ≥ 80 years, respectively, in the PsO + cohort, and from 2.13 to 13.95 per 1000 PY, respectively, in the PsO– cohort, but the aIRRs tended to decrease with increasing age. The incidence rate of HZ varied widely by PsO therapy, from 3.08 per 1000 PY among 2376 patients who received IL-23 inhibitors (aIRR: 0.44, 95% CI 0.26–0.75 vs the PsO– cohort) to 18.29 per 1000 PY (aIRR: 2.01, 95% CI 1.45–2.80) among 1229 on phototherapy (Fig. S2).
Unadjusted incidence rates and aIRRs of HZ in the PsO + vs PsO– cohorts, overall and by age at index. aIncidence rates were calculated by dividing the number of incident HZ events during the observation period by the patient-time observed. Patients were followed until HZ diagnosis or censored at the first of: receipt of HZ vaccine or end of data availability. The PsO + cohort included 144,115 patients (10,188, 16,817, 20,505, 26,961, 32,864, 26,220, 10,560 for ages 18–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years, respectively); the PsO– cohort included 23,837,237 patients (4,487,143, 4,083,218, 3,658,704, 3,704,033, 3,901,319, 2,587,543, 1,415,277 for ages 18–29, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years, respectively). The mean ± SD observation period in the PsO + cohort was 22.8 ± 13.9 months (19.4 ± 12.9, 20.8 ± 13.6, 22.3 ± 14.0, 22.2 ± 13.9, 22.3 ± 13.7, 25.9 ± 14.0, 25.5 ± 13.8 months across age groups); in the PsO– cohort 20.9 ± 15.7 months (16.7 ± 14.0, 18.2 ± 14.8, 19.9 ± 15.5, 20.7 ± 15.6, 21.9 ± 15.5, 29.3 ± 15.9, 27.5 ± 15.9 months across age groups). baIRRs were calculated using the PROC GENMOD procedure for GLMs assuming a Poisson distribution and log link, accounting for the propensity score of being diagnosed with PsO and relevant baseline characteristics. The GLM for the overall cohort adjusted for a propensity score based on the following baseline characteristics: index year, age at index, sex, race and ethnicity, region, insurance type, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the following characteristics (doubly robust adjustment): index year, age at index, race and ethnicity, insurance type, CCI, use of specific PsO treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs. For the age stratifications, age at index was excluded from the propensity score and doubly robust adjustment by design. aIRR adjusted incidence rate ratio, CCI Charlson comorbidity index, CI confidence interval, ED emergency department, GLM generalized linear model, HZ herpes zoster, PsO psoriasis, PY patient-years, SD standard deviation
Elevated HRU and Costs Among Patients with PsO and HZ
For the HRU and cost analyses, 1859 patients were included in the PsO + /HZ + cohort and 78,664 in the PsO + /HZ– cohort (Fig. S3). Patients in the PsO + /HZ + vs PsO + /HZ– cohort were older (mean age 65.3 vs 58.3 years), more often had Medicare Advantage (58.0% vs 42.0%), had a higher comorbidity burden (mean CCI 1.3 vs 0.9), more frequently had comorbidities potentially associated with HZ (15.3% vs 11.5%), and had higher mean all-cause healthcare costs during baseline ($39,815 vs $30,069) (Table 1). There were 1709 PsO + /HZ + patients and 75,446 PsO + /HZ– patients with ≥ 3 months of follow-up; and 1081 and 32,963, respectively, with ≥ 12 months of follow-up.
The unadjusted numbers of each HRU category (outpatient visits, ED visits, and inpatient admissions) were higher in the PsO + /HZ + vs PsO + /HZ– cohorts for all three outcomes at all three time points, e.g., during month: 2.83 vs 1.30 outpatient visits per patient; 0.17 vs 0.06 ED visits per patient; 0.04 vs 0.01 inpatient admissions per patient (Fig. 3). After accounting for baseline covariates, all three HRU categories were significantly higher in the PsO + /HZ + cohort during 1 and 3 months, e.g., during month: outpatient visits: aIRR: 1.96, 95% CI 1.86–2.06; ED visits: aIRR: 3.18, 95% CI 2.58–3.93; inpatient admissions: aIRR: 2.19, 95% CI 1.69–2.85. Outpatient and ED visits during 12 months were also significantly higher in the PsO + /HZ + cohort. When the analysis was restricted to patients with ≥ 12 months of follow-up, the results were similar (Fig. S4).
HRU in the PsO + /HZ + vs PsO + /HZ– cohorts during the 1, 3, and 12 months after indexa. aFor the 1 month after index, data from all patients (1859 PsO + /HZ + and 78,664 PsO + /HZ–) were used; for the 3 months after index, 1709 PsO + /HZ + and 75,446 PsO + /HZ– with ≥ 3-month follow-up; for the 12 months after index, 1,081 PsO + /HZ + and 32,963 PsO + /HZ– with ≥ 12-month follow-up. bHRU encounters were calculated as the mean number of HRU events per patient during the period of interest. caIRRs were calculated using the PROC GENMOD procedure for GLMs assuming a negative binomial distribution and log link, accounting for the propensity score of being in the PsO + /HZ + cohort (for the PsO + /HZ– cohort) and relevant baseline characteristics. The GLM was adjusted for a propensity score based on the following baseline characteristics: index year, age at index, sex, race and ethnicity, region, insurance type, having only one PsO diagnosis prior to index, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the following characteristics (doubly robust adjustment): index year, age at index, CCI, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs. aIRR adjusted incidence rate ratio, CCI Charlson comorbidity index, CI confidence interval, ED emergency department, GLM generalized linear model, HRU healthcare resource use, HZ herpes zoster, PsO psoriasis
Mean all-cause costs during month after the index date were higher in the PsO + /HZ + vs PsO + /HZ– cohort ($5020 vs $2715 per patient; adjusted cost difference: $1390; 95% CI $842–$1964; Fig. 4a). This difference was driven by inpatient admission costs (adjusted cost difference: $760; 95% CI $438–$1066), but the adjusted cost differences in outpatient and ED visits were also significant ($263; 95% CI $164–$385 and $204; 95% CI $150–$261, respectively). During the 3 months after the index date, costs remained higher in the PsO + /HZ + vs PsO + /HZ– cohort ($12,305 vs $8256; adjusted cost difference: $1422; 95% CI $280–$2889; Fig. 4b). This difference was driven by inpatient admission costs (adjusted cost difference: $865; 95% CI $227–$2140), but the adjusted cost differences in outpatient and ED visits were also significant ($368; 95% CI $130–$606 and $193; 95% CI $113–$287, respectively). By 12 months after index, per-patient mean costs were still numerically higher in the PsO + /HZ + cohort, but the adjusted cost difference was only $17 with high uncertainty around the point estimate (95% CI − $3535 to $4313) (Fig. 4c). Cost results for the subset of patients with ≥ 12 months of follow-up are shown in Fig. S5.
All-cause costs in the PsO + /HZ + vs PsO + /HZ– cohorts during a 1 month, b 3 months, and c 12 months after index. aCost differences were estimated using the two-part modeling approach: (1) the probability of observing a positive cost was modeled using logistic regression; (2) a GLM with a gamma distribution and log link was used to predict costs among patients with positive costs. Both models included the patients’ propensity scores and relevant baseline characteristics. The 95% CIs were estimated from nonparametric bootstrap procedures with 499 replications. The GLM adjusted for a propensity score based on the following baseline characteristics: index year, age at index, sex, race and ethnicity, region, insurance type, having only one PsO diagnosis prior to index, CCI, any comorbidity associated with HZ, additional immunosuppressive conditions, use of specific PsO treatment type, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs, as well as the following characteristics (doubly robust adjustment): index year, age at index, insurance type, CCI, inpatient costs, outpatient costs, ED costs, other medical costs, and pharmacy costs. bIncluding skilled nursing facilities, home care services, hospice, vision care, and durable medical equipment. CCI Charlson comorbidity index, CI confidence interval, ED emergency department, GLM generalized linear model, HZ herpes zoster, PsO psoriasis, SD standard deviation, USD United States dollars
Mean HZ-related medical costs were $1222, $1517, and $1822 during 1, 3, and 12 months after index, respectively, in the PsO + /HZ + cohort (Fig. 5). Costs were driven by inpatient admission costs, but most HZ-related use was in the outpatient setting with 1.38–1.89 HZ-related visits per patient across the three time periods (compared with 0.11–0.12 ED visits; and 0.03–0.04 inpatient admissions; data not shown).
HZ-related medical costs in the PsO + /HZ + cohort during 1, 3, and 12 months after index. aIncluding skilled nursing facilities, home care services, hospice, vision care, and durable medical equipment. Values are 6, 8, and 13 at 1, 3, and 12 months, respectively. ED emergency department, HZ herpes zoster, PsO psoriasis, SD standard deviation, USD United States dollars
Discussion
This retrospective claims-based study estimated that the incidence of HZ was significantly higher in patients with PsO than in patients without PsO (11.35 vs 7.67 per 1000 PY; aIRR: 1.21; 95% CI 1.16–1.25). HRU and costs were also significantly higher among patients with PsO and HZ vs those with PsO but no HZ (e.g., means of $5020 vs $2715 during month after index; adjusted cost difference: $1390; 95% CI $842–$1964).
HZ Incidence
Our finding that patients in the PsO + cohort had a higher incidence of HZ than those in the PsO– cohort (aIRR: 1.21; 95% CI 1.16–1.25) is in line with a previous US study (8.03 vs 4.82 cases per 1000 PY) [11] and various studies outside the US: adjusted hazard ratio [aHR]: 1.08; 95% CI 1.05–1.11 [10], aHR: 1.22; 95% CI 1.12–1.33 [8], and aHR: 1.29; 95% CI 1.07–1.56 [9]. However, another study reported no increased risk of HZ among patients with PsO vs immunocompetent individuals [33]. Interestingly, one of the studies reported similar HZ risk for patients with mild and severe PsO (aHR: 1.23 and 1.19, respectively) [8], while two reported higher aHRs with more severe PsO (1.61 [severe] vs 1.20 [mild] [9]; 1.17 [moderate-to-severe] vs 1.07 [mild] [10]).
We did not study HZ incidence by PsO severity but did find differences in HZ incidence in the PsO + cohort by PsO therapy (Fig. S2), which may be used as a proxy for disease severity. These data should be interpreted with caution due to confounding factors, small patient counts in some groups, and because groupings were only based on the medications used at the end of follow-up. Patients on interleukin inhibitors had lower or similar HZ incidences to the PsO– cohort, while those on any other therapy had higher HZ incidences than the PsO– cohort. Other studies have reported that HZ risk is increased in patients taking systemic corticosteroids [13, 15, 16], TNF-alpha inhibitors [12, 16], JAK inhibitors [15, 16], and various combination treatments [13,14,15,16], but not in those on interleukin inhibitors [13, 15, 16], acitretin [16], or phototherapy [14, 16].
Lastly, the incidence rates of HZ increased with age in the PsO + and PsO– cohorts, which is in line with other studies of patients with PsO [8, 9, 11] and the general population [21]. In a US database study from 2005 to 2009, the incidence of HZ among patients with PsO increased from 5.3 per 1000 PY among patients aged 18–49 years to 15.4 per 1000 PY among those aged ≥ 65 years [11]. In another study outside the US, HZ incidence increased from 5.5 per 1000 PY among patients aged 20–39 years to 20.4 per 1000 PY among those aged ≥ 60 years [8]. Similarly, in another non-US study, the incidence of HZ increased from 1.4 per 1000 PY among patients aged 20–39 years to 9.3 per 1000 PY among those aged ≥ 60 years [9]. This indicates that older patients with PsO have a greater HZ disease burden. Interestingly, the aIRR tended to decrease with increasing age. As the background risk of HZ increases with age, we may conclude that PsO may increase the risk of HZ more meaningfully among younger adults.
HRU and Cost
Patients in the PsO + /HZ + vs PsO + /HZ– cohort had significantly higher HRU across all visit types (outpatient visits, ED visits, and inpatient admissions) during 1 and 3 months after index (Fig. 3). This resulted in significant adjusted cost differences during 1 and 3 months ($1390; 95% CI $842–$1964 and $1422; 95% CI $280–$2889, respectively; Fig. 4). By 12 months, the adjusted cost difference had fallen to $17, although there was a high degree of uncertainty (95% CI − $3535 to $4313). This high uncertainty in the estimated cost difference may be due to the high variability in costs within cohorts and the relatively modest sample size of the PsO + /HZ + cohort with ≥ 12 months of follow-up. HZ-related costs were high during month after index ($1222). Among patients with longer follow-up, mean HZ-related costs during 3 and 12 months were $1517 and $1822, respectively. These results are in line with HZ being an acute disease, with most of the cost occurring during month, although patients who went on to develop complications such as postherpetic neuralgia or HZ ophthalmicus may have required longer-term medical care. Of note, patients in the PsO + /HZ + cohort had higher mean baseline medical costs than those in the PsO + /HZ– cohort ($39,815 vs $30,069), which could imply that higher healthcare utilizers were more likely to be diagnosed with HZ.
To our knowledge, no other studies on HRU and costs of HZ among patients with PsO have been published. However, two US studies have reported annual mean all-cause healthcare costs of $10,965 (2014 USD) [31] and $11,062 (2019 USD) [30] per patient with PsO but without PsA. Others have reported annual mean all-cause healthcare costs of $13,731 (2012 USD) [34] and $21,481 (2014 USD) [31] per patient with moderate-to-severe PsO. The mean annual costs in our PsO + /HZ– cohort ($30,069 during baseline; $30,809 during follow-up [2021 USD]) were considerably higher than these estimates. This could be because: (1) patients in the current study were older than those in the earlier studies (mean age of 58 years vs mid-40s) [30, 31, 34]; (2) patients in our study likely had longer-standing PsO disease as our index date was any time after a first PsO diagnosis, while the other studies defined index as first PsO diagnosis; and (3) inflation.
Various studies have reported on the costs of HZ in different populations in the US. In 2009, Yawn et al. [35] reported that, during the 3 weeks before to 3 months after an HZ diagnosis, patients in a community population had means of 2.39 outpatient visits, 0.20 ED visits, and 0.07 inpatient admissions attributable to HZ, and had an HZ-attributable mean cost of $1112 (2006 USD). This is similar to the HZ-related cost in the current study during 3 months after index ($1517 [2021 USD]) after accounting for inflation.
Meyers et al. [36] reported incremental costs among immunocompetent patients aged ≥ 50 years with HZ compared with matched controls without HZ. Their mean adjusted incremental healthcare costs were $979 and $1270 (2013 USD) for 1 and 3 months, respectively [36]. Our adjusted cost differences at these time points were $1390 and $1422 (2021 USD), respectively. Meyers et al. [37] also reported incremental costs among immunocompromised patients aged ≥ 50 years with HZ compared with matched controls without HZ. Their mean unadjusted incremental healthcare costs were $561 and $1087 (2013 USD) for 1 and 3 months, respectively.
Other studies have reported on the additional HRU and costs observed in patients with COPD [38] or rheumatoid arthritis [32] with vs without HZ. The estimated adjusted cost differences were $2983 and $3404 during month, respectively. These are higher than in the current study, likely due to different population characteristics. However, all three studies showed a high burden of HZ among patients with comorbid conditions, with much of the costs accruing soon after an HZ diagnosis.
Strengths and Limitations
Strengths of the current study include the novelty of the HRU and cost findings, breadth of our sample, relatively recent data, large sample size, and robust analytical approach used for comparative analyses. The propensity score adjustment approach allowed us to maximize the sample size, as opposed to propensity score matching, in which patients without matches would have been removed, thereby reducing the overall study sample size, and potentially reducing generalizability. While multivariable models and doubly robust propensity score adjustment may arrive at similar conclusions, propensity score adjustment is more robust to model misspecification relative to multivariable models alone.
However, this study has several limitations to consider when interpreting the results. The administrative claims in CDM are primarily generated for the payment of health services and do not include certain clinical variables, e.g., disease severity, physician notes, patient-reported outcomes, or quality of life measures. The lack of medical record review may have resulted in residual confounding and misclassification, given the complexities of the PsO population. More generally, HRU and cost calculations may be subject to omissions, missing data, or coding errors. Clinical encounters and pharmacy claims for which a particular diagnosis code is not used to specify the condition(s) for which the medical act is performed may lead to the underreporting of HRU associated with these conditions.
HZ-related costs may accumulate shortly before a patient’s HZ diagnosis during the prodromal period of disease [35]. Thus, any HZ-related HRU and cost prior to a patient’s HZ diagnosis would not have been included, so HRU and associated costs may have been underestimated. Instead, some of these costs may have been captured in the baseline all-cause cost. Although ICD-10-CM codes for acute HZ episodes have been shown to have high positive predictive values [39], it is possible that the use of one HZ-associated claim may not result in an accurate estimation of HZ incidence. There is also the possibility that HZ could have been misdiagnosed as PsO [40]. Also, data were derived from CDM, which provides data from commercial and Medicare Advantage with Part D plans. CDM may not be generalizable to patients not covered by commercial and Medicare Advantage insurance programs, such as Medicaid or patients without insurance, or patients outside the US.
Conclusions
Patients with PsO (without PsA) had a higher incidence of HZ than those without psoriatic disease. This finding may be related to their underlying disease, immunosuppressive treatments for PsO, or may be influenced by both. Patients with PsO and HZ had higher HRU and costs than those with PsO without HZ during 1 and 3 months after an HZ diagnosis. Findings from this study help to bridge a gap in the literature and, considering the high prevalence of PsO in the general population and the burden of HZ among patients with PsO, are important for understanding the impact of HZ in this population.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines (upon approval of proposals submitted to www.clinicalstudydatarequest.com). To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
References
Lee YW, Park EJ, Kwon IH, Kim KH, Kim KJ. Impact of psoriasis on quality of life: relationship between clinical response to therapy and change in health-related quality of life. Ann Dermatol. 2010;22(4):389–96.
Armstrong AW, Mehta MD, Schupp CW, Gondo GC, Bell SJ, Griffiths CEM. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157(8):940–6.
Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–70.
Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81(3):775–804.
Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–86.
Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–72.
Summa C, Patel P, Kesselman MM, Demory BM. Psoriasis: to vaccinate or not to vaccinate? Cureus. 2021;13(6): e15860.
Min C, Yoo DM, Kim M, Choi HG. Increased risk of herpes zoster in patients with psoriasis: a longitudinal follow-up study using a national sample cohort. Australas J Dermatol. 2021;62(2):183–9.
Tsai SY, Chen HJ, Lio CF, et al. Increased risk of herpes zoster in patients with psoriasis: a population-based retrospective cohort study. PLoS ONE. 2017;12(8): e0179447.
Takeshita J, Shin DB, Ogdie A, Gelfand JM. Risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis in the United Kingdom. J Invest Dermatol. 2018;138(8):1726–35.
Chen SY, Suaya JA, Li Q, et al. Incidence of herpes zoster in patients with altered immune function. Infection. 2014;42(2):325–34.
Zou A, Chen Y, Shi N, Ye Y. Risk of herpes zoster associated with biological therapies for psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Medicine (Baltimore). 2021;100(40): e27368.
Hagberg KW, Persson R, Vasilakis-Scaramozza C, et al. Herpes zoster, Hepatitis C, and tuberculosis risk with apremilast compared to biologics, DMARDs and corticosteroids to treat psoriasis and psoriatic arthritis. Clin Epidemiol. 2020;12:153–61.
Shalom G, Zisman D, Bitterman H, et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500,000 person-year study. JAMA Dermatol. 2015;151(5):533–8.
Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2019;81(1):102–10.
Chiu HY, Hung YT, Huang SW, Huang YH. Comparative risk of herpes zoster in patients with psoriatic disease on systemic treatments: a systematic review and network meta-analysis. Ther Adv Chronic Dis. 2022;13:20406223221091188.
Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84(3):274–80.
Johnson RW, Bouhassira D, Kassianos G, Leplège A, Schmader KE, Weinke T. The impact of herpes zoster and post-herpetic neuralgia on quality-of-life. BMC Med. 2010;8:37.
Matthews S, De Maria A, Passamonti M, et al. The economic burden and impact on quality of life of herpes zoster and postherpetic neuralgia in individuals aged 50 years or older in Italy. Open Forum Infect Dis. 2019;6(2):ofz007.
Centers for Disease Control and Prevention (CDC). Shingles (herpes zoster). https://www.cdc.gov/shingles/hcp/clinical-overview.html. Accessed 17 Nov 2022.
Curran D, Callegaro A, Fahrbach K, et al. Meta-Regression of Herpes Zoster Incidence Worldwide. Infect Dis Ther. 2022;11(1):389–403.
Forbes HJ, Bhaskaran K, Thomas SL, Smeeth L, Clayton T, Langan SM. Quantification of risk factors for herpes zoster: Population based case-control study. BMJ. 2014;348: g2911.
Singer D, Thompson-Leduc P, Poston S, et al. Incidence of herpes zoster in patients with rheumatoid arthritis in the United States: a retrospective cohort study. J Rheumatol 2023;50(7):873–80.
Singer D, Thompson-Leduc P, Gupta D, et al. Incidence and risk of herpes zoster in patients with ulcerative colitis and Crohn’s disease in the USA. Gastroenterol Rep (Oxf). 2023;11:goad016.
Muñoz-Quiles C, Lopez-Lacort M, Diez-Domingo J. Risk and impact of herpes zoster among COPD patients: a population-based study, 2009–2014. BMC Infect Dis. 2018;18(1):203.
Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1–30 (quiz CE2-4).
Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–8.
Anderson TC, Masters NB, Guo A, et al. Use of recombinant zoster vaccine in immunocompromised adults aged ≥19 years: recommendations of the advisory committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–4.
Centers for Disease Control and Prevention. Clinical Considerations for Use of Recombinant Zoster Vaccine (RZV, Shingrix) in Immunocompromised Adults Aged ≥19 Years. https://www.cdc.gov/shingles/vaccination/immunocompromised-adults.html. Accessed 6 Dec 2022.
Merola JF, Dennis N, Chakravarty SD, et al. Healthcare utilization and costs among patients with psoriasis and psoriatic arthritis in the USA - a retrospective study of claims data from 2009 to 2020. Clin Rheumatol. 2021;40(10):4061–70.
Al Sawah S, Foster SA, Goldblum OM, et al. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20(9):982–90.
Singer D, Thompson-Leduc P, Poston S, et al. Clinical and Economic Burden of Herpes Zoster in Patients with Rheumatoid Arthritis: A Retrospective Cohort Study Using Administrative Claims. Rheumatol Ther. 2023;10(4):933–50.
Yanni EA, Ferreira G, Guennec M, et al. Burden of herpes zoster in 16 selected immunocompromised populations in England: a cohort study in the Clinical Practice Research Datalink 2000–2012. BMJ Open. 2018;8(6): e020528.
Feldman SR, Zhao Y, Shi L, Tran MH. Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(10):874–88.
Yawn BP, Itzler RF, Wollan PC, Pellissier JM, Sy LS, Saddier P. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84(9):787–94.
Meyers JL, Madhwani S, Rausch D, Candrilli SD, Krishnarajah G, Yan S. Analysis of real-world health care costs among immunocompetent patients aged 50 years or older with herpes zoster in the United States. Hum Vaccine Immunother. 2017;13(8):1861–72.
Meyers JL, Candrilli SD, Rausch DA, Yan S, Patterson BJ, Levin MJ. Cost of herpes zoster and herpes zoster-related complications among immunocompromised individuals. Vaccine. 2018;36(45):6810–8.
Ghaswalla P, Thompson-Leduc P, Cheng WY, et al. Increased health care resource utilization and costs associated with herpes zoster among patients aged ≥50 years with chronic obstructive pulmonary disease in the United States. Chronic Obstr Pulm Dis. 2021;8(4):502–16.
Klompas M, Kulldorff M, Vilk Y, Bialek SR, Harpaz R. Herpes zoster and postherpetic neuralgia surveillance using structured electronic data. Mayo Clin Proc. 2011;86(12):1146–53.
Trondina D, Bollati Delclós G, Manzorro FP. Guttaded psoriasis vs herpes zoster. Aten Primaria. 2020;52(9):651–2.
Medical Writing/Editorial Assistance
The authors would like to thank Aruna Muthukumar and Justin Chun (Analysis Group, Inc.) for having provided help with statistical programming and exploratory analyses and for having provided analytical support on this study, respectively. The authors would also like to thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination, on behalf of GSK. Jenny Lloyd (Compass Healthcare Communications Ltd., on behalf of GSK) provided medical writing support.
Funding
GlaxoSmithKline Biologicals SA funded this study (GSK study identifier: VEO-000258 and paid the Rapid Service Fee) and was involved in all stages of study conduct, including analysis of the data. GlaxoSmithKline Biologicals SA also took in charge all costs associated with the development and publication of this manuscript.
Author information
Authors and Affiliations
Contributions
DS, PTL, SM, DG, WYC, SRS, MS, EH, MSD, and SP participated in the design of the study. WYC participated in the collection of the data; and all authors were involved in the analyses and interpretation of the study. All authors participated in the development of this manuscript and in its critical review, with important intellectual contributions. All authors had full access to the data and gave approval before submission. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The work described was carried out in accordance with the recommendations of the International Committee of Medical Journal Editors for conduct, reporting, editing, and Publication of scholarly work in medical journals.
Corresponding author
Ethics declarations
Conflict of Interest
David Singer, Nikita Stempniewicz, and Sara Poston are employees of, and hold shares in, GSK. Philippe Thompson-Leduc, Deepshekhar Gupta, Wendy Y. Cheng, Selvam R. Sendhil, Manasvi Sundar, Ella Hagopian, and Mei Sheng Duh are employees of Analysis Group, Inc., a consulting firm that has received funding from GSK for the conduct of this study. Siyu Ma declares to have received postdoctoral fellowship grant from GSK during the conduct of the study. The authors declare no other financial and non-financial relationships and activities.
Ethical Approval
The study employed a retrospective cohort study design using a large administrative claims database. Data were de-identified and comply with the requirements of the Health Insurance Portability and Accountability Act. Institutional Review Board review and approval was therefore not required, as per United States Department of Health and Human Services regulation for the protection of human subjects in research (45 CFR 46, https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46). The study has been conducted in accordance with the guiding principles of the Declaration of Helsinki. As only existing de-identified data has been analyzed and as patients have not been contacted during the course of this study, informed consent process is not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Singer, D., Thompson-Leduc, P., Ma, S. et al. Burden of Herpes Zoster Among Patients with Psoriasis in the United States. Dermatol Ther (Heidelb) 13, 2649–2668 (2023). https://doi.org/10.1007/s13555-023-00988-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13555-023-00988-y